DIC DISSEMINATED INTRAVASCULAR COAGULATION DIC IS A CLINICOPATHOLOGICAL




















- Slides: 29

DIC DISSEMINATED INTRAVASCULAR COAGULATION (DIC) IS A CLINICOPATHOLOGICAL SYNDROME THAT IS CHARACTERIZED BY SYSTEMIC ACTIVATION OF COAGULATION AND FIBRINOLYSIS, CONSUMPTION OF PLATELETS AND COAGULATION FACTORS, AND GENERATION OF FIBRIN CLOTS THAT CAN LEAD TO ISCHEMIC ORGAN DAMAGE OR FAILURE

DIC A continuum in severity with definable phases characterized by initial localization and compensation (non-overt or compensated DIC) that progresses into widespread dysregulation of coagulation and fibrinolysis (overt or decompensated DIC).

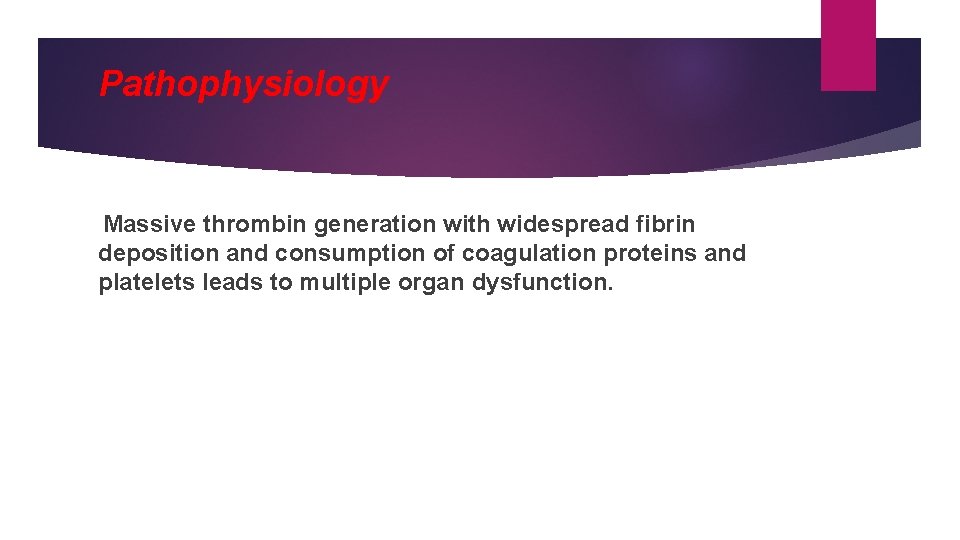
Pathophysiology Massive thrombin generation with widespread fibrin deposition and consumption of coagulation proteins and platelets leads to multiple organ dysfunction.

