CTD ICHCTD Format for Marketing Authorization Dossier Amir













![3. 2. S. 2 Manufacture 3. 2. S. 2. 1 Manufacturers [name, manufacturer] November 3. 2. S. 2 Manufacture 3. 2. S. 2. 1 Manufacturers [name, manufacturer] November](https://slidetodoc.com/presentation_image_h/c7dcd08b8ded4b53dfb23cab7a777b91/image-25.jpg)
![3. 2. S. 2 Manufacture 3. 2. S. 2. 1 Manufacturers [name, manufacturer] November 3. 2. S. 2 Manufacture 3. 2. S. 2. 1 Manufacturers [name, manufacturer] November](https://slidetodoc.com/presentation_image_h/c7dcd08b8ded4b53dfb23cab7a777b91/image-26.jpg)


































- Slides: 83

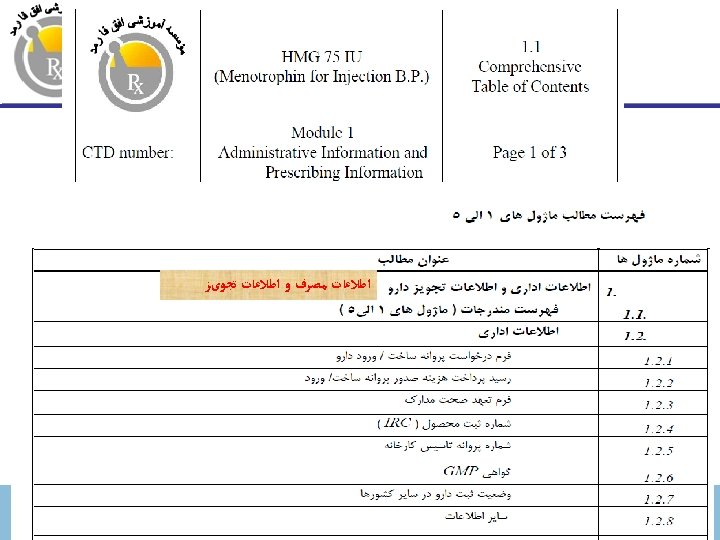
ﺑﺮﺍی پﺮﻭﻧﺪﻩ ﺛﺒﺖ ﺩﺍﺭﻭ CTD ﻓﺮﻣﺖ ICH-CTD Format for Marketing Authorization Dossier Amir Mehdizadeh, Ph. D. a_mehdizadeh@yahoo. com www. ofoghpharmed. com ICH-CTD Dr. Mehdizadeh


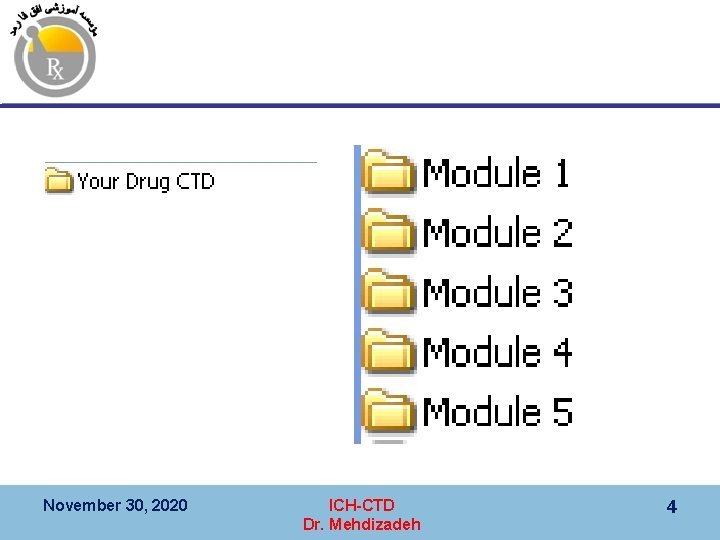
ﻣﺎژﻮﻝ ﺗﺸکیﻞ ﺷﺪﻩ ﺍﺳﺖ 5 ﺍﺯ CTD پﺮﻭﻧﺪﻩ Module Information 1 Administrative and prescribing information (region specific) 2 Summaries and overview 3 Quality 4 Non-clinical study reports 5 Clinical study reports November 30, 2020 ICH-CTD Dr. Mehdizadeh 3

November 30, 2020 ICH-CTD Dr. Mehdizadeh 4

November 30, 2020 ICH-CTD Dr. Mehdizadeh 5


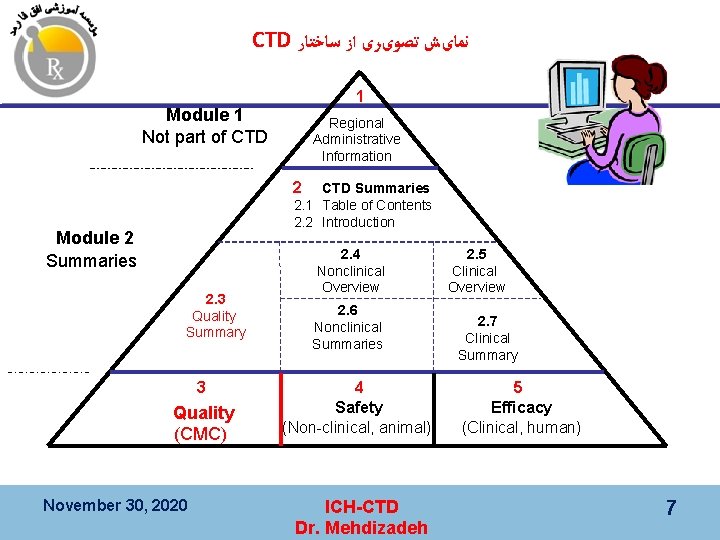
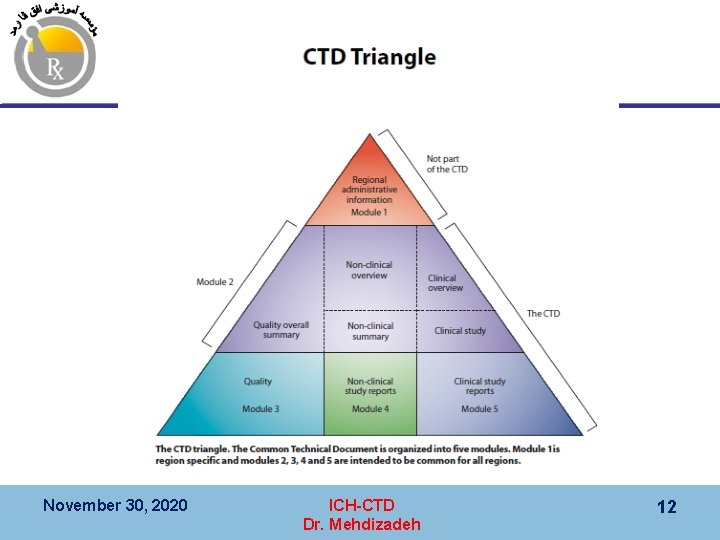
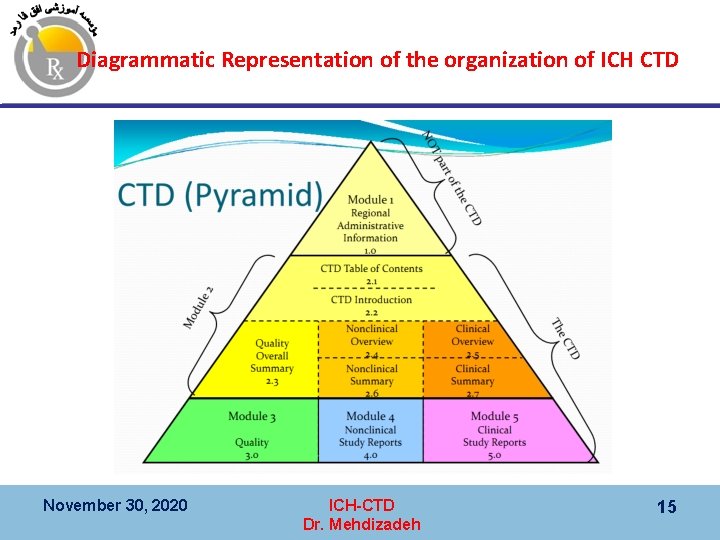
CTD ﻧﻤﺎیﺶ ﺗﺼﻮیﺮی ﺍﺯ ﺳﺎﺧﺘﺎﺭ 1 Module 1 Not part of CTD Regional Administrative Information 2 CTD Summaries 2. 1 Table of Contents 2. 2 Introduction Module 2 Summaries 2. 3 Quality Summary 3 Quality (CMC) November 30, 2020 2. 4 Nonclinical Overview 2. 6 Nonclinical Summaries 4 Safety (Non-clinical, animal) ICH-CTD Dr. Mehdizadeh 2. 5 Clinical Overview 2. 7 Clinical Summary 5 Efficacy (Clinical, human) 7

