CTD Dossier Preparation K Srikantha Reddy Sr ManagerRegulatory


























- Slides: 46

CTD Dossier Preparation K. Srikantha Reddy Sr. Manager-Regulatory Affairs Medreich Limited Srikanth. k@medreich. com

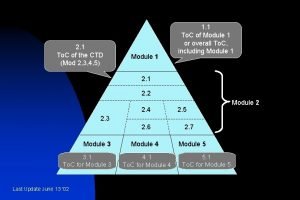
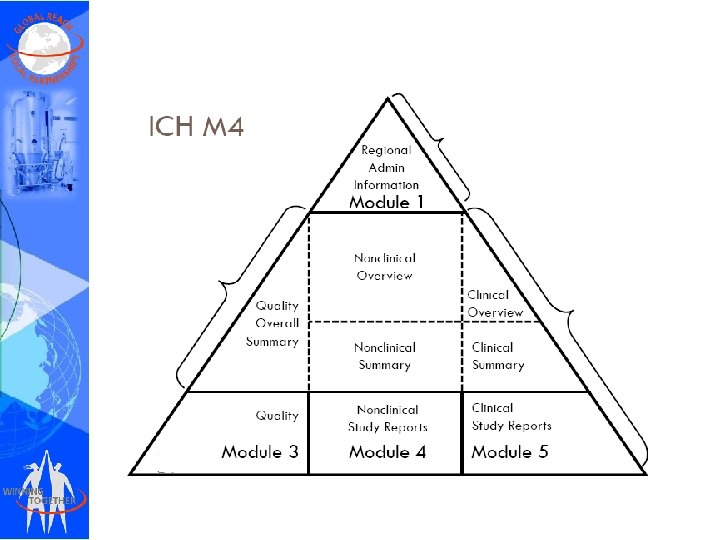
CTD Dossier Preparation • CTD (Common Technical Document) contains 5 modules • • • Module – 1 Module – 2 Module – 3 Module – 4 Module – 5

DMF Drug Master File (DMF) is a submission to the Food and Drug Administration (FDA) that may be used to provide confidential detailed information about facilities, processes, or articles used in the manufacturing, processing, packaging, and storing of one or more human drugs. The information contained in the DMF may be used to support following, – – Investigational New Drug Application (IND), New Drug Application (NDA), Abbreviated New Drug Application (ANDA), Export Application.

ANDA: • An Abbreviated New Drug Application (ANDA) is an application for a U. S. generic drug approval for an existing licensed medication or approved drug. • The ANDA contains data which when submitted to FDA's Center For drug Evaluation and Research (CDER), Office of Generic Drugs, provides for the review and ultimate approval of a generic drug product. Once approved, an applicant may manufacture and market the generic drug product to provide a safe, effective, low cost alternative to the American public.


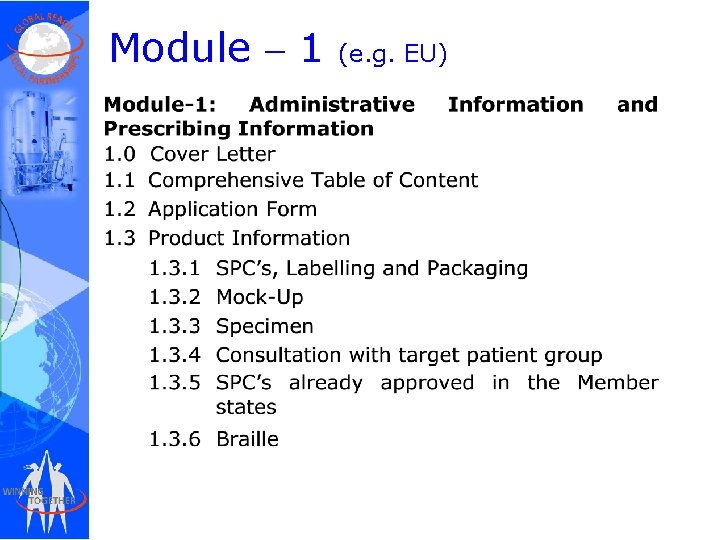
Module – 1 (e. g. EU)

