Define Cation a positively charged ion Anion a



![Lewis Dots for Ionic compounds 1+ [Li] [ 1 F] 2+ [Ca ] [O Lewis Dots for Ionic compounds 1+ [Li] [ 1 F] 2+ [Ca ] [O](https://slidetodoc.com/presentation_image_h2/bcfd3f29fc14d26c76b44e3dcd5a6743/image-9.jpg)



- Slides: 37


Define: › Cation – a positively charged ion › Anion – a negatively charged ion › Oxidation number – the charge on an ion › Electronegativity – How strongly an atom attracts other electrons. › Lone Pair - Those two valence electrons in a Lewis Dot structure that are NOT bonded. › Bonding Pair - Two valence electrons shared between atoms that ARE bonded.

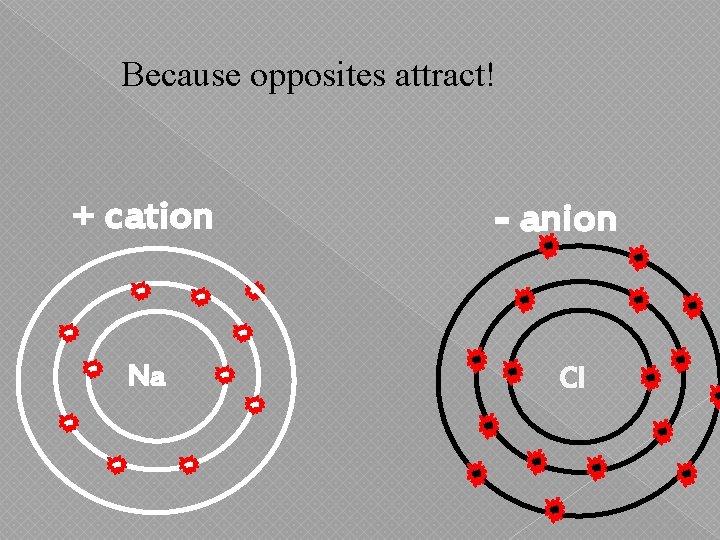
An atom that gains one or more electrons will have a NEGATIVE __________ charge. An atom that loses one or more electrons will have a POSITIVE __________ charge. An atom that gains or loses one or more electrons is ION___ called an _____. CATION A positive ion is called a _______ and a negative ion is called an ________. ANION

Ionic Bonds ELECTRONS to another to Atoms will transfer one or more ________ form the bond. COMPLETE Each atom is left with a ________ outer shell… that is (usually) with 8 electrons METAL ion with a positive An ionic bond forms between a ______ NONMETAL charge and a ________ ion with a negative charge.

Because opposites attract! + cation Na - anion Cl

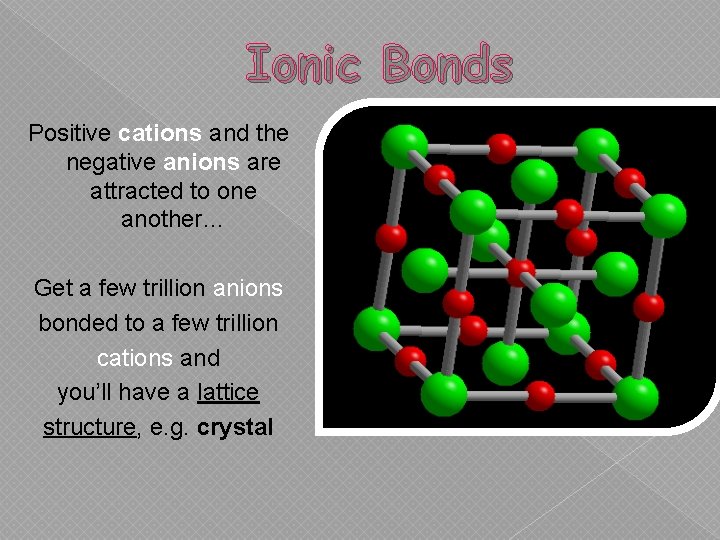
Ionic Bonds Positive cations and the negative anions are attracted to one another… Get a few trillion anions bonded to a few trillion cations and you’ll have a lattice structure, e. g. crystal