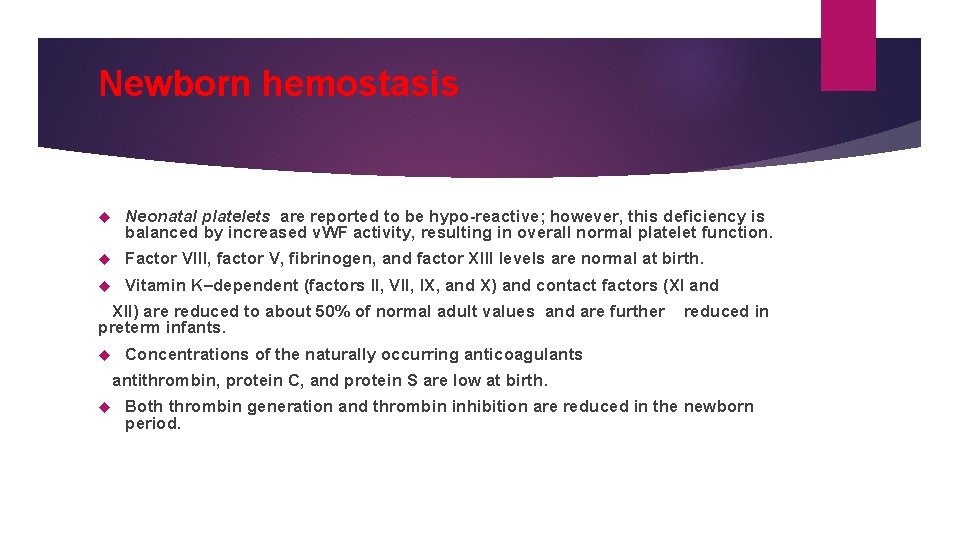
Newborn hemostasis Neonatal platelets are reported to be hypo-reactive; however, this deficiency is balanced by increased v. WF activity, resulting in overall normal platelet function. Factor VIII, factor V, fibrinogen, and factor XIII levels are normal at birth. Vitamin K–dependent (factors II, VII, IX, and X) and contact factors (XI and XII) are reduced to about 50% of normal adult values and are further preterm infants. reduced in Concentrations of the naturally occurring anticoagulants antithrombin, protein C, and protein S are low at birth. Both thrombin generation and thrombin inhibition are reduced in the newborn period.

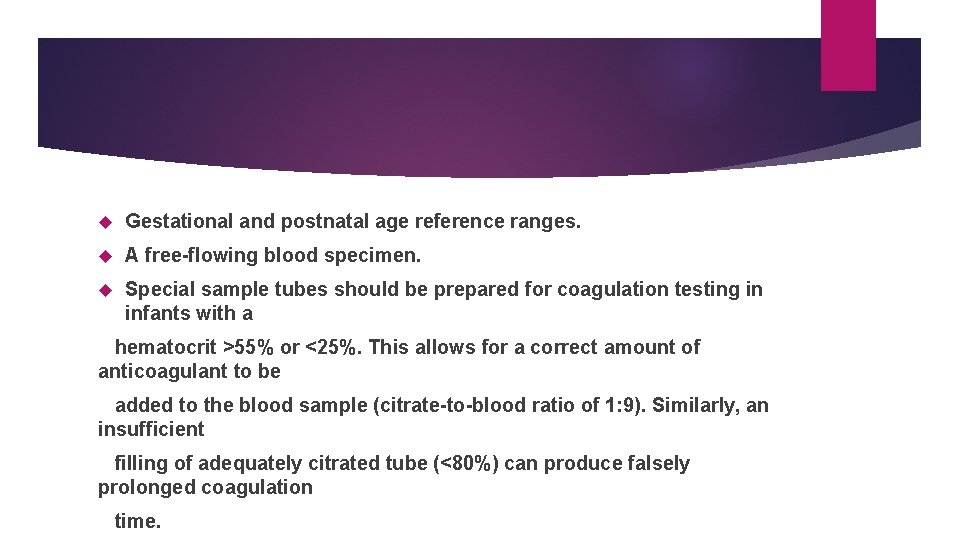
Gestational and postnatal age reference ranges. A free-flowing blood specimen. Special sample tubes should be prepared for coagulation testing in infants with a hematocrit >55% or <25%. This allows for a correct amount of anticoagulant to be added to the blood sample (citrate-to-blood ratio of 1: 9). Similarly, an insufficient filling of adequately citrated tube (<80%) can produce falsely prolonged coagulation time.