ﻧﺎﻣﻪ ﺩﺭﺧﻮﺍﺳﺖ 1 -0 Cover letter November 30, 2020 ICH-CTD Dr. Mehdizadeh 8

November 30, 2020 ICH-CTD Dr. Mehdizadeh 9


ﺧﻼﺻﻪ ﻫﺎ ،CTD 2 ﻣﺎژﻮﻝ Module 2 (Summaries) ICH-CTD Dr. Mehdizadeh

November 30, 2020 ICH-CTD Dr. Mehdizadeh 12

ﺧﻼﺻﻪ ﻫﺎ ، CTD 2 ﻣﺎژﻮﻝ Module 2 (Summaries) کیﻔیﺖ ،3 § ﺧﻼﺻﻪ ﻣﺎژﻮﻝ § Quality (Module 3) Overall Summary, ﻣﻄﺎﻟﻌﺎﺕ ﻏیﺮﺑﺎﻟیﻨی ، 4 § ﻧگﺎﻩ کﻠی ﻭ ﺧﻼﺻﻪ ﻣﺎژﻮﻝ § Non-clinical (Module 4) Overview/Summaries, and ﻣﻄﺎﻟﻌﺎﺕ ﺑﺎﻟیﻨی ،5 § ﻧگﺎﻩ کﻠی ﻭ ﺧﻼﺻﻪ ﻣﺎژﻮﻝ § Clinical (Module 5) Overview/Summaries. November 30, 2020 ICH-CTD Dr. Mehdizadeh 13

کیﻔیﺖ ،CTD 3 ﻣﺎژﻮﻝ Module 3, Quality (CMC) ICH-CTD Dr. Mehdizadeh

Diagrammatic Representation of the organization of ICH CTD November 30, 2020 ICH-CTD Dr. Mehdizadeh 15

ICH/CTD Guidance § M 4: Organization of the CTD § M 4 Q: The CTD — Quality § M 4 S: The CTD — Safety § M 4 E: The CTD — Efficacy. November 30, 2020 ICH-CTD Dr. Mehdizadeh 16

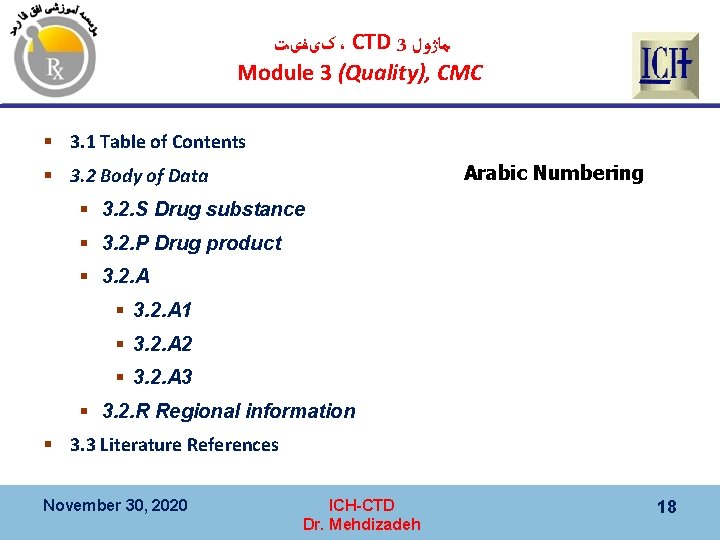
کیﻔیﺖ ، CTD 3 ﻣﺎژﻮﻝ Module 3 (Quality), CMC § Documentation of § Chemical, and § Pharmaceutical on § active substance and/or medicinal product is provided in Quality Report, Module 3. November 30, 2020 ICH-CTD Dr. Mehdizadeh 17

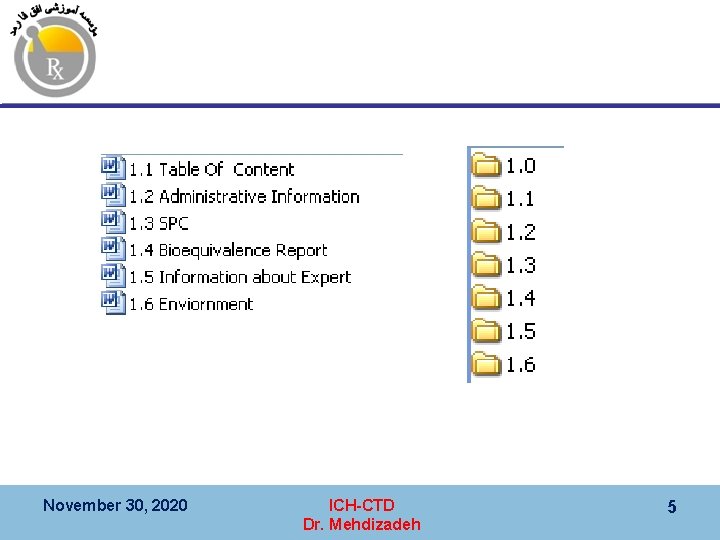
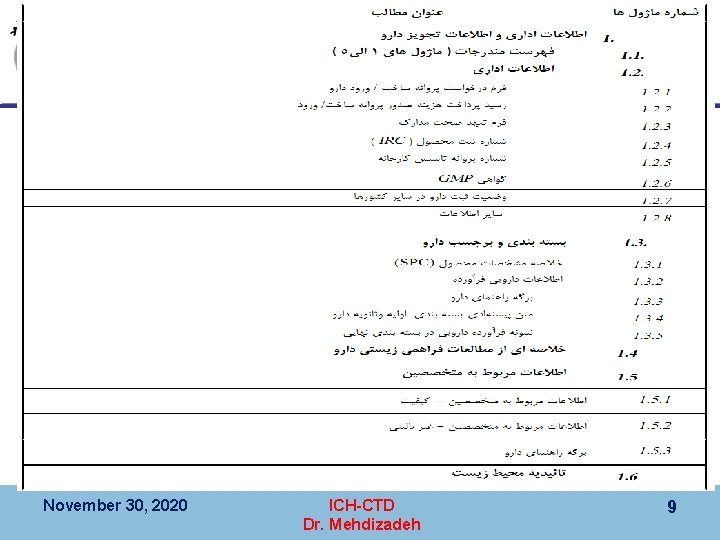
کیﻔیﺖ ، CTD 3 ﻣﺎژﻮﻝ Module 3 (Quality), CMC § 3. 1 Table of Contents Arabic Numbering § 3. 2 Body of Data § 3. 2. S Drug substance § 3. 2. P Drug product § 3. 2. A 1 § 3. 2. A 2 § 3. 2. A 3 § 3. 2. R Regional information § 3. 3 Literature References November 30, 2020 ICH-CTD Dr. Mehdizadeh 18