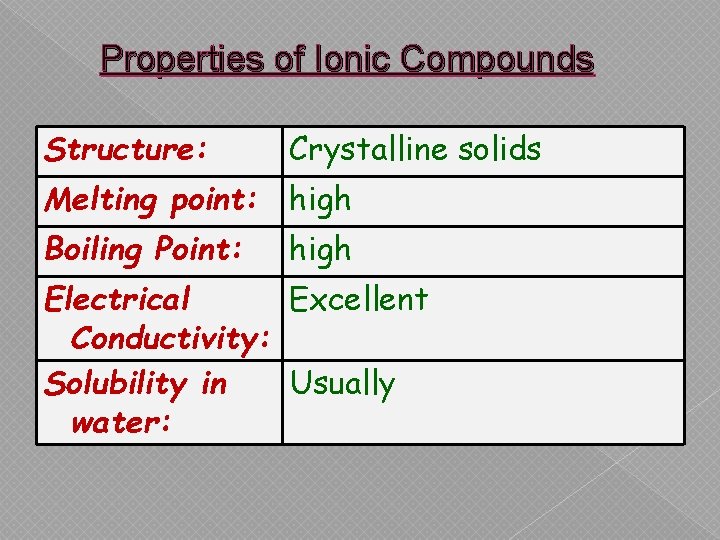
Properties of Ionic Compounds Structure: Crystalline solids Melting point: high Boiling Point: high Electrical Excellent Conductivity: Solubility in Usually water:

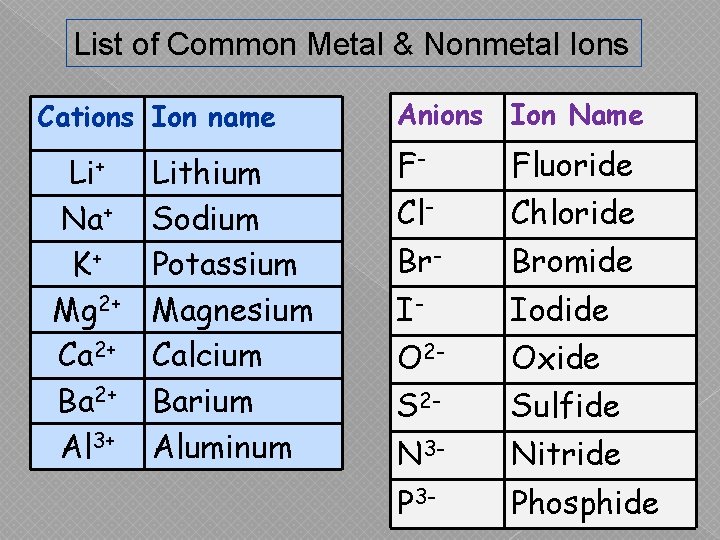
List of Common Metal & Nonmetal Ions Cations Ion name Li+ Na+ K+ Mg 2+ Ca 2+ Ba 2+ Al 3+ Lithium Sodium Potassium Magnesium Calcium Barium Aluminum Anions Ion Name F- Fluoride Cl- Chloride Br- Bromide I- Iodide O 2 - Oxide S 2 - Sulfide N 3 - Nitride P 3 - Phosphide
![Lewis Dots for Ionic compounds 1 Li 1 F 2 Ca O Lewis Dots for Ionic compounds 1+ [Li] [ 1 F] 2+ [Ca ] [O](https://slidetodoc.com/presentation_image_h2/bcfd3f29fc14d26c76b44e3dcd5a6743/image-9.jpg)
Lewis Dots for Ionic compounds 1+ [Li] [ 1 F] 2+ [Ca ] [O 2+ [Ca ] 2[ F 2] 1]

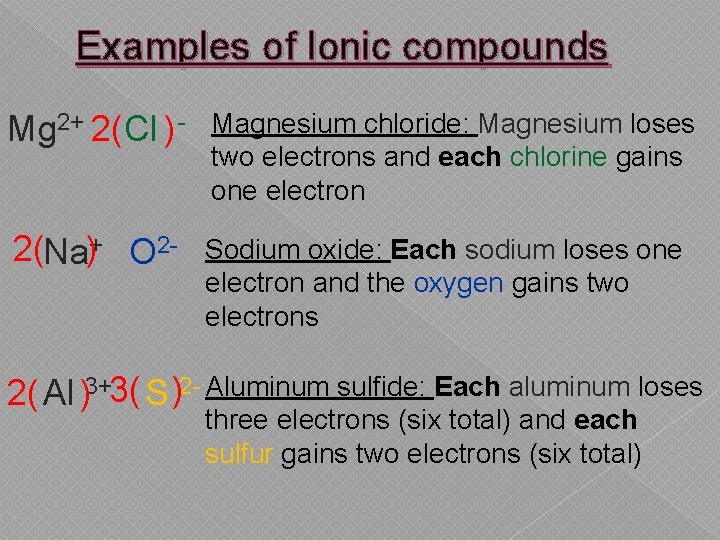
Examples of Ionic compounds Mg 2+ 2(Cl ) - Magnesium chloride: Magnesium loses two electrons and each chlorine gains one electron 2(Na)+ O 2 - Sodium oxide: Each sodium loses one electron and the oxygen gains two electrons 2( Al )3+3( S )2 - Aluminum sulfide: Each aluminum loses three electrons (six total) and each sulfur gains two electrons (six total)

Covalent Bonds SHARE one or more electrons with each other to form Atoms ______ the bond. COMPLETE Each atom is left with a ________ outer shell. NONMETAL A covalent bond forms between two _________.

