The Dossier Developing Effective Academy of Managed Care














- Slides: 30

The Dossier Developing Effective Academy of Managed Care Pharmacy and Global Value Dossiers Caitlin Rothermel, MA, MPH 1

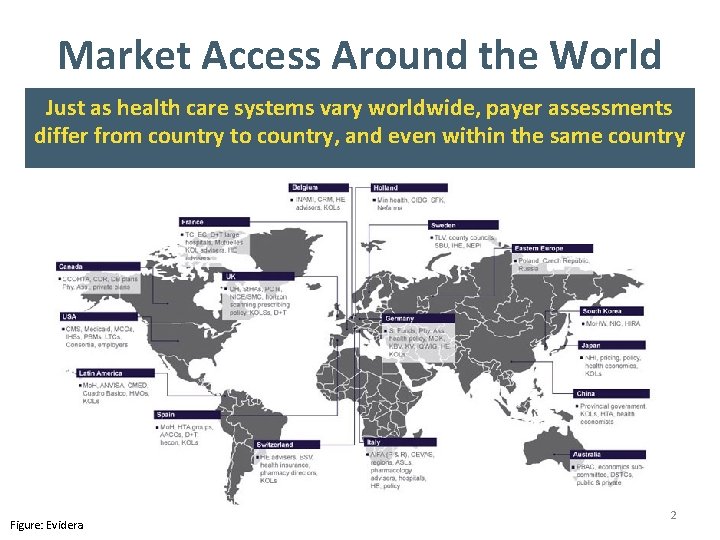
Market Access Around the World Just as health care systems vary worldwide, payer assessments differ from country to country, and even within the same country Figure: Evidera 2

Global Market Access: Key Issues To successfully launch a drug in multiple markets, you have to understand the particular requirements of each payer • Worldwide, payers weigh data in different ways when deciding whether to pay for a new drug, and what its price should be • Public agencies look at clinical benefit, budget impact, and overall costeffectiveness (CE) – The UK evaluates clinical and budget impact, but bases its decisions on CE – A German agency looks separately at clinical benefit and affordability, weighting both to decide – A Swedish agency evaluates costs and benefits through a societal lens • Private agencies look at clinical benefit and budget impact – This includes most private U. S. payers • Dossiers address the common concerns and information needs of a range of payers 3

What Is A Global Value Dossier (GVD)? With increasing medical costs, aging populations, and budget deficits, countries and payers need to develop objective requirements for coverage and reimbursement • A GVD is a comprehensive, internal document used to communicate the value of a product to payers and other relevant stakeholders • It includes available evidence on the global burden of the relevant illness, as well as the clinical, economical, and humanistic value of the new treatment • It provides a comprehensive reference base that can be tailored for use in different markets • Typically, GVDs are developed by global headquarters with local affiliate input; they are generally prepared in a modular format • GVDs are also internal tools for product launch education—to ensure that all team members are on the same page in terms of value messaging; they can also serve as the basis to develop Health Technology Assessments 4

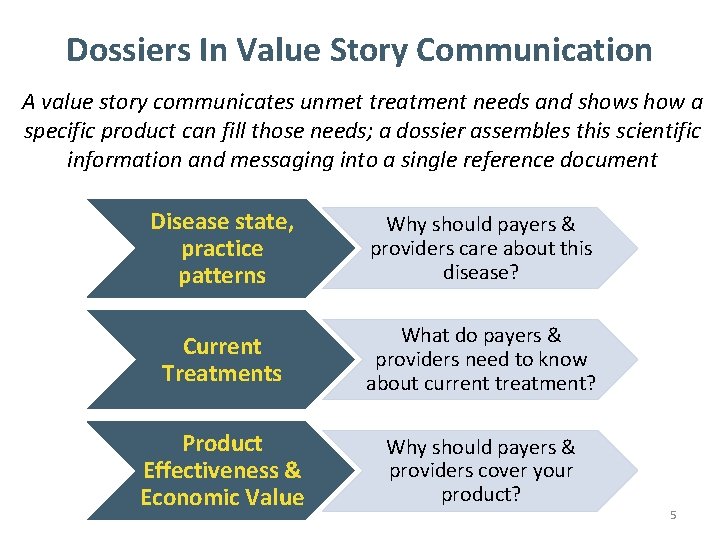
Dossiers In Value Story Communication A value story communicates unmet treatment needs and shows how a specific product can fill those needs; a dossier assembles this scientific information and messaging into a single reference document Disease state, practice patterns Why should payers & providers care about this disease? Current Treatments What do payers & providers need to know about current treatment? Product Effectiveness & Economic Value Why should payers & providers cover your product? 5