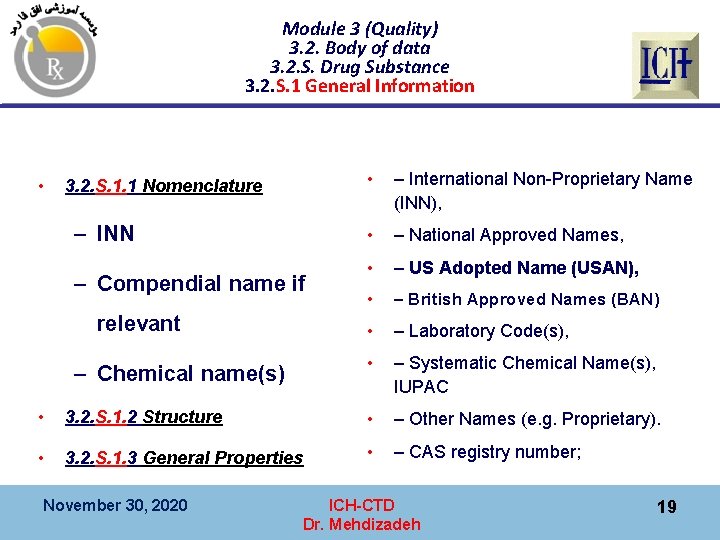
Module 3 (Quality) 3. 2. Body of data 3. 2. S. Drug Substance 3. 2. S. 1 General Information • 3. 2. S. 1. 1 Nomenclature – INN – Compendial name if relevant – Chemical name(s) • – International Non-Proprietary Name (INN), • – National Approved Names, • – US Adopted Name (USAN), • – British Approved Names (BAN) • – Laboratory Code(s), • – Systematic Chemical Name(s), IUPAC • 3. 2. S. 1. 2 Structure • – Other Names (e. g. Proprietary). • 3. 2. S. 1. 3 General Properties • – CAS registry number; November 30, 2020 ICH-CTD Dr. Mehdizadeh 19

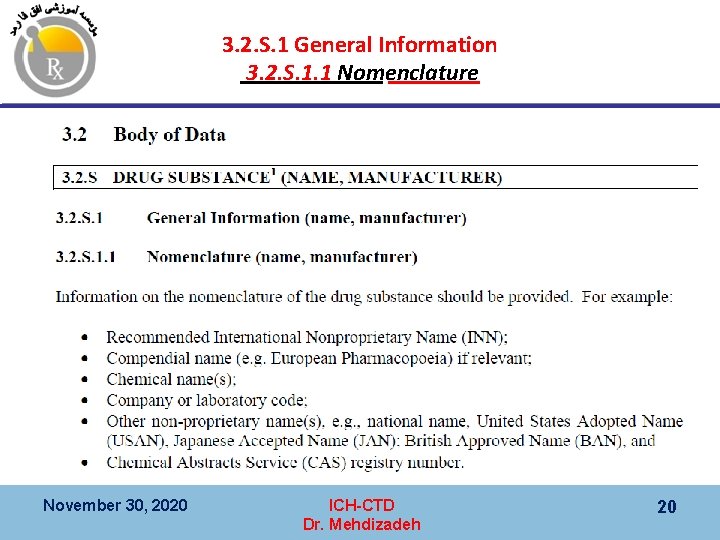
3. 2. S. 1 General Information 3. 2. S. 1. 1 Nomenclature November 30, 2020 ICH-CTD Dr. Mehdizadeh 20

3. 2. S. 1 General Information 3. 2. S. 1. 1 Nomenclature November 30, 2020 ICH-CTD Dr. Mehdizadeh 21

3. 2. S. 1 General Information 3. 2. S. 1. 2 Structure November 30, 2020 ICH-CTD Dr. Mehdizadeh 22

3. 2. S. 1 General Information 3. 2. S. 1. 3 General Properties November 30, 2020 ICH-CTD Dr. Mehdizadeh 23

Module 3 (Quality) 3. 2. Body of data 3. 2. S. Drug Substance 3. 2. S. 2 Manufacture § 3. 2. S. 2. 1 Manufacturers [name, manufacturer] § 3. 2. S. 2. 2 Manufacturing Process and Process Controls § 3. 2. S. 2. 3 Control of Materials § 3. 2. S. 2. 4 Controls of Critical Steps and Intermediates § 3. 2. S. 2. 5 Process Validation and/or Evaluation § 3. 2. S. 2. 6 Manufacturing Process Development November 30, 2020 ICH-CTD Dr. Mehdizadeh 24
![3 2 S 2 Manufacture 3 2 S 2 1 Manufacturers name manufacturer November 3. 2. S. 2 Manufacture 3. 2. S. 2. 1 Manufacturers [name, manufacturer] November](https://slidetodoc.com/presentation_image_h/c7dcd08b8ded4b53dfb23cab7a777b91/image-25.jpg)
3. 2. S. 2 Manufacture 3. 2. S. 2. 1 Manufacturers [name, manufacturer] November 30, 2020 ICH-CTD Dr. Mehdizadeh 25
![3 2 S 2 Manufacture 3 2 S 2 1 Manufacturers name manufacturer November 3. 2. S. 2 Manufacture 3. 2. S. 2. 1 Manufacturers [name, manufacturer] November](https://slidetodoc.com/presentation_image_h/c7dcd08b8ded4b53dfb23cab7a777b91/image-26.jpg)
3. 2. S. 2 Manufacture 3. 2. S. 2. 1 Manufacturers [name, manufacturer] November 30, 2020 ICH-CTD Dr. Mehdizadeh 26

3. 2. S. 2 Manufacture 3. 2. S. 2. 2 Manufacturing Process and Process Controls November 30, 2020 ICH-CTD Dr. Mehdizadeh 27

3. 2. S. 2 Manufacture 3. 2. S. 2. 2 Manufacturing Process and Process Controls November 30, 2020 ICH-CTD Dr. Mehdizadeh 28

3. 2. S. 2 Manufacture 3. 2. S. 2. 2 Manufacturing Process and Process Controls November 30, 2020 ICH-CTD Dr. Mehdizadeh 29

3. 2. S. 2 Manufacture 3. 2. S. 2. 2 Manufacturing Process and Process Controls November 30, 2020 ICH-CTD Dr. Mehdizadeh 30

3. 2. S. 2 Manufacture 3. 2. S. 2. 2 Manufacturing Process and Process Controls November 30, 2020 ICH-CTD Dr. Mehdizadeh 31

3. 2. S. 2 Manufacture 3. 2. S. 2. 2 Manufacturing Process and Process Controls November 30, 2020 ICH-CTD Dr. Mehdizadeh 32

3. 2. S. 2 Manufacture 3. 2. S. 2. 3 Control of Materials November 30, 2020 ICH-CTD Dr. Mehdizadeh 33

3. 2. S. 2 Manufacture 3. 2. S. 2. 3 Control of Materials November 30, 2020 ICH-CTD Dr. Mehdizadeh 34