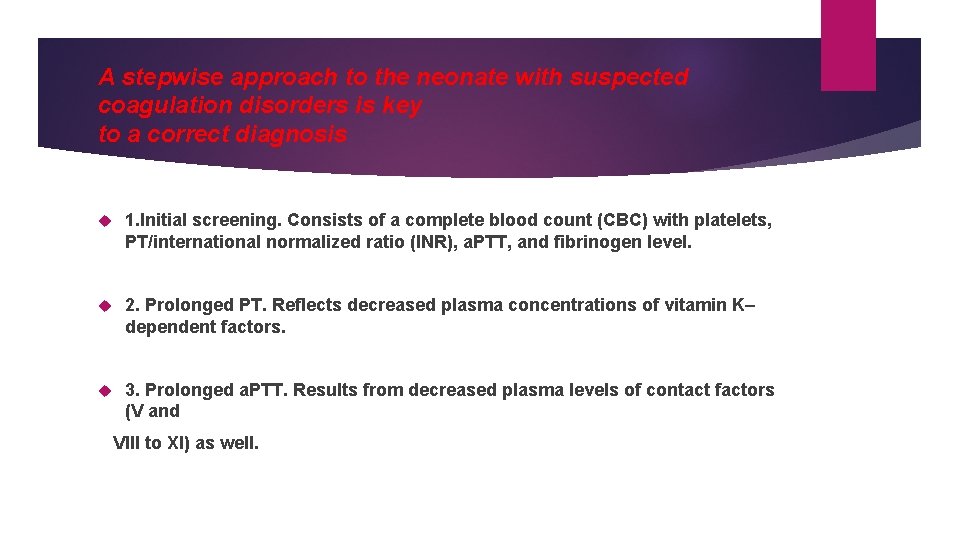
A stepwise approach to the neonate with suspected coagulation disorders is key to a correct diagnosis 1. Initial screening. Consists of a complete blood count (CBC) with platelets, PT/international normalized ratio (INR), a. PTT, and fibrinogen level. 2. Prolonged PT. Reflects decreased plasma concentrations of vitamin K– dependent factors. 3. Prolonged a. PTT. Results from decreased plasma levels of contact factors (V and VIII to XI) as well.

4. Bleeding neonate who has no laboratory abnormality , Factor XIII and α 2 antiplasmin activity should be assessed. 5. Elevated D-dimers, as an acute phase reaction in all patients with infection or systemic inflammatory response syndrome (SIRS). A negative d -dimer assay is relatively accurate in ruling out thrombosis.

Diagnosis Thrombocytopenia Elevated fibrin degradation products (FDP) or D-dimers Prolonged PT, prolonged a. PTT, prolonged thrombin clotting time

Serial testing may be necessary for the diagnosis, given the dynamic nature of DIC Fibrinogen is an acute-phase reactant; plasma fibrinogen can remain well within the normal range despite ongoing consumption

Clinical presentation Persistent oozing from the umbilical stump, Excessive bleed-ing from peripheral venipuncture/heelstick sites, Large caput succedaneum and cephalhematoma or subgaleal hemorrhage occurring without significant birth trauma history Prolonged bleeding following circumcision Intracranial hemorrhage in a term or late preterm infant without history of birth trauma

Gastrointestinal bleeding must be distinguished from swallowed maternal blood Pulmonary hemorrhages are most frequently hemorrhagic pulmonary edema not associated with specific coagulation anomaly. Major abdominal organ bleeding such as liver or spleen are more often related to traumatic injury or local lesion (such as teratoma) than any coagulopathy.

Overt DIC Excessive bleeding Microvascular thrombosis Hemorrhagic symptoms include petechiae and bruising, oozing from venipuncture sites, bleeding from traumatic and surgical wounds, and in severe cases, bleeding involving internal organs Thrombosis typically manifests as biochemical and/or clinical evidence of end-organ dysfunction.

Maternal, family, and neonatal history A history of any prior pregnancies and their outcomes can be a clue for disorders such as neonatal alloimmune thrombocytopenia. Maternal medication use can also lead to immune-mediated thrombocytopenia. Parental ethnic background and whethere is consanguinity are significant. Perinatal complications can result in coagulation activation and disseminated intravascular coagulation (DIC).