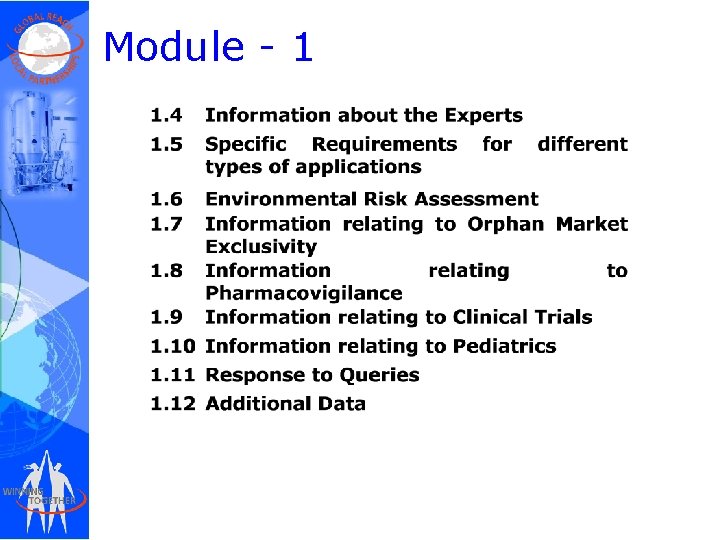
Module - 1

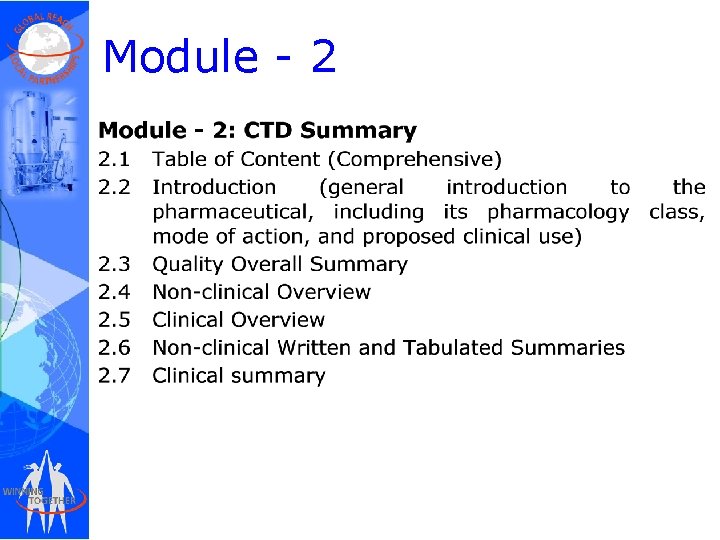
Module - 2

Module - 2

Module - 2

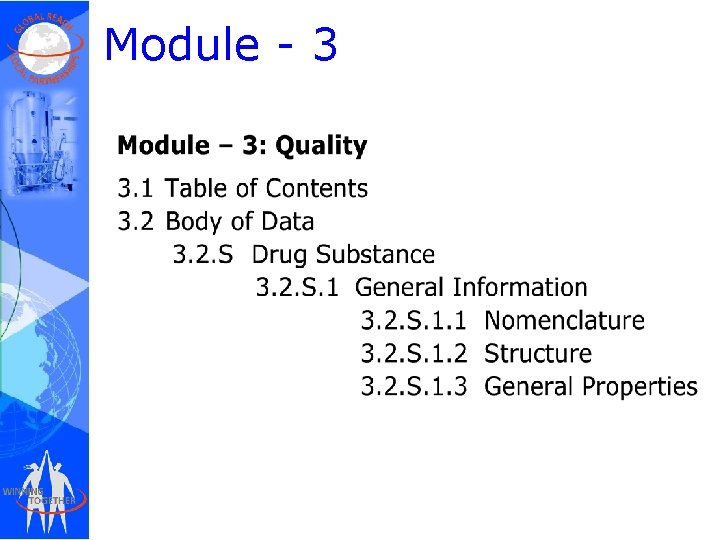
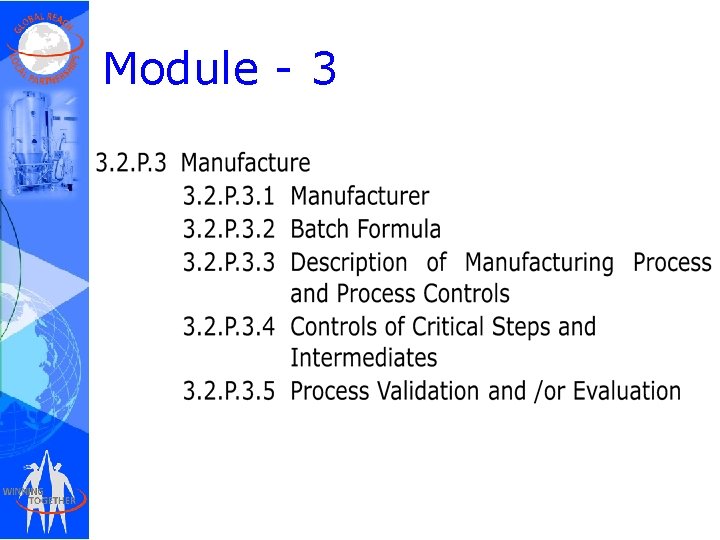
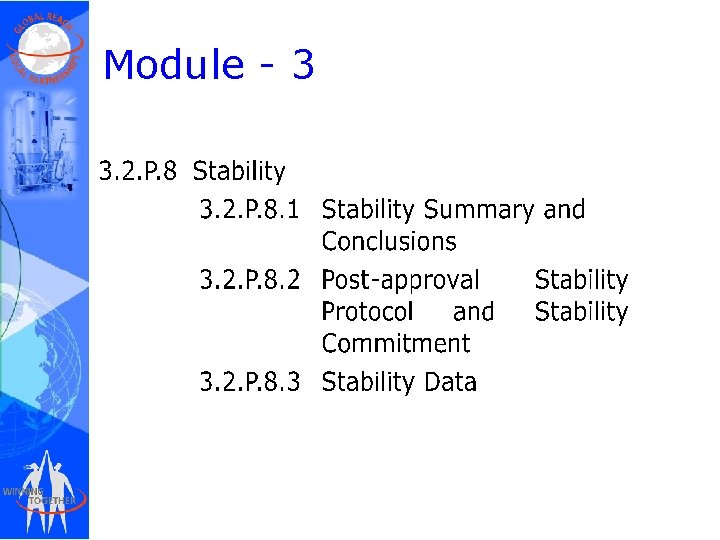
Module - 3