3. 2. S. 2 Manufacture 3. 2. S. 2. 3 Control of Materials November 30, 2020 ICH-CTD Dr. Mehdizadeh 35

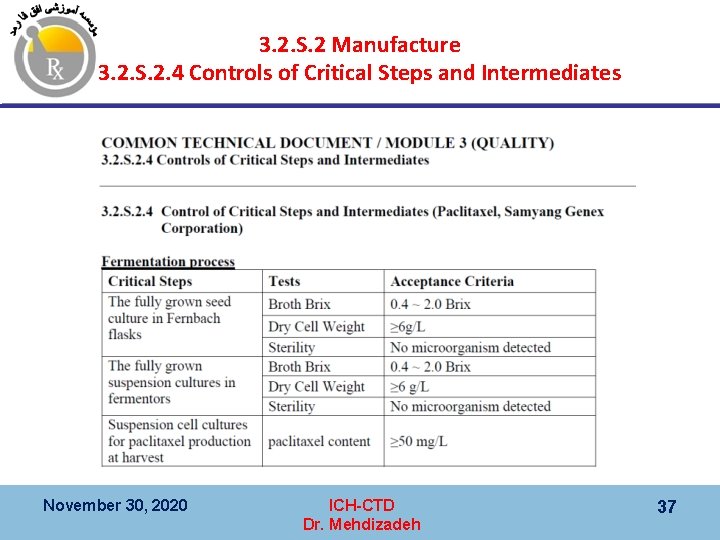
3. 2. S. 2 Manufacture 3. 2. S. 2. 4 Controls of Critical Steps and Intermediates November 30, 2020 ICH-CTD Dr. Mehdizadeh 36

3. 2. S. 2 Manufacture 3. 2. S. 2. 4 Controls of Critical Steps and Intermediates November 30, 2020 ICH-CTD Dr. Mehdizadeh 37

3. 2. S. 2 Manufacture 3. 2. S. 2. 5 Process Validation November 30, 2020 ICH-CTD Dr. Mehdizadeh 38

3. 2. S. 2 Manufacture 3. 2. S. 2. 6 Manufacturing Process Development November 30, 2020 ICH-CTD Dr. Mehdizadeh 39

3. 2. S. 2 Manufacture 3. 2. S. 2. 6 Manufacturing Process Development November 30, 2020 ICH-CTD Dr. Mehdizadeh 40

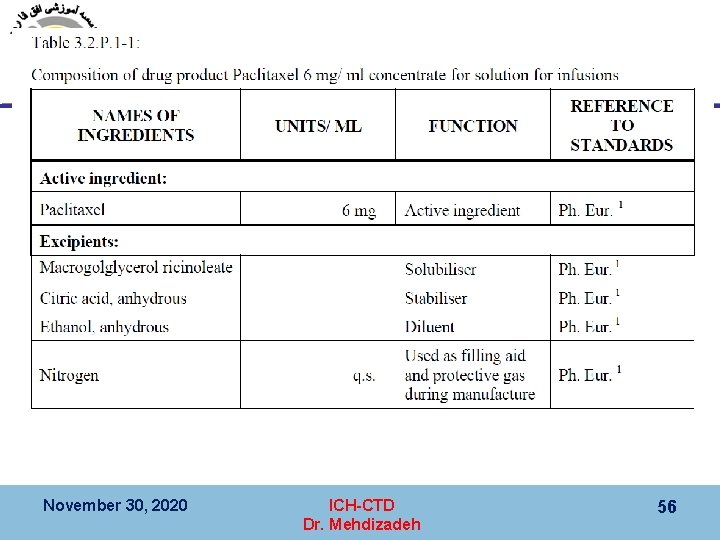
Module 3 (Quality) 3. 2. Body of data 3. 2. S. Drug Substance 3. 2. S. 3 Characterization § 3. 2. S. 3. 1 Elucidation of Structure and other Characteristics § 3. 2. S. 3. 2 Impurities November 30, 2020 ICH-CTD Dr. Mehdizadeh 41

3. 2. S. 3 Characterization 3. 2. S. 3. 1 Elucidation of Structure and other Characteristics November 30, 2020 ICH-CTD Dr. Mehdizadeh 42

3. 2. S. 3 Characterization 3. 2. S. 3. 1 Elucidation of Structure and other Characteristics November 30, 2020 ICH-CTD Dr. Mehdizadeh 43

3. 2. S. 3 Characterization 3. 2. S. 3. 2 Impurities November 30, 2020 ICH-CTD Dr. Mehdizadeh 44

3. 2. S. 3 Characterization 3. 2. S. 3. 2 Impurities November 30, 2020 ICH-CTD Dr. Mehdizadeh 45

Module 3 (Quality) 3. 2. Body of data 3. 2. S. Drug Substance 3. 2. S. 4 Control of Drug Substance § 3. 2. S. 4. 1 Specification § 3. 2. S. 4. 2 Analytical Procedures § 3. 2. S. 4. 3 Validation of Analytical Procedures § 3. 2. S. 4. 4 Batch Analyses § 3. 2. S. 4. 5 Justification of Specification November 30, 2020 ICH-CTD Dr. Mehdizadeh 46

Module 3 (Quality) 3. 2. Body of data 3. 2. S. Drug Substance 3. 2. S. 5 Reference Standards or Materials § 3. 2. S. 5 Reference Standards or Materials [name, manufacturer] November 30, 2020 ICH-CTD Dr. Mehdizadeh 47

Module 3 (Quality) 3. 2. Body of data 3. 2. S. Drug Substance 3. 2. S. 6 Container Closure System § 3. 2. S. 6 Container Closure System [name, manufacturer] November 30, 2020 ICH-CTD Dr. Mehdizadeh 48

3. 2. S. 6 Container Closure System November 30, 2020 ICH-CTD Dr. Mehdizadeh 49

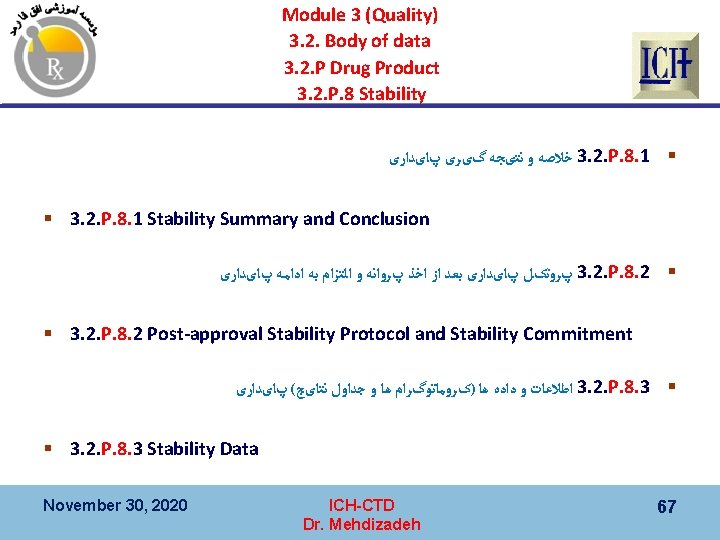
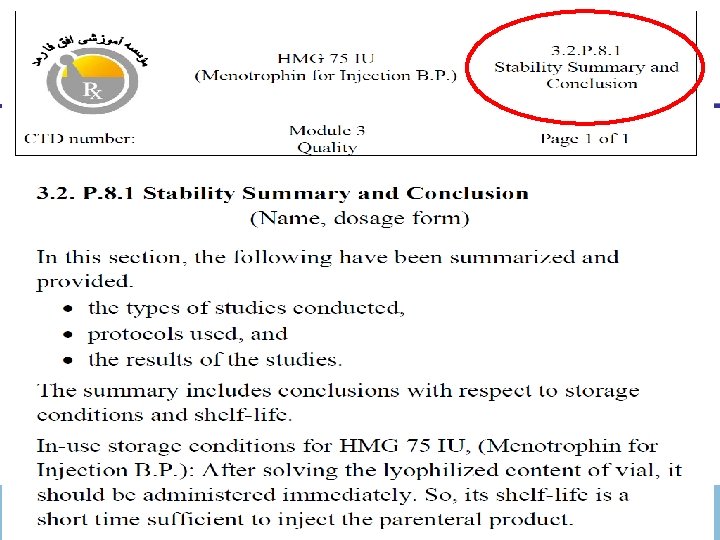
Module 3 (Quality) 3. 2. Body of data 3. 2. S. Drug Substance 3. 2. S. 7 Stability § 3. 2. S. 7. 1 Stability Summary and Conclusions § 3. 2. S. 7. 2 Post-approval (on going) Stability Protocol and Stability Commitment § 3. 2. S. 7. 3 Stability Data November 30, 2020 ICH-CTD Dr. Mehdizadeh 50