Sharing is caring! O O

Covalent Bonds � Electrons are shared between nonmetals & other nonmetals › Can share 1, 2, or 3 electrons › Neither atom is “strong” enough to swipe It… so they share it.

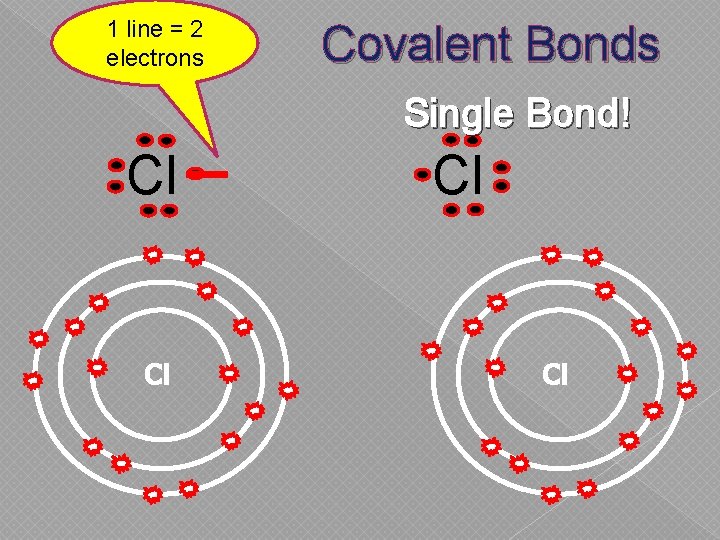
1 line = 2 electrons Covalent Bonds Single Bond! Cl Cl

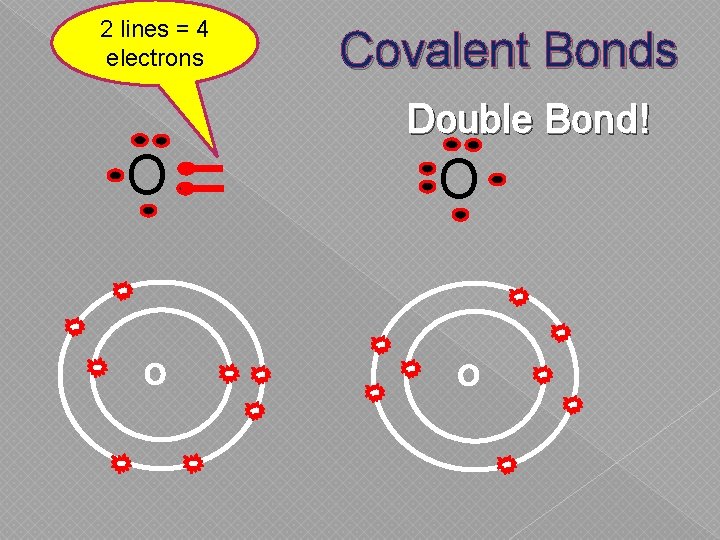
2 lines = 4 electrons Covalent Bonds Double Bond! O O

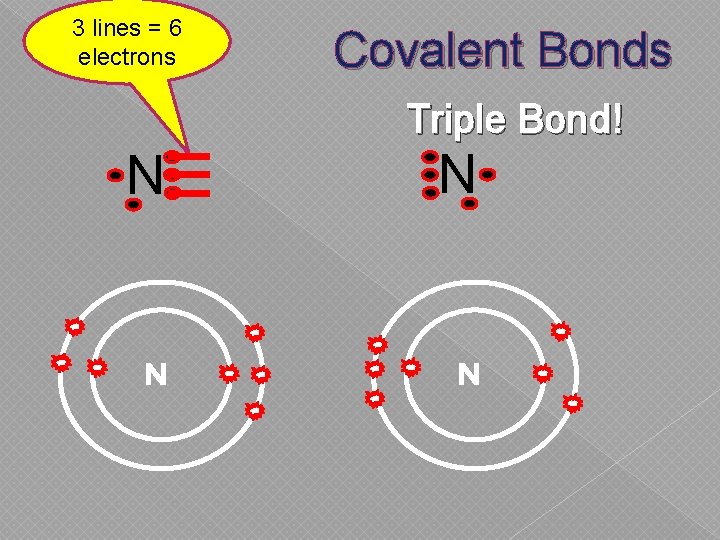
3 lines = 6 electrons Covalent Bonds Triple Bond! N N