Module - 3

Module - 3

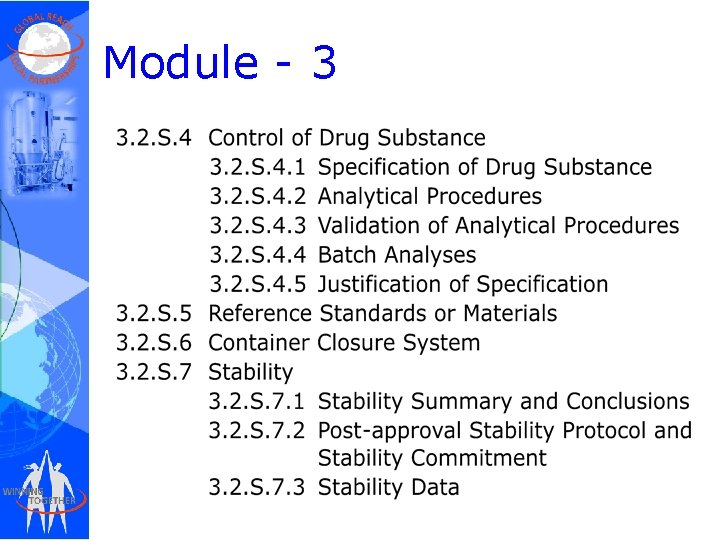
Module - 3

Module - 3

Module - 3

Module - 3

Module - 3

Module - 3

Module - 4

Module - 4

Module - 4

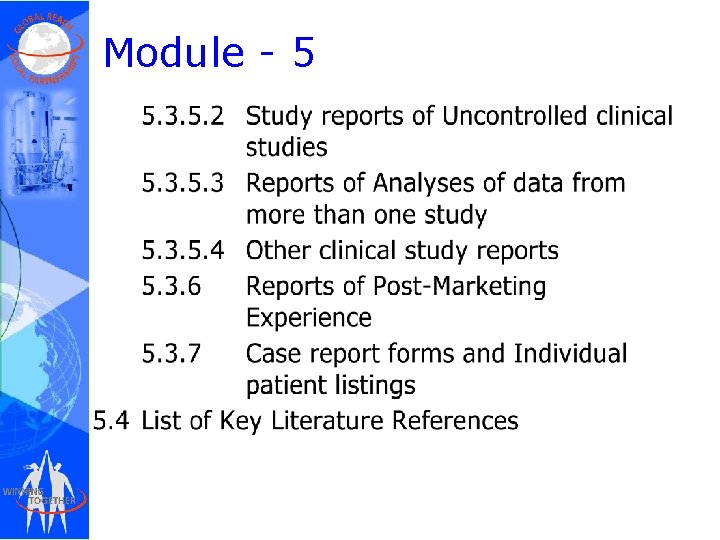
Module - 5

Module - 5

Module - 5

e. CTD (Version 3. 2. 2) 06. 05. 2011 K. Srikantha Reddy Sr. Manager-Regulatory Affairs Medreich Limited Srikanth. k@medreich. com

e. CTD • e. CTD – electronic Common Technical Document • The e. CTD is the electronic equivalent to the CTD. • Regulatory Perspective • “The e. CTD is defined as an interface for industry to agency transfer of regulatory information while at the same time taking into consideration the facilitation of the creation, review, lifecycle management and archival of the electronic submission. ” • Common structure for Modules 2 through 5 • Agency specific requirements for Modules 1

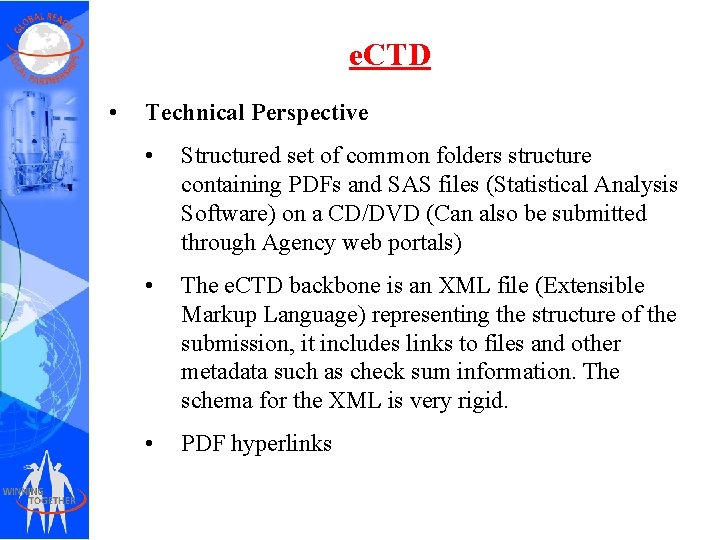