Physical examination An otherwise normal neonate with thrombocytopenia is suggestive of alloimmune thrombocytopenia. Skeletal abnormalities such as absence of thumb or radii are important clues for conditions such as thrombocytopenia with absent radii or Fanconi anemia. Cardiac defects may be associated with factor V deficiency. Delayed cord separation and persistent oozing from the umbilical stump is typical for infants with defective fibrinogen production or function and factor XIII deficiency. Acquired consumptive coagulopathy is generally a secondary event in a “sick” acting infant. Bacterial or viral infection and metabolic disorders (such as tyrosinemia)

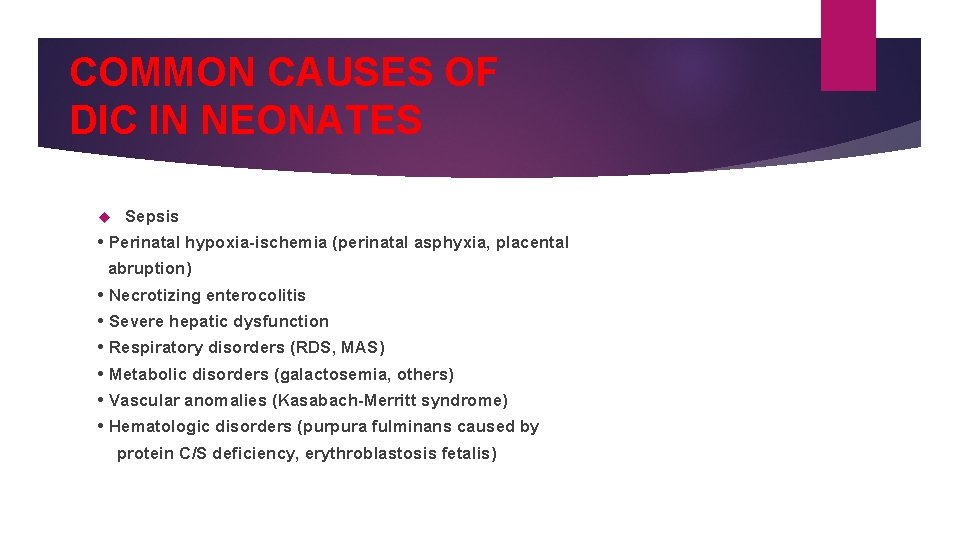
COMMON CAUSES OF DIC IN NEONATES Sepsis • Perinatal hypoxia-ischemia (perinatal asphyxia, placental abruption) • Necrotizing enterocolitis • Severe hepatic dysfunction • Respiratory disorders (RDS, MAS) • Metabolic disorders (galactosemia, others) • Vascular anomalies (Kasabach-Merritt syndrome) • Hematologic disorders (purpura fulminans caused by protein C/S deficiency, erythroblastosis fetalis)

The presence of DIC in a neonate without any evidence of sepsis or history of asphyxia should warrant the evaluation for a capillary hemangioma.

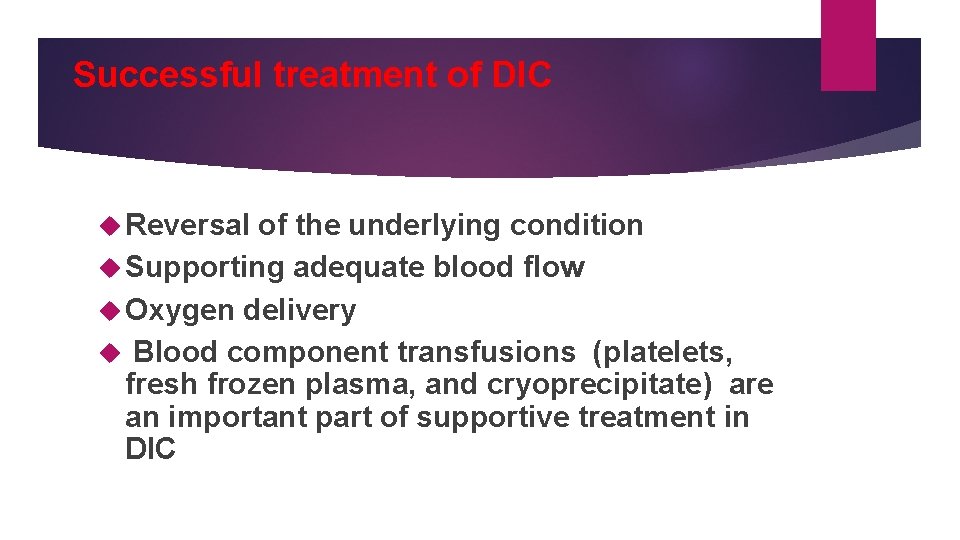
Successful treatment of DIC Reversal of the underlying condition Supporting adequate blood flow Oxygen delivery Blood component transfusions (platelets, fresh frozen plasma, and cryoprecipitate) are an important part of supportive treatment in DIC