Module 3 (Quality) 3. 2. Body of data 3. 2. P Drug Product (Name, Dosage Form) ICH-CTD Dr. Mehdizadeh

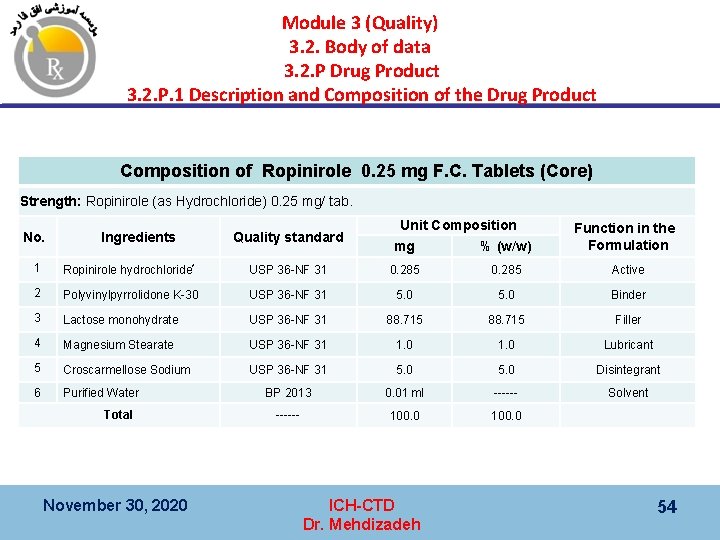
Module 3 (Quality) 3. 2. Body of data 3. 2. P Drug Product 3. 2. P. 1 Description and Composition of the Drug Product § ﺗﻮﺻیﻒ ﺷکﻞ ﺩﺍﺭﻭیی § Description of the dosage form ﻭ ﺭﻓﺮﺍﻧﺲ ، ﻋﻤﻠکﺮﺩ آﻨﻬﺎ ،( ﻣﻘﺪﺍﺭ ﺩﺭ ﻫﺮ ﻭﺍﺣﺪ ﻭ ﺩﺭ ﺻﻮﺭﺕ ﺩﺍﺷﺘﻦ ﺍکﺴﺲ ، § ﺗﺮکیﺐ )ﻟیﺴﺖ ﻣﻮﺍﺩ ﻣﺘﺸکﻠﻪ ﻓﺎﺭﻣﺎکﻮپﻪ یﺎ ﻣﺸﺨﺼﺎﺕ ﺳﺎﺯﻧﺪﻩ § Composition (i. e. , list of all components, amount on a per unit basis (including overages, if any) their function, and a reference (e. g. , compendial monographs or manufacturer’s specifications) § ﺗﻮﺻیﻒ ﺣﻼﻝ ﻫﻤﺮﺍﻩ ﺣﻞ کﻨﻨﺪﻩ § Description of accompanying reconstitution diluents ( § ﻧﻮﻉ ﺑﺴﺘﻪ ﺑﻨﺪی )ﻇﺮﻑ ﻭ ﺩﺭپﻮﺵ § Type of container and closure used for the dosage form ﺩﺍﺷﺘﻪ ﺑﺎﺷﺪ P ﺣﻼﻝ ﻫﻤﺮﺍﻩ ﺑﺎیﺪ یک ﻗﺴﻤﺖ ﺟﺪﺍ : § ﺗﻮﺟﻪ § Note: For a drug product supplied with reconstitution diluent(s), the information on the diluent(s) should be provided in a separate part “P”. November 30, 2020 ICH-CTD Dr. Mehdizadeh 52

Module 3 (Quality) 3. 2. Body of data 3. 2. P Drug Product 3. 2. P. 1 Description and Composition of the Drug Product November 30, 2020 ICH-CTD Dr. Mehdizadeh 53

Module 3 (Quality) 3. 2. Body of data 3. 2. P Drug Product 3. 2. P. 1 Description and Composition of the Drug Product Composition of Ropinirole 0. 25 mg F. C. Tablets (Core) Strength: Ropinirole (as Hydrochloride) 0. 25 mg/ tab. No. Ingredients Quality standard Unit Composition mg % (w/w) Function in the Formulation 1 Ropinirole hydrochloride* USP 36 -NF 31 0. 285 Active 2 Polyvinylpyrrolidone K-30 USP 36 -NF 31 5. 0 Binder 3 Lactose monohydrate USP 36 -NF 31 88. 715 Filler 4 Magnesium Stearate USP 36 -NF 31 1. 0 Lubricant 5 Croscarmellose Sodium USP 36 -NF 31 5. 0 Disintegrant 6 Purified Water BP 2013 0. 01 ml ------ Solvent ------ 100. 0 Total November 30, 2020 ICH-CTD Dr. Mehdizadeh 54

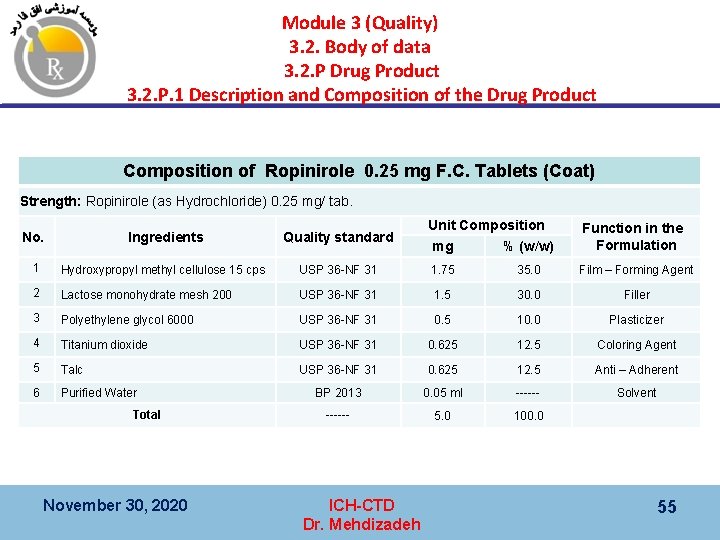
Module 3 (Quality) 3. 2. Body of data 3. 2. P Drug Product 3. 2. P. 1 Description and Composition of the Drug Product Composition of Ropinirole 0. 25 mg F. C. Tablets (Coat) Strength: Ropinirole (as Hydrochloride) 0. 25 mg/ tab. No. Ingredients Quality standard 1 Hydroxypropyl methyl cellulose 15 cps 2 Unit Composition Function in the Formulation mg % (w/w) USP 36 -NF 31 1. 75 35. 0 Film – Forming Agent Lactose monohydrate mesh 200 USP 36 -NF 31 1. 5 30. 0 Filler 3 Polyethylene glycol 6000 USP 36 -NF 31 0. 5 10. 0 Plasticizer 4 Titanium dioxide USP 36 -NF 31 0. 625 12. 5 Coloring Agent 5 Talc USP 36 -NF 31 0. 625 12. 5 Anti – Adherent 6 Purified Water BP 2013 0. 05 ml ------ Solvent ------ 5. 0 100. 0 Total November 30, 2020 ICH-CTD Dr. Mehdizadeh 55