e. CTD • Technical Perspective • Structured set of common folders structure containing PDFs and SAS files (Statistical Analysis Software) on a CD/DVD (Can also be submitted through Agency web portals) • The e. CTD backbone is an XML file (Extensible Markup Language) representing the structure of the submission, it includes links to files and other metadata such as check sum information. The schema for the XML is very rigid. • PDF hyperlinks

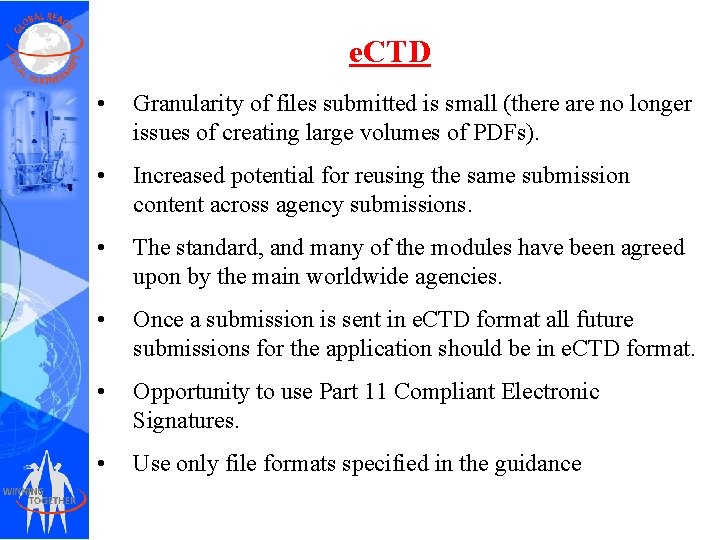
e. CTD • Granularity of files submitted is small (there are no longer issues of creating large volumes of PDFs). • Increased potential for reusing the same submission content across agency submissions. • The standard, and many of the modules have been agreed upon by the main worldwide agencies. • Once a submission is sent in e. CTD format all future submissions for the application should be in e. CTD format. • Opportunity to use Part 11 Compliant Electronic Signatures. • Use only file formats specified in the guidance