Goals Platelets >50, 000– 100, 000/μL PT <3 seconds above the upper limit of normal, Fibrinogen >100 mg/d. L

Fresh frozen plasma In bleeding patients with DIC and prolonged (PT) and (a. PTT), administration (FFP) may be useful. It should not be instituted based on laboratory tests alone but should be considered in those with active bleeding and in those requiring an invasive procedure. There is no evidence that infusion of plasma stimulates the ongoing activation of coagulation

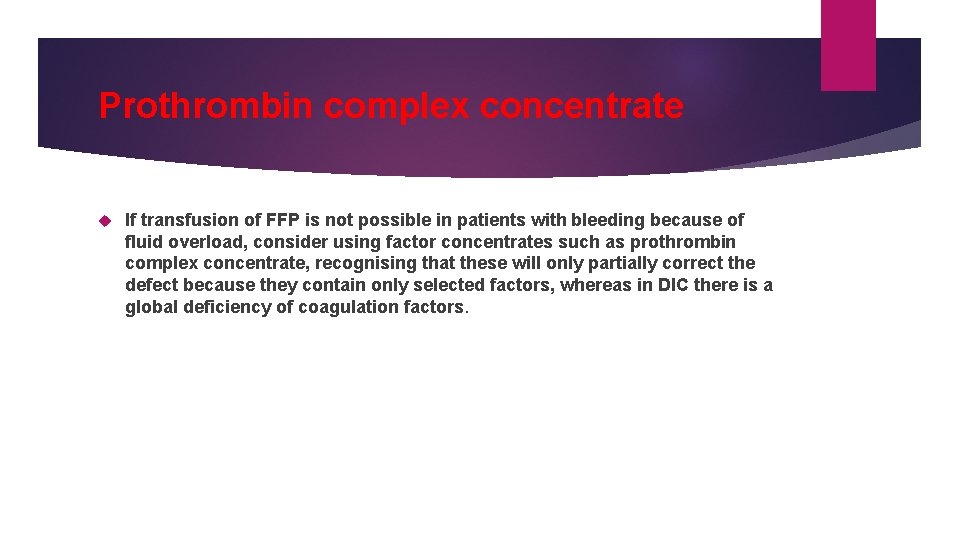
Prothrombin complex concentrate If transfusion of FFP is not possible in patients with bleeding because of fluid overload, consider using factor concentrates such as prothrombin complex concentrate, recognising that these will only partially correct the defect because they contain only selected factors, whereas in DIC there is a global deficiency of coagulation factors.

Transfusion of platelets or plasma (components) in patients with DIC should not primarily be based on laboratory results and should in general be reserved for patients who present with bleeding. In patients with DIC and bleeding or at high risk of bleeding (e. g. postoperative patients or patients due to undergo an invasive procedure) and a platelet count of <50 x 10(9)/l transfusion of platelets should be considered In non-bleeding patients with DIC, prophylactic platelet transfusion is not given unless it is perceived that there is a high risk of bleeding

Fibrinogen concentrate Severe hypofibrinogenaemia (<1 g/l) that persists despite FFP replacement may be treated with fibrinogen concentrate or cryoprecipitate.