November 30, 2020 ICH-CTD Dr. Mehdizadeh 56

Module 3 (Quality) 3. 2. Body of data 3. 2. P Drug Product 3. 2. P. 2 Pharmaceutical Development (name, dosage form) ، ﺩﺭﺏ - ﺳیﺴﺘﻢ ﻇﺮﻑ ، ﻓﺮآیﻨﺪ ﺳﺎﺧﺖ ، ﻓﺮﻣﻮﻻﺳیﻮﻥ ، § ﻣﻄﺎﻟﻌﺎﺕ ﻣﺮﺑﻮﻁ ﺑﻪ ﺗﻌییﻦ ﺷکﻞ ﺩﺍﺭﻭﺋی . ﻣﺸﺨﺼﺎﺕ ﻣیکﺮﻭﺑی ﺩﺭ ﺗﻮﺳﻌﻪ ﺩﺍﺭﻭﺋی ﺍﻧﺠﺎﻡ ﻣی ﺷﻮﺩ § Information on the development studies conducted to establish that the dosage form, the formulation, manufacturing process, container closure system, microbiological attributes. November 30, 2020 ICH-CTD Dr. Mehdizadeh 57

Module 3 (Quality) 3. 2. Body of data 3. 2. P Drug Product 3. 2. P. 2 Pharmaceutical Development (name, dosage form) § The studies described here are distinguished from routine control tests conducted according to specifications. ﻭ ﺍﺻﻮﻻ کیﻔیﺖ ﻣﺤﺼﻮﻝ ﺍﺛﺮ گﺬﺍﺭﻧﺪ ، کﺎﺭﺍﺋی ﻣﺤﺼﻮﻝ ، § پﺎﺭﺍﻣﺘﺮﻫﺎی ﺑﺤﺮﺍﻧی کﻪ ﻣی ﺗﻮﺍﻧﻨﺪ ﺩﺭ ﺗکﺮﺍﺭپﺬیﺮی ﻣﺤﺼﻮﻝ . ﺩﺭ ﺗﻮﺳﻌﻪ ﺩﺍﺭﻭﺋی ﻫﺪﺍیﺖ ﻣی ﺷﻮﻧﺪ § Additionally, this section should identify and describe the formulation and process attributes (critical parameters) that can influence batch reproducibility, product performance and drug product quality. § Supportive data & results from specific studies or published literature can be included within or attached to Pharmaceutical Development section. § Additional supportive data can be referenced to the relevant nonclinical or clinical sections of the application. November 30, 2020 ICH-CTD Dr. Mehdizadeh 58

Module 3 (Quality) 3. 2. Body of data 3. 2. P Drug Product 3. 2. P. 2 Pharmaceutical Development (name, dosage form) § 3. 2. P. 2. 1 Components of the Drug Product (name, dosage form) Ø 3. 2. P. 2. 1. 1 Drug Substance (name, dosage form) Ø 3. 2. P. 2. 1. 2 Excipients (name, dosage form) § 3. 2. P. 2. 2 Drug Product Ø 3. 2. P. 2. 2. 1 Formulation Development (name, dosage form) Ø 3. 2. P. 2. 2. 2 Overages [name, dosage form] Ø 3. 2. P. 2. 2. 3 Physicochemical Properties (name, dosage form) § 3. 2. P. 2. 3 Manufacturing Process Development (name, dosage form) § 3. 2. P. 2. 4 Container Closure System (name, dosage form) § 3. 2. P. 2. 5 Microbiological Attributes (name, dosage form) § 3. 2. P. 2. 6 Compatibility (name, dosage form) November 30, 2020 ICH-CTD Dr. Mehdizadeh 59

Module 3 (Quality) 3. 2. Body of data 3. 2. P Drug Product 3. 2. P. 3 Manufacture (name, dosage form) § 3. 2. P. 3. 1 Manufacturers (name, dosage form) § 3. 2. P. 3. 2 Batch Formula (name, dosage form) § 3. 2. P. 3. 3 Description of Manufacturing Process and Process Controls § 3. 2. P. 3. 4 Controls of Critical Steps (name, dosage form) § 3. 2. P. 3. 5 Process Validation and/or Evaluation (name, dosage form) November 30, 2020 ICH-CTD Dr. Mehdizadeh 60

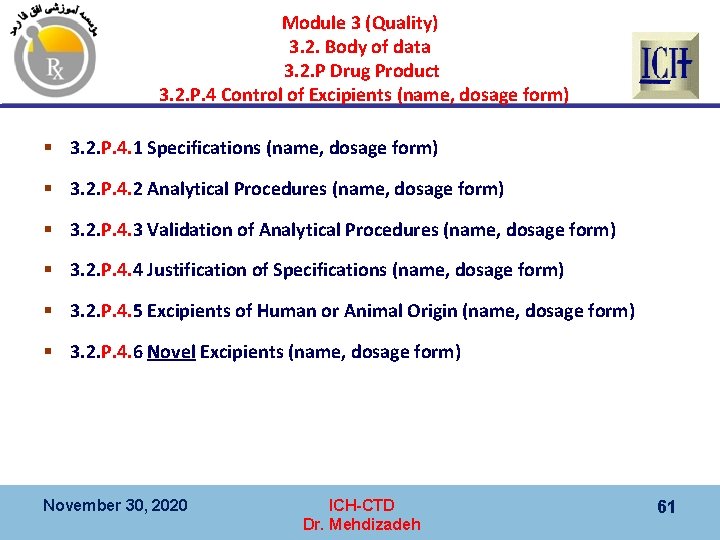
Module 3 (Quality) 3. 2. Body of data 3. 2. P Drug Product 3. 2. P. 4 Control of Excipients (name, dosage form) § 3. 2. P. 4. 1 Specifications (name, dosage form) § 3. 2. P. 4. 2 Analytical Procedures (name, dosage form) § 3. 2. P. 4. 3 Validation of Analytical Procedures (name, dosage form) § 3. 2. P. 4. 4 Justification of Specifications (name, dosage form) § 3. 2. P. 4. 5 Excipients of Human or Animal Origin (name, dosage form) § 3. 2. P. 4. 6 Novel Excipients (name, dosage form) November 30, 2020 ICH-CTD Dr. Mehdizadeh 61

Module 3 (Quality) 3. 2. Body of data 3. 2. P Drug Product 3. 2. P. 5 Control of Drug Product (name, dosage form) § 3. 2. P. 5. 1 Specifications (name, dosage form) § 3. 2. P. 5. 2 Analytical Procedures (name, dosage form) § 3. 2. P. 5. 3 Validation of Analytical Procedures (name, dosage form) § 3. 2. P. 5. 4 Batch Analyses (name, dosage form) § 3. 2. P. 5. 5 Characterization of Impurities (name, dosage form) § 3. 2. P. 5. 6 Justification of Specifications (name, dosage form) November 30, 2020 ICH-CTD Dr. Mehdizadeh 62