e. CTD Benefits • Easy to distribute and review • More efficient use of resources, less cost and stress to the organization • Highly organized electronic table of contents • Searchable • Self-validating • Integrated document and life-cycle management • Cross submission integration • Living document • New, replace, append & delete

How it is different to Paper/Document CTD • Overall Table of contents provided in XML (Extensible Markup Language) • Utility files to enable technical conformance and viewing • Submission Folders, XML and Utility Files are created automatically if an e. CTD builder is used. • Generally high level of granularity in documents • Structure is more precise • Lifecycle Management of the submission is easier.

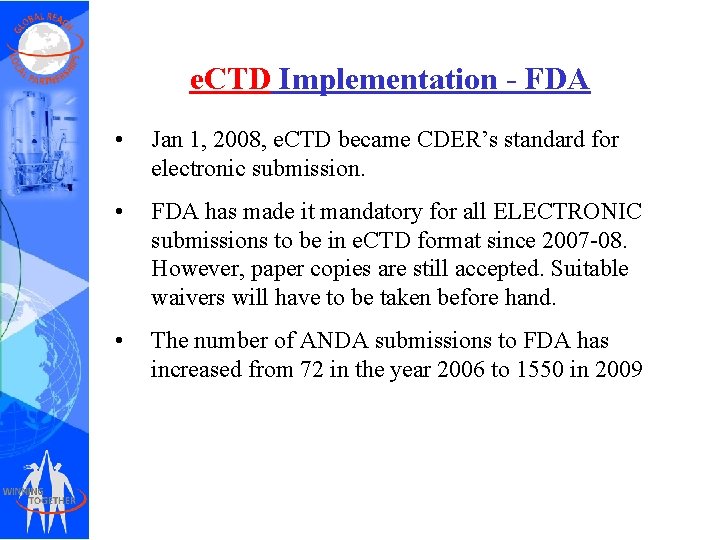
e. CTD Implementation - FDA • Jan 1, 2008, e. CTD became CDER’s standard for electronic submission. • FDA has made it mandatory for all ELECTRONIC submissions to be in e. CTD format since 2007 -08. However, paper copies are still accepted. Suitable waivers will have to be taken before hand. • The number of ANDA submissions to FDA has increased from 72 in the year 2006 to 1550 in 2009

e. CTD Implementation - EU (http: //esubmission. emea. europa. eu/) • Requirements on Electronic submissions (Nees (Non-e. CTD electronic submission, Version 2. 0 March-2010) and e. CTD) and paper documentation for New Application within MRP, DCP or National procedure – Refer CMDh/085/2008/Rev 7 October 2010) • From 1 st July 2010, the EU M 1 v 1. 4 must be used for all e. CTD submissions for all European procedures,