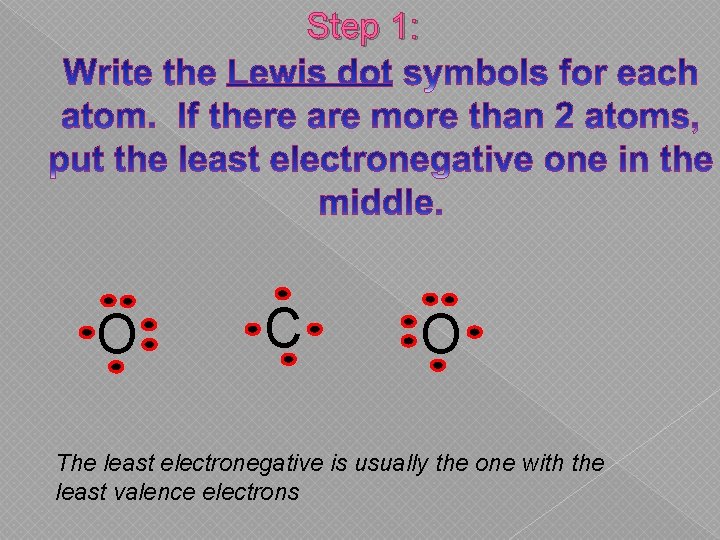
Step 1: O C O The least electronegative is usually the one with the least valence electrons