Heparin In cases of DIC where thrombosis predominates, such as arterial or venous thromboembolism, severe purpura fulminans associated with acral ischemia or vascular skin infarction, therapeutic doses of heparin should be considered. In these patients where there is perceived to be a co-existing high risk of bleeding there may be benefits in using continuous infusion unfractionated heparin (UFH) due to its short half-life and reversibility

Heparin In critically ill, non-bleeding patients with DIC, prophylaxis for venous thromboembolism with prophylactic doses of heparin or low molecular weight heparin is recommended

Anticoagulant therapy is not generally used. The benefit has not proved to out-weigh the added risk for hemorrhage. The use of activated protein C is controversial (increased risk of ICH).

Recombinant factor VIIa (r. FVIIa; 40– 300 mcg/kg) This has been used successfully to treat severe bleeding in infants with DIC. In the presence of endothelial damage, r. FVIIa binds to exposed tissue factor to activate factor X and thus generate thrombin. The potential for thrombotic complications makes its use limited to life-threatening hemorrhagic situations

Recombinant human activated protein C Consider treating patients with severe sepsis and DIC with recombinant human activated protein C (continuous infusion, 24 microg/kg/h for 4 d). Patients at high risk of bleeding should not be given recombinant human activated protein C. Current manufacturers guidance advises against using this product in patients with platelet counts of <30 x 10(9)/l. In the event of invasive procedures, administration of recombinant human activated protein C should be discontinued shortly before the intervention (elimination half-life approximately 20 min) and may be resumed a few hours later, dependent on the clinical situation.

Except for the use of protein C concentrates in purpura fulminans owing to protein C deficiency, anticoagulant concentrates (protein C, antithrombin), recombinant factor VIIa, and recombinant tissue plasminogen activator are not generally. indicated for the routine treatment of DIC

Antithrombin cannot be recommended. In general, patients with DIC should not be treated with antifibrinolytic agents. Patients with DIC that is characterised by a primary hyperfibrinolytic state and who present with severe bleeding could be treated with lysine analogues, such as tranexamic acid (e. g. 1 g every 8 h).
 Disseminated intravascular coagulation pathophysiology
Disseminated intravascular coagulation pathophysiology Dic labs
Dic labs Two node loop instability
Two node loop instability Liquido intravascular
Liquido intravascular Esquema de liquidos corporales
Esquema de liquidos corporales Intravascular hemolytic anemia
Intravascular hemolytic anemia Disforese
Disforese Ivus
Ivus Liquido intravascular
Liquido intravascular Ivus
Ivus Generalidades de la mecanica corporal
Generalidades de la mecanica corporal Espaço intravascular
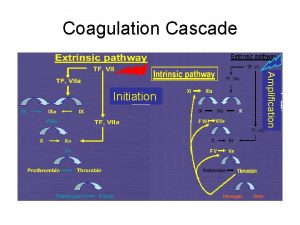
Espaço intravascular Coagulation pathway made easy
Coagulation pathway made easy Describe the mechanism of coagulation of blood
Describe the mechanism of coagulation of blood Plasma and serum
Plasma and serum Coagulation profile test
Coagulation profile test Coagulation disorders
Coagulation disorders Dextrinisation food examples
Dextrinisation food examples Coagulation casecade
Coagulation casecade Necrose de liquefaction
Necrose de liquefaction Coagulation eggs definition
Coagulation eggs definition Coagulation of blood flow chart
Coagulation of blood flow chart Desiccation diathermy
Desiccation diathermy Coagulate egg
Coagulate egg Loading or coagulation
Loading or coagulation Vasoconstriction
Vasoconstriction Coagulation cascade
Coagulation cascade Slide method clotting time
Slide method clotting time Coagulation profile test
Coagulation profile test Antifibrinolytic drugs
Antifibrinolytic drugs