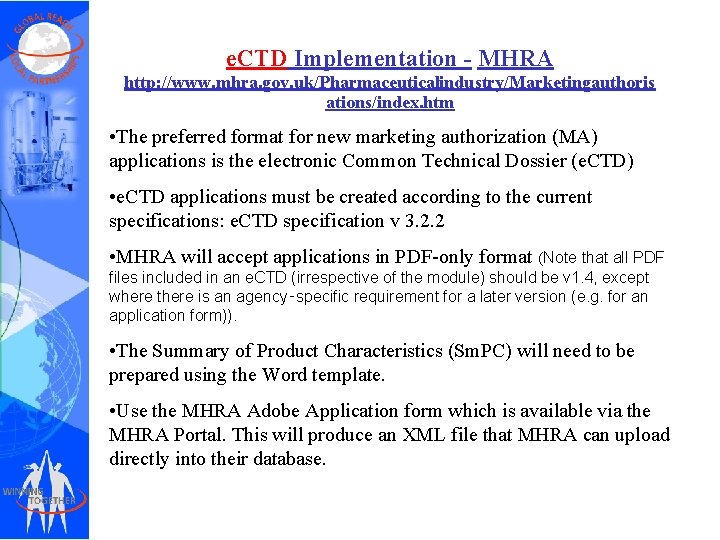
e. CTD Implementation - MHRA http: //www. mhra. gov. uk/Pharmaceuticalindustry/Marketingauthoris ations/index. htm • The preferred format for new marketing authorization (MA) applications is the electronic Common Technical Dossier (e. CTD) • e. CTD applications must be created according to the current specifications: e. CTD specification v 3. 2. 2 • MHRA will accept applications in PDF-only format (Note that all PDF files included in an e. CTD (irrespective of the module) should be v 1. 4, except where there is an agency‑specific requirement for a later version (e. g. for an application form)). • The Summary of Product Characteristics (Sm. PC) will need to be prepared using the Word template. • Use the MHRA Adobe Application form which is available via the MHRA Portal. This will produce an XML file that MHRA can upload directly into their database.

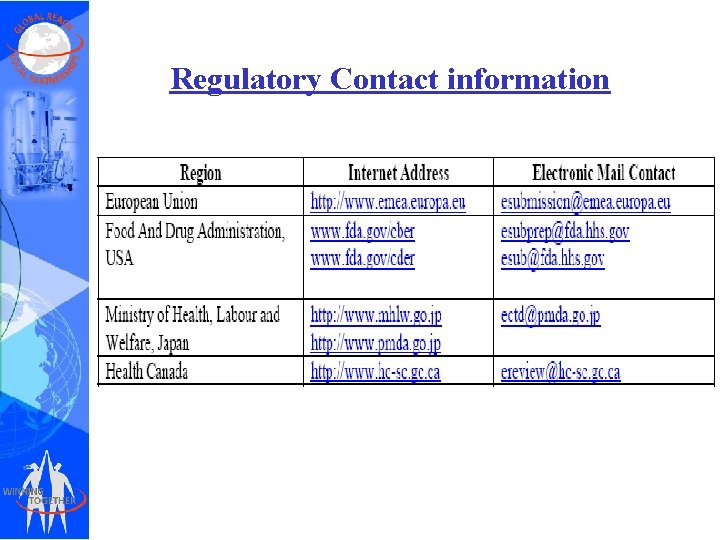
Regulatory Contact information

e. CTD Modules • When making an electronic submission, each document should be provided as a separate file. • The documents, whether for a marketing application, an investigational application, or a related submission, should be organized based on the five modules in the CTD: • Module 1 includes administrative information and prescribing information, • Module 2 includes CTD summary documents, • Module 3 includes information on quality, • Module 4 includes the nonclinical study reports, and • Module 5 includes the clinical study reports.