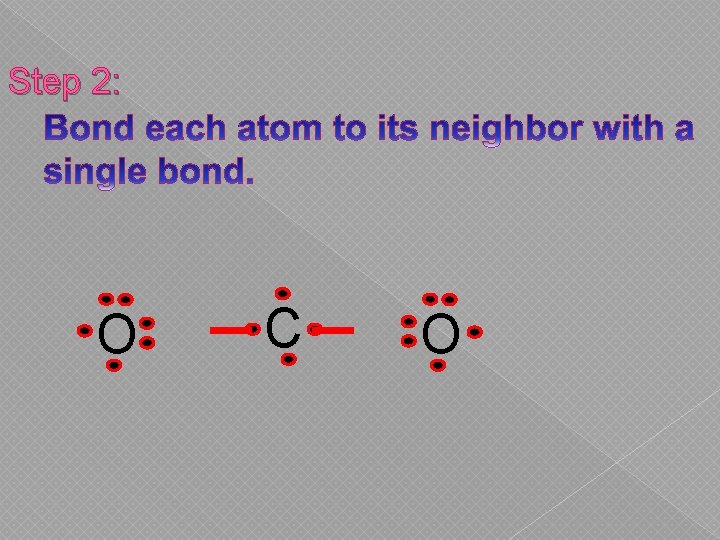
Step 2: O C O

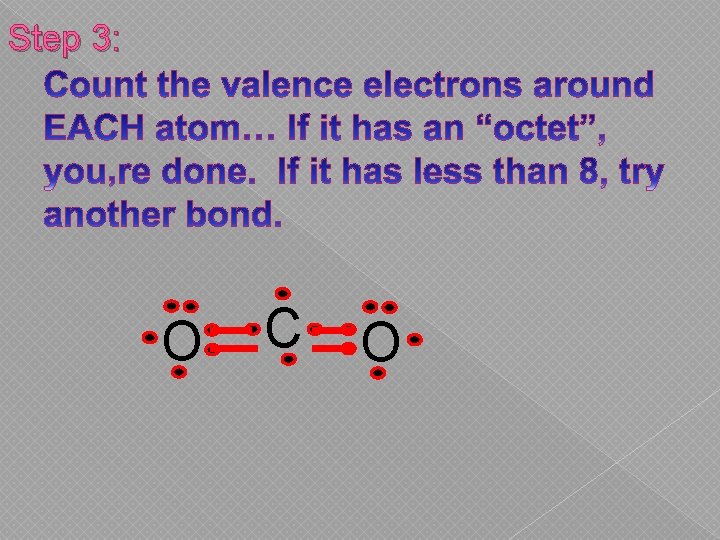
Step 3: O C O

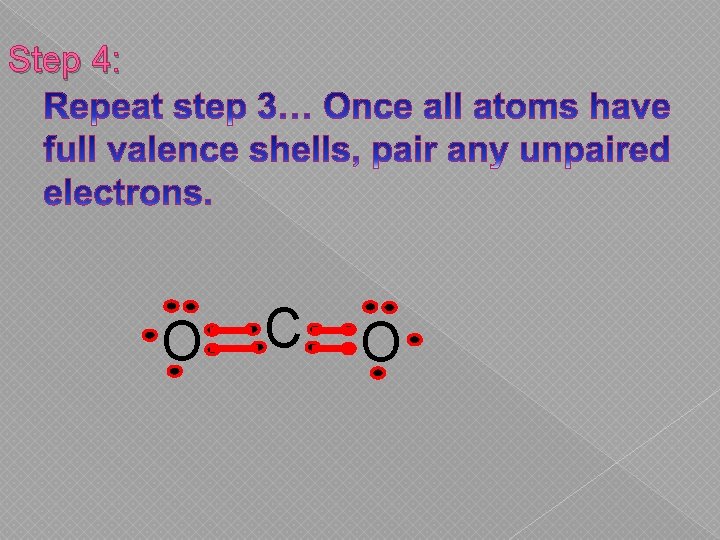
Step 4: O C O

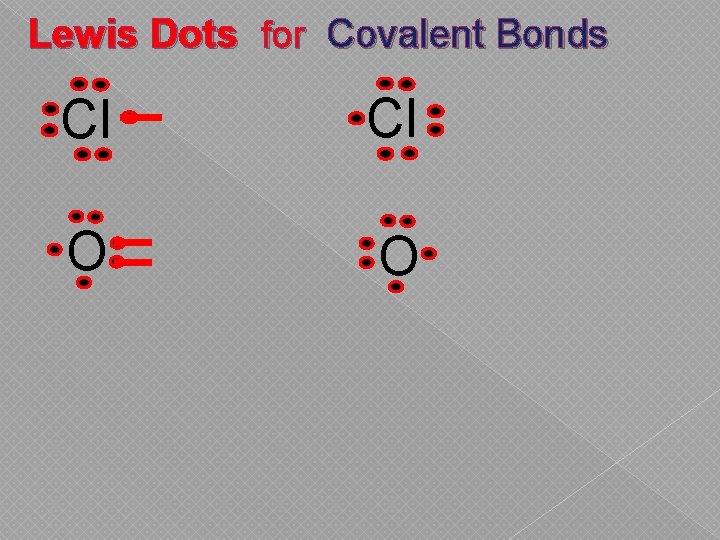
Lewis Dots for Covalent Bonds Cl Cl O O

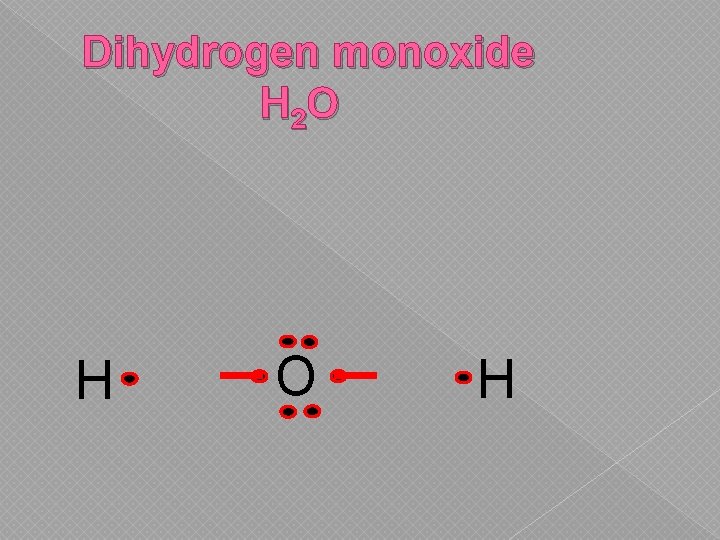
Dihydrogen monoxide H 2 O H

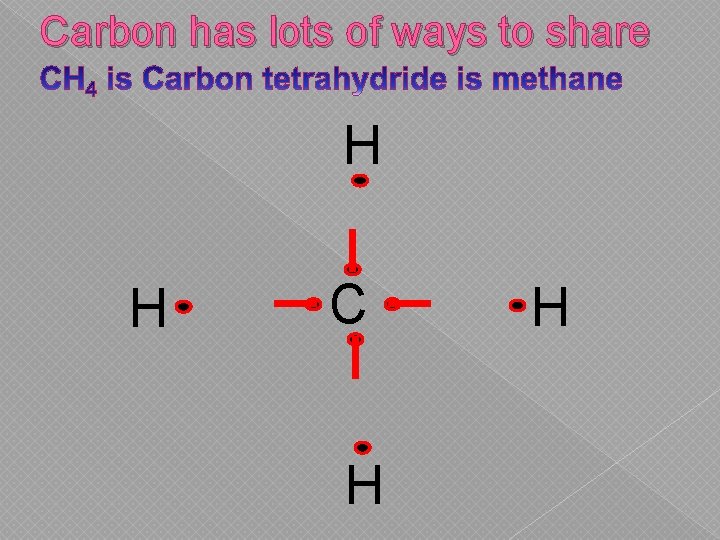
Carbon has lots of ways to share H H C H H