What Are Value Messages? • Value messages are developed in collaboration with clinical, marketing, commercial, and other departments • Each message should be supported by scientifically accurate evidence • Message testing may include primary research with payers, physicians, and patients • Draft value hypotheses should be prepared early in product development (~Phase 2) to help guide clinical development and the selection of trial endpoints • Final messages are written after clinical data are available Barrows S. ISPOR 14 th Annual International Meeting, May 2009. 6

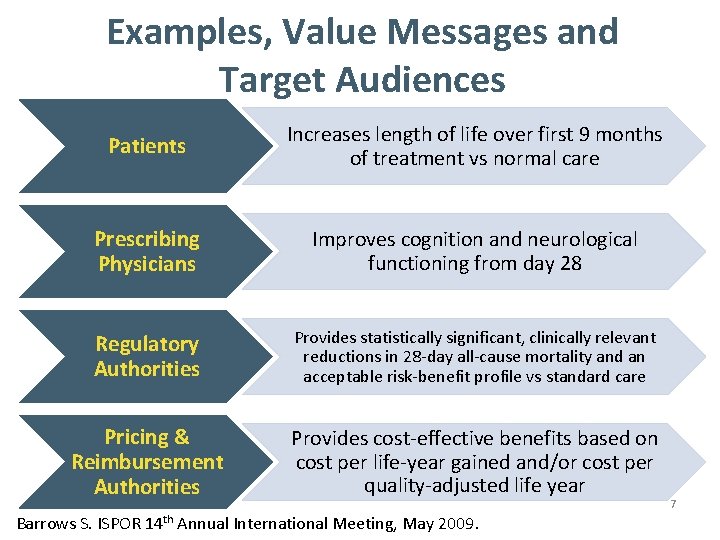
Examples, Value Messages and Target Audiences Patients Increases length of life over first 9 months of treatment vs normal care Prescribing Physicians Improves cognition and neurological functioning from day 28 Regulatory Authorities Provides statistically significant, clinically relevant reductions in 28 -day all-cause mortality and an acceptable risk-benefit profile vs standard care Pricing & Reimbursement Authorities Provides cost-effective benefits based on cost per life-year gained and/or cost per quality-adjusted life year Barrows S. ISPOR 14 th Annual International Meeting, May 2009. 7

Global Value Dossier What’s In the Dossier? (1 of 3) 1. Introduction • Purpose of/how to use the GVD 2. Burden of Disease (The Problem) • Disease background • Epidemiology, including prevalence/incidence, mortality, comorbidities • Humanistic burden (eg, health-related quality of life, functional status, symptoms) • Unmet treatment need with current therapies • Sources of information: systematic literature review of peerreviewed literature, national/international professional organizations, federally sponsored research organizations Rycroft CE. J Eur Med Writers Assn. 2010; 19(3): 211 -212. 8

Global Value Dossier What’s In the Dossier? (2 of 3) 3. Product Value (The Solution) • Clinical value: efficacy and effectiveness, safety and tolerability • Patient-reported outcomes, quality of life value: healthrelated quality of life, functional status, compliance, satisfaction/preference, caregiver burden • Economic value: cost-effectiveness, budget impact, associated decrease in health care utilization • Sources of information: sponsored and other studies of product Rycroft CE. J Eur Med Writers Assn. 2010; 19(3): 211 -212. 9

Global Value Dossier What’s In the Dossier? (3 of 3) 4. Country-specific Information • Enables adaptation of GVD for different markets and populations; includes: Epidemiology Economic and health-related quality of life burden Country-specific clinical considerations, including key comparators Country-specific economic considerations, including reference drugs – Country-specific value message considerations (value may vary by country related to differences in available comparators, treatment guidelines, and physician awareness of adverse events) – – Rycroft CE. J Eur Med Writers Assn. 2010; 19(3): 211 -212. 10

Writing GVDs: Practical Issues • Generally no predefined format, but still highly structured and visually appealing – Movement towards “i. GVDs” – to view on tablets and mobile devices • Brevity and organization are key! Avoid the urge to use the GVD as a “data dump” • Typically developed in collaboration with multiple stakeholders—ie, many people provide input (and revisions) 11