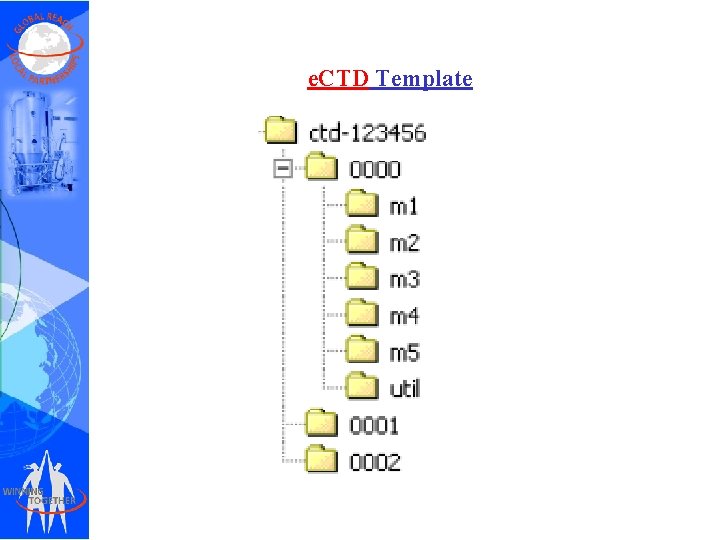
e. CTD Template

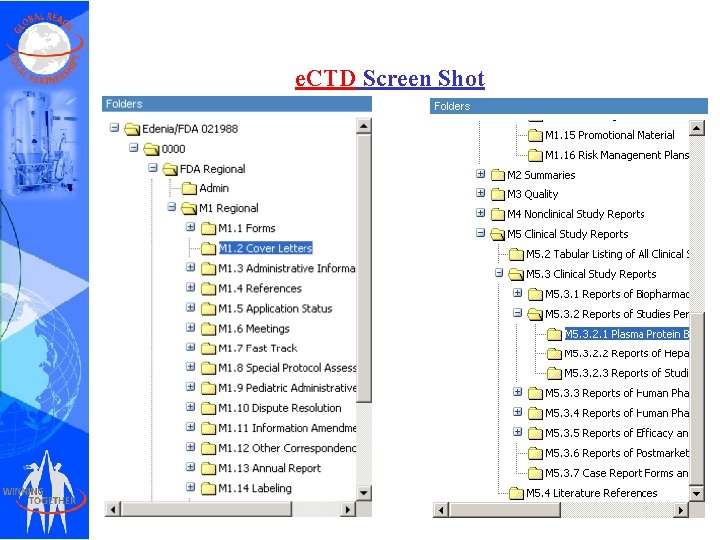
e. CTD Screen Shot

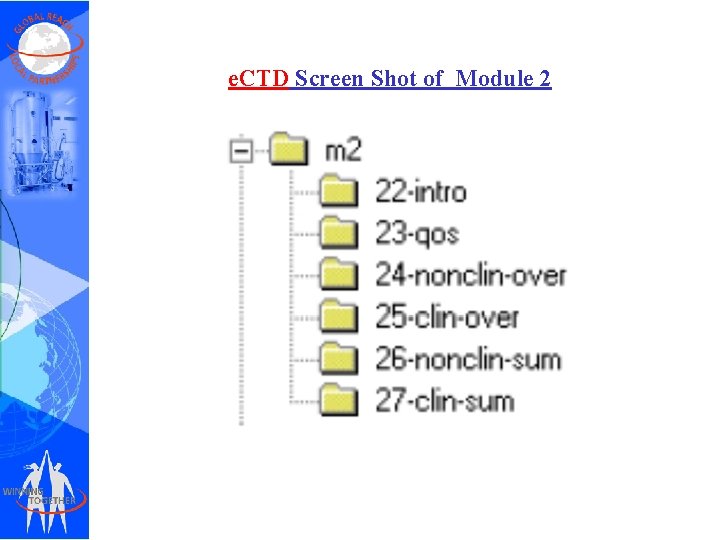
e. CTD Screen Shot of Module 2

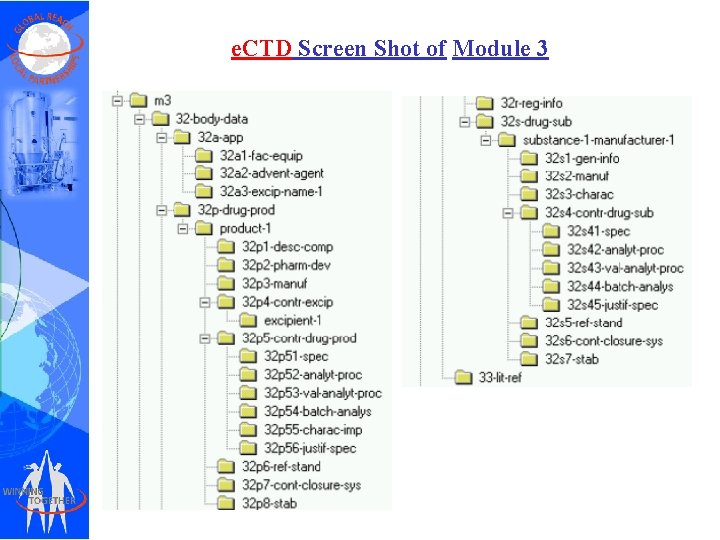
e. CTD Screen Shot of Module 3

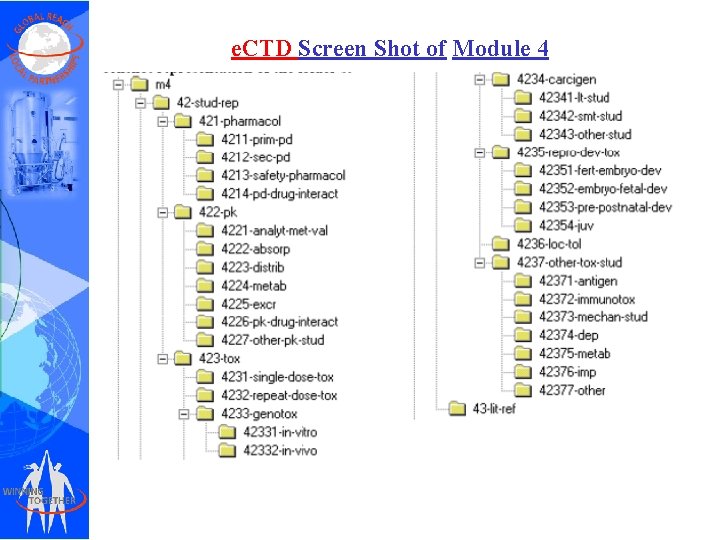
e. CTD Screen Shot of Module 4

e. CTD Screen Shot of Module 5

e. CTD Screen Shot of Module 5

e. CTD Screen Shot of Module 5

e. CTD Management Software • e. CTDXPress – Image Solutions –http: //www imagesolutions. com • Master. Control Submissions Gateway™ - Master Control, http: //www. mastercontrol. com • Liquent’s EZsubs® software solution, http: //www. liquent. com/ • Data Farm, http: //www. datafarminc. com/ • Take solution : www. Pharma. Ready. com • Lorenz Life Sciences : www. lorenz. cc

Thank You SRIKANTH. K
 Prof raj reddy
Prof raj reddy Jessica reddy x
Jessica reddy x Ketan reddy
Ketan reddy Konda madhava reddy
Konda madhava reddy Bheemarjuna reddy tamma
Bheemarjuna reddy tamma Raj reddy inventions
Raj reddy inventions Dr dn reddy
Dr dn reddy Conduit metaphor examples
Conduit metaphor examples I am woman helen reddy lyrics meaning
I am woman helen reddy lyrics meaning Swarith
Swarith Do93
Do93 Gesfarma
Gesfarma Ctd module 4
Ctd module 4 Ctd
Ctd Ctd module 3