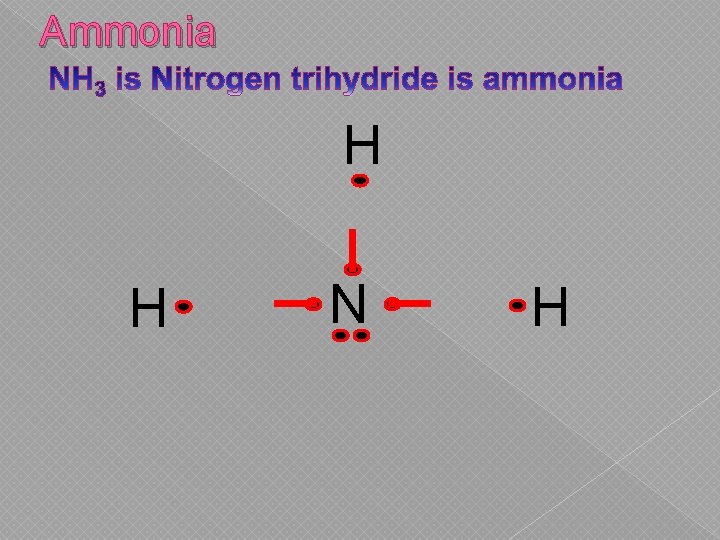
Ammonia H H N H

Your turn… � I 2 � S 2 � P 2 � OF 2 � NF 3 � Si. H 4

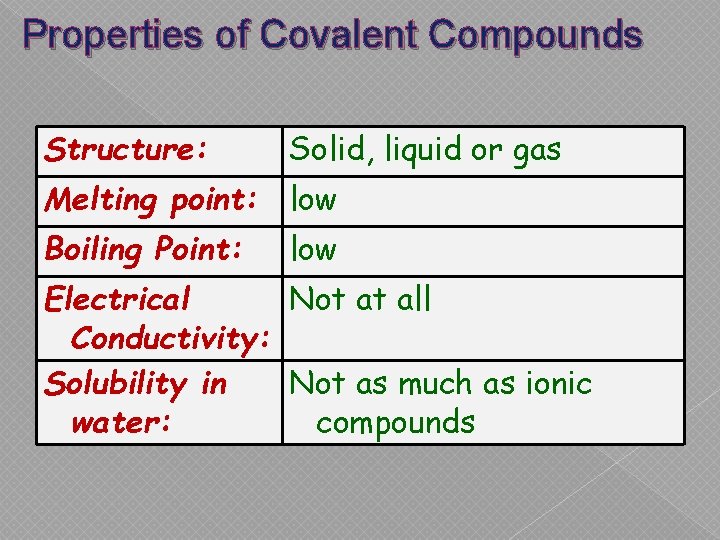
Properties of Covalent Compounds Structure: Solid, liquid or gas Melting point: low Boiling Point: low Electrical Not at all Conductivity: Solubility in Not as much as ionic water: compounds

Non-Polar Covalent Bonds � electronegativity difference between 0. 0 - 0. 3 › …usually it’s the same diatomic element bonding to itself. � Or if the middle atom is pulled symmetrically in all direction › Ex. O 2, N 2, CO 2, CH 4

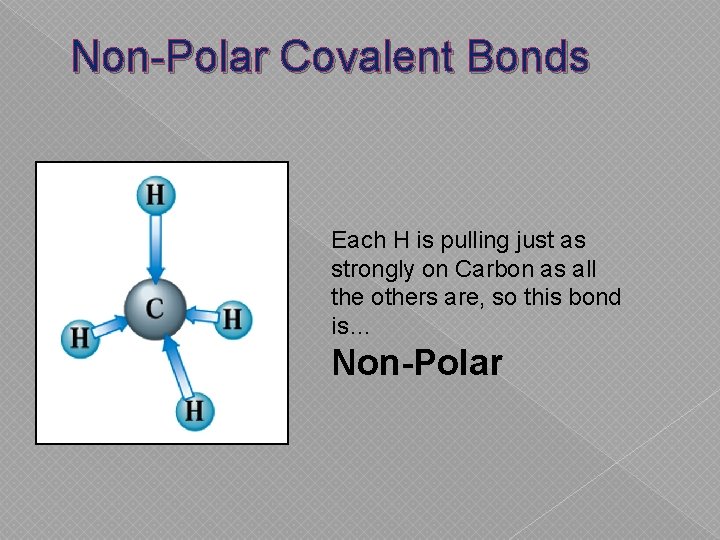
Non-Polar Covalent Bonds Each H is pulling just as strongly on Carbon as all the others are, so this bond is… Non-Polar

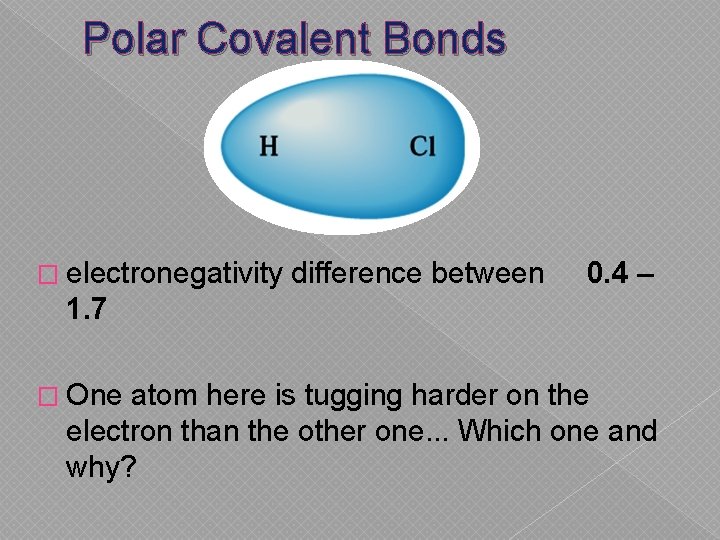
Polar Covalent Bonds � electronegativity difference between 0. 4 – 1. 7 � One atom here is tugging harder on the electron than the other one. . . Which one and why?