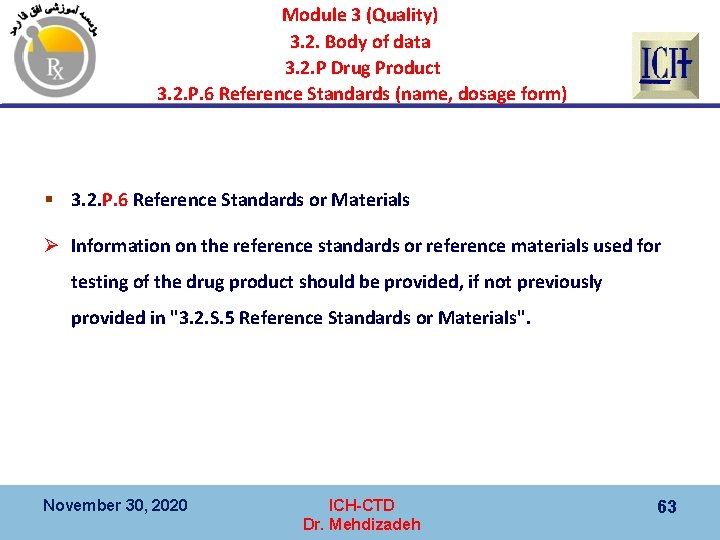
Module 3 (Quality) 3. 2. Body of data 3. 2. P Drug Product 3. 2. P. 6 Reference Standards (name, dosage form) § 3. 2. P. 6 Reference Standards or Materials Ø Information on the reference standards or reference materials used for testing of the drug product should be provided, if not previously provided in "3. 2. S. 5 Reference Standards or Materials". November 30, 2020 ICH-CTD Dr. Mehdizadeh 63

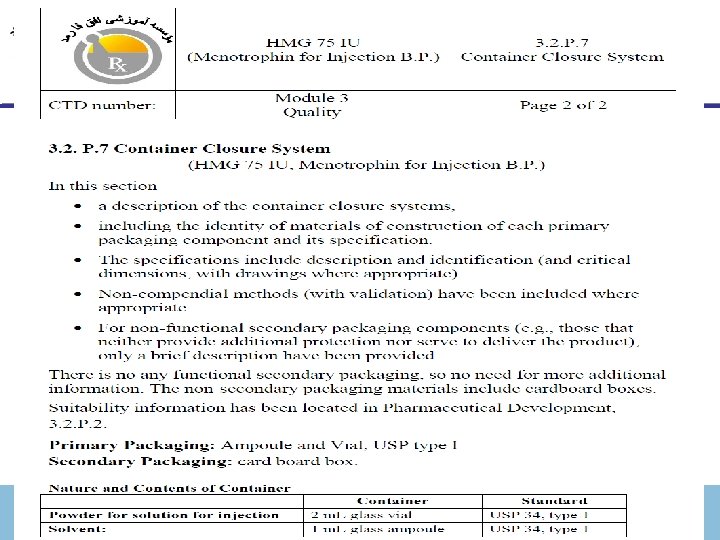
Module 3 (Quality) 3. 2. Body of data 3. 2. P Drug Product 3. 2. P. 7 Container Closure System (name, dosage form). § ﺗﻮﺻیﻒ ﻣﺨﺘﺼﺮی ﺍﺯ ﻇﺮﻑ ﺍﺳﺘﻔﺎﺩﻩ ﺷﺪﻩ ﺩﺭ ﺍیﻦ ﻗﺴﻤﺖ آﻮﺭﺩﻩ ﺷﻮﺩ § A brief description of the containers to be used should be given. § ﺍگﺮ ﺑﺴﺘﻪ ﺑﻨﺪی ﺛﺎﻧﻮیﻪ ﻣﺜﻞ ﺟﻌﺒﻪ ﻣﻘﻮﺍﺋی ﺑﺮﺍی ﻣﻮﺍﺩ ﺣﺴﺎﺱ ﺑﻪ ﻧﻮﺭ ﺩﺭ آﻤپﻮﻝ ﺷﻔﺎﻑ ﺑﺴﺘﻪ ﺑﻨﺪی ﺍﺳﺘﻔﺎﺩﻩ ﻣی ﺷﻮﻧﺪ . ﻻﺯﻡ ﺍﺳﺖ ﺩﺭ ﺍیﻦ ﻗﺴﻤﺖ ﺫکﺮ ﺷﻮﺩ § It is also helpful if any outer packages to be used are also mentioned in appropriate cases e. g. cardboard cartons to provide light protection for photosensitive material packed in clear glass ampoules. November 30, 2020 ICH-CTD Dr. Mehdizadeh 64

November 30, 2020 ICH-CTD Dr. Mehdizadeh 65

Module 3 (Quality) 3. 2. Body of data 3. 2. P Drug Product 3. 2. P. 7 Containers (name, dosage form) Ø A description of the container closure systems should be provided, including the identity of materials of construction of each primary packaging component and its specification. Ø The specifications should include description and identification (and critical dimensions, with drawings where appropriate). Non-compendial methods (with validation) should be included where appropriate. Ø For non-functional secondary packaging components (e. g. , those that neither provide additional protection nor serve to deliver the product), only a brief description should be provided. Ø For functional secondary packaging components, additional information should be provided. Ø Suitability information should be located in 3. 2. P. 2. November 30, 2020 ICH-CTD Dr. Mehdizadeh 66


November 30, 2020 ICH-CTD Dr. Mehdizadeh 68

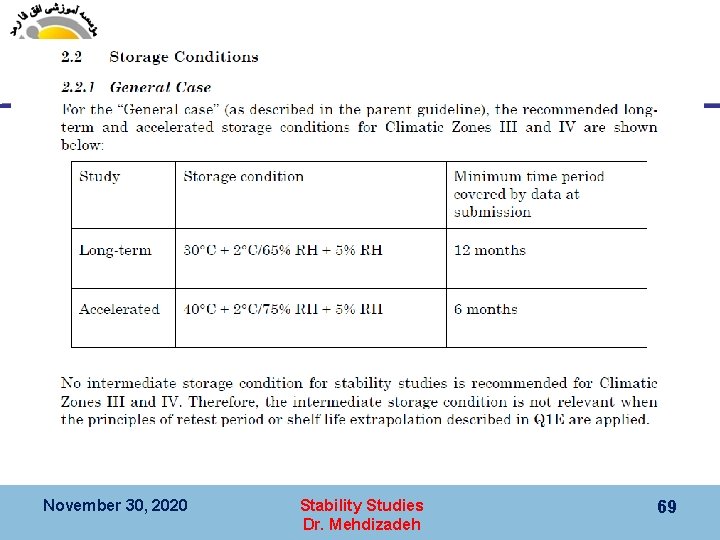
November 30, 2020 Stability Studies Dr. Mehdizadeh 69