Ctd module 3 Ctd module 5 table of contents
Ctd module 5 table of contents Ctd modules
Ctd modules Ctd modules
Ctd modules Ctd royston
Ctd royston Ctd triangle
Ctd triangle Time delay procedures
Time delay procedures Ctd
Ctd Ctd modules
Ctd modules Civ 6 ctd
Civ 6 ctd Ctd triangle
Ctd triangle Ctd structure
Ctd structure Prophet ctd
Prophet ctd Ctd sections
Ctd sections Studietoelage opvolgen
Studietoelage opvolgen Dossier pevl
Dossier pevl Dossier tecnico pedagogico dgert
Dossier tecnico pedagogico dgert Bounass
Bounass Diaporama dossier e13
Diaporama dossier e13 Exemple dossier bp coiffure coupe transformation
Exemple dossier bp coiffure coupe transformation Dossier bac pro
Dossier bac pro Plan oral brevet
Plan oral brevet Value dossier
Value dossier Dossier sponsoring equitation
Dossier sponsoring equitation Dossier permanent audit exemple
Dossier permanent audit exemple Exemple dossier u62 bts mv
Exemple dossier u62 bts mv Collection biologique
Collection biologique Dossier formativo cogeaps
Dossier formativo cogeaps Dossier bts muc
Dossier bts muc Studietoelagen dossier opvolgen
Studietoelagen dossier opvolgen Veterinary medicinal product dossier
Veterinary medicinal product dossier Lyne beaudoin
Lyne beaudoin Conclusion dossier raep
Conclusion dossier raep