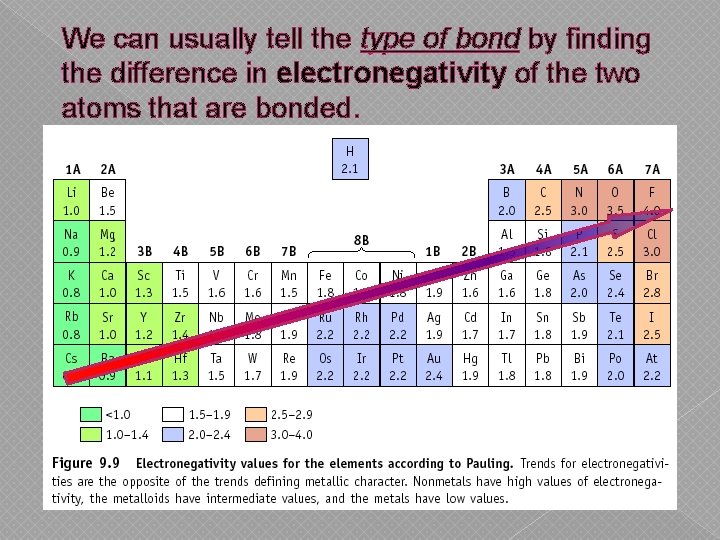
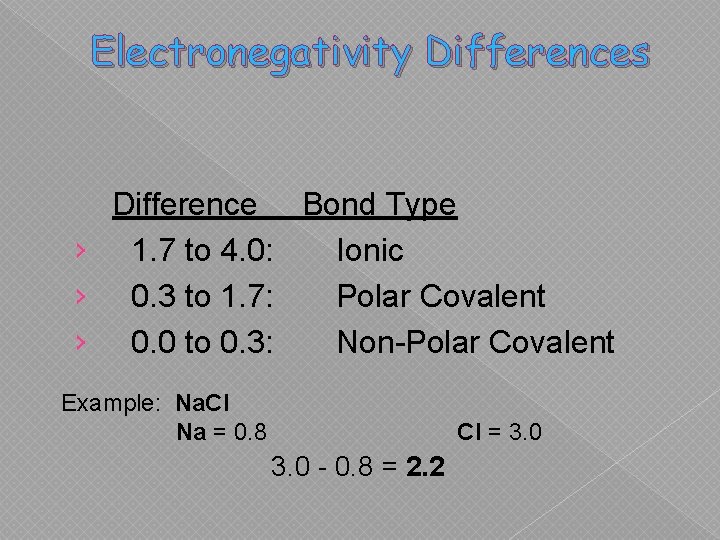
We can usually tell the type of bond by finding the difference in electronegativity of the two atoms that are bonded.

Electronegativity Differences Difference Bond Type › 1. 7 to 4. 0: Ionic › 0. 3 to 1. 7: Polar Covalent › 0. 0 to 0. 3: Non-Polar Covalent Example: Na. Cl Na = 0. 8 Cl = 3. 0 - 0. 8 = 2. 2