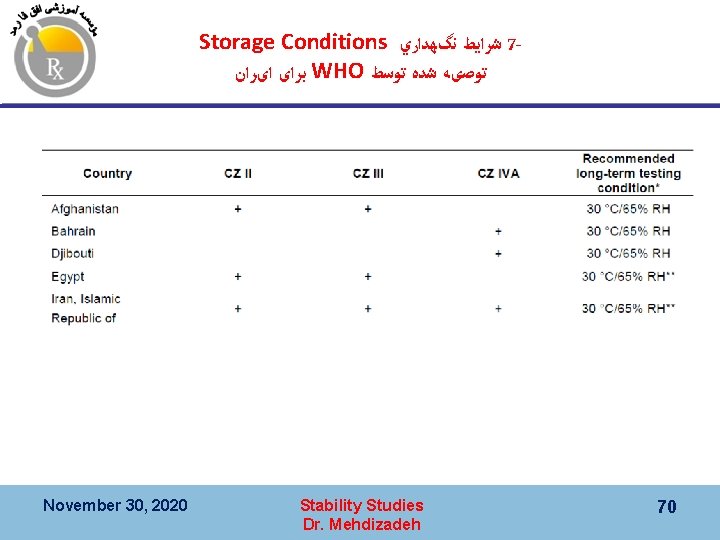
Storage Conditions ﺷﺮﺍﻳﻂ ﻧگﻬﺪﺍﺭﻱ 7 ﺑﺮﺍی ﺍیﺮﺍﻥ WHO ﺗﻮﺻیﻪ ﺷﺪﻩ ﺗﻮﺳﻂ November 30, 2020 Stability Studies Dr. Mehdizadeh 70

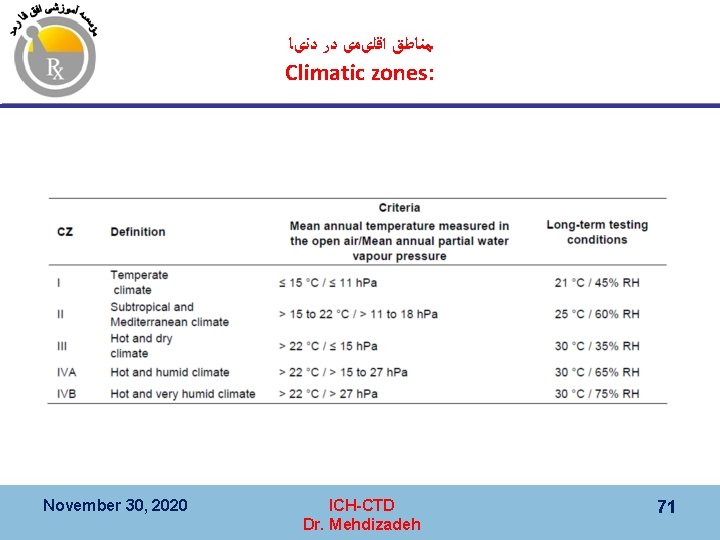
ﻣﻨﺎﻃﻖ ﺍﻗﻠیﻤی ﺩﺭ ﺩﻧیﺎ Climatic zones: November 30, 2020 ICH-CTD Dr. Mehdizadeh 71

MODULE 3 Quality (3. 2. A, 3. 2. R) § 3. 2. A APPENDICES Ø 3. 2. A. 1 Facilities and Equipment (name, manufacturer) Ø 3. 2. A. 2 Adventitious Agents Safety Evaluation (name, dosage form, manufacturer) Ø Information assessing the risk with respect to potential contamination with adventitious agents should be provided in this section. Ø 3. 2. A. 3 Novel Excipients (name, manufacturer) § 3. 2. R Regional information (name, manufacturer) § 3. 3 Literature references (name, manufacturer) November 30, 2020 ICH-CTD Dr. Mehdizadeh 72

MODULE 3 Quality (3. 2. R) § 3. 2. R Regional information Ø Executed Batch Record (U. S. , in English) Ø Method Validation Package (U. S. , EDQM) Ø Comparability Protocols (U. S. ) Ø Process Validation Scheme (EU) Ø Medical Device (EU) Ø Certificate of Suitability § For Iran (FDO): Ø Not applicable. November 30, 2020 ICH-CTD Dr. Mehdizadeh 73

ﺗﻮﺟﻪ § Note: Ø Irritation test (Animal): Module 4 Ø In-vitro: Module 5 Ø In-vivo: Module 5 Ø Bioequivalence: Module 5 Ø Irritation test (Human): Module 5 November 30, 2020 ICH-CTD Dr. Mehdizadeh 74

3. 2. P. 4. 6 Novel Excipients (name, dosage form) § For excipient(s) used for the first time in a drug product or by a new route of administration, full details of manufacture, characterisation, and controls, with cross references to supporting safety data (nonclinical and/or clinical) should be provided according to the drug substance format. § (Details in 3. 2. A. 3). November 30, 2020 ICH-CTD Dr. Mehdizadeh 75

November 30, 2020 ICH-CTD Dr. Mehdizadeh 76

Module 3 (Quality) 3. 3. Literatures § 3. 3 LITERATURE REFERENCES November 30, 2020 ICH-CTD Dr. Mehdizadeh 77

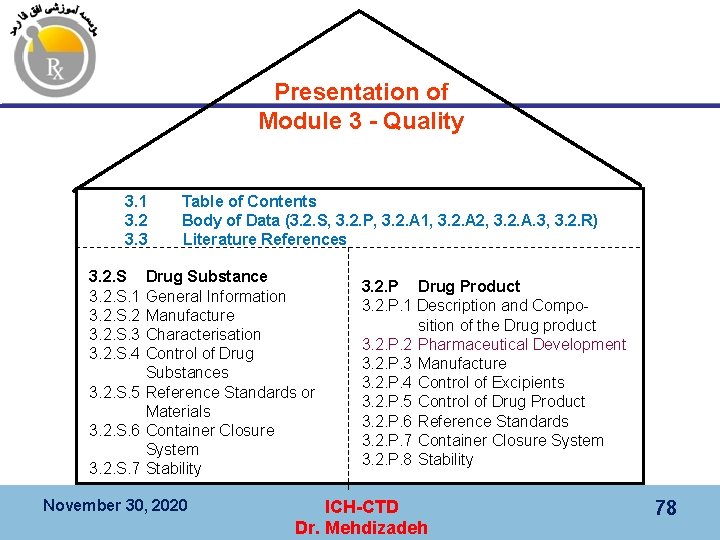
Presentation of Module 3 - Quality 3. 1 3. 2 3. 3 Table of Contents Body of Data (3. 2. S, 3. 2. P, 3. 2. A 1, 3. 2. A 2, 3. 2. A. 3, 3. 2. R) Literature References 3. 2. S. 1 3. 2. S. 2 3. 2. S. 3 3. 2. S. 4 Drug Substance General Information Manufacture Characterisation Control of Drug Substances 3. 2. S. 5 Reference Standards or Materials 3. 2. S. 6 Container Closure System 3. 2. S. 7 Stability November 30, 2020 3. 2. P Drug Product 3. 2. P. 1 Description and Composition of the Drug product 3. 2. P. 2 Pharmaceutical Development 3. 2. P. 3 Manufacture 3. 2. P. 4 Control of Excipients 3. 2. P. 5 Control of Drug Product 3. 2. P. 6 Reference Standards 3. 2. P. 7 Container Closure System 3. 2. P. 8 Stability ICH-CTD Dr. Mehdizadeh 78

Module 4 § Module 4: Non- clinical Study Reports § 4. 1 Table of Contents § 4. 2 Study Reports § 4. 2. 1 Pharmacology § 4. 2. 2 Pharmacokonetics § 4. 2. 3 Toxicology § 4. 3 Literature references November 30, 2020 ICH-CTD Dr. Mehdizadeh 79

November 30, 2020 ICH-CTD Dr. Mehdizadeh 80

Module 5: Clinical Study reports § 5. 1 Table of Contents § 5. 2 Tabular Listing of All Clinical Studies § 5. 3 Clinical Study Reports § 5. 4 Literature References November 30, 2020 ICH-CTD Dr. Mehdizadeh 81

November 30, 2020 ICH-CTD Dr. Mehdizadeh 82