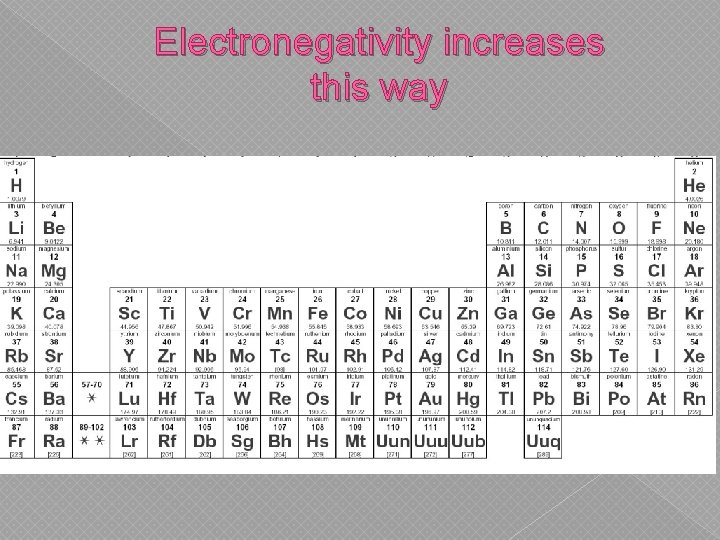
Electronegativity increases this way

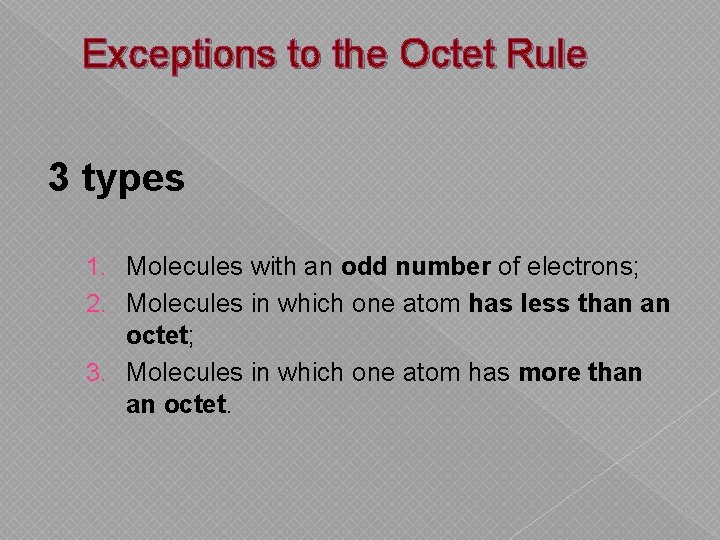
Exceptions to the Octet Rule 3 types 1. Molecules with an odd number of electrons; 2. Molecules in which one atom has less than an octet; 3. Molecules in which one atom has more than an octet.

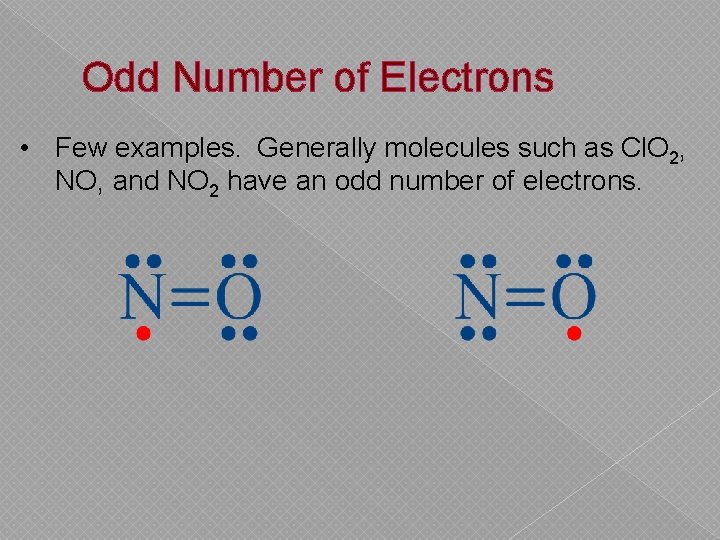
Odd Number of Electrons • Few examples. Generally molecules such as Cl. O 2, NO, and NO 2 have an odd number of electrons.

Less than an Octet • Relatively rare. • Molecules with less than an octet are typical for compounds of Groups 1 A, 2 A, and 3 A. • Most typical example is BF 3. • Formal charges indicate that the Lewis structure with an incomplete octet is more important than the ones with double bonds.

More than an Octet • This is the largest class of exceptions. • Atoms from the 3 rd period onwards can accommodate more than an octet. • Beyond the third period, the d-orbitals are low enough in energy to participate in bonding and accept the extra electron density.

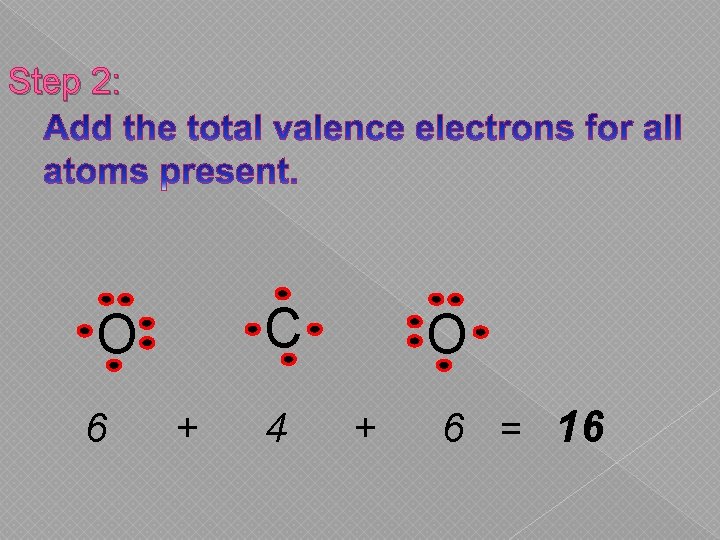
Step 2: C O 6 + 4 O + 6 = 16