Market Access In the U. S. , the key healthcare payers are a mix of governmental and private organizations • U. S. Centers for Medicare and Medicaid Services • Managed Care Organizations • Indian Health Service • Department of Veterans Affairs • Pharmacy Benefit Management • Consortia, Employers • Individual Market 12

What Is the AMCP Dossier? • The AMCP Dossier is the U. S. industry standard by which managed care organizations request evidence-based information to evaluate pharmaceuticals, biologics, and vaccines formulary placement, coverage, and reimbursement decisions • The AMCP Dossier Format provides a standardized template to present clinical and economic evidence – Developed to improve clarity and transparency, to streamline evidence acquisition and decision-makers’ review process – Users include national and regional health insurers, pharmacy benefit managers, federal agencies, hospital systems, and state Medicaid agencies—representing >150 million Americans AMCP: Academy of Managed Care Pharmacy 13

Placeholder: AMCP Dossier Format Release/Update History • 1999 – Academy of Managed Care Pharmacy (AMCP) Committee organized • 2000 – AMCP Format Version 1. 0 – AMCP Board of Directors approves AMCP Format • 2002 – AMCP Format Version 2. 0 • 2005 – AMCP Format Version 2. 1 • 2009 – AMCP Format Version 3. 0 – Substantial format revisions from version 2. 0 to 3. 0 • 2013 – AMCP Format Version 3. 1 – Updated to include: • Companion Diagnostic Tests • Comparative Effectiveness Research • Specialty Pharmaceuticals • 2015/2016 – Next update likely? Jackson J. AMCP Format for Formulary Submissions (slide presentation). February 2014. 14

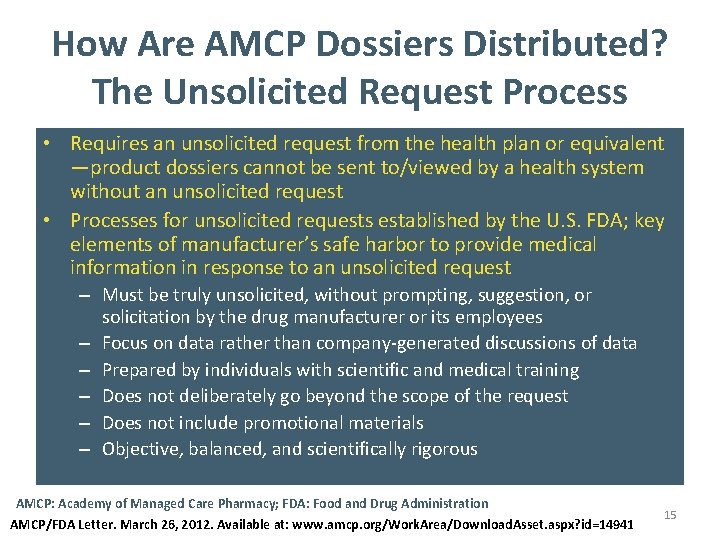
How Are AMCP Dossiers Distributed? The Unsolicited Request Process • Requires an unsolicited request from the health plan or equivalent —product dossiers cannot be sent to/viewed by a health system without an unsolicited request • Processes for unsolicited requests established by the U. S. FDA; key elements of manufacturer’s safe harbor to provide medical information in response to an unsolicited request – Must be truly unsolicited, without prompting, suggestion, or solicitation by the drug manufacturer or its employees – Focus on data rather than company-generated discussions of data – Prepared by individuals with scientific and medical training – Does not deliberately go beyond the scope of the request – Does not include promotional materials – Objective, balanced, and scientifically rigorous AMCP: Academy of Managed Care Pharmacy; FDA: Food and Drug Administration AMCP/FDA Letter. March 26, 2012. Available at: www. amcp. org/Work. Area/Download. Asset. aspx? id=14941 15

AMCP Dossier: Not a Promotional Document “The quality and comprehensiveness of evidence within the dossiers remains an issue. Plans are warning that if the evidence dossiers are hijacked by commercial interests in the companies or from outside, they will not use them. They desire a scientific communication—not promotion. ” -Sean Sullivan University of Washington AMCP: Academy of Managed Care Pharmacy Sullivan S. FDA Unsolicited Request and the AMCP Format. ISPOR 2012. Available at: http: //www. ispor. org/meetings/Washington. DC 0512/releasedpresentations/IP 4 -Sullivan. pdf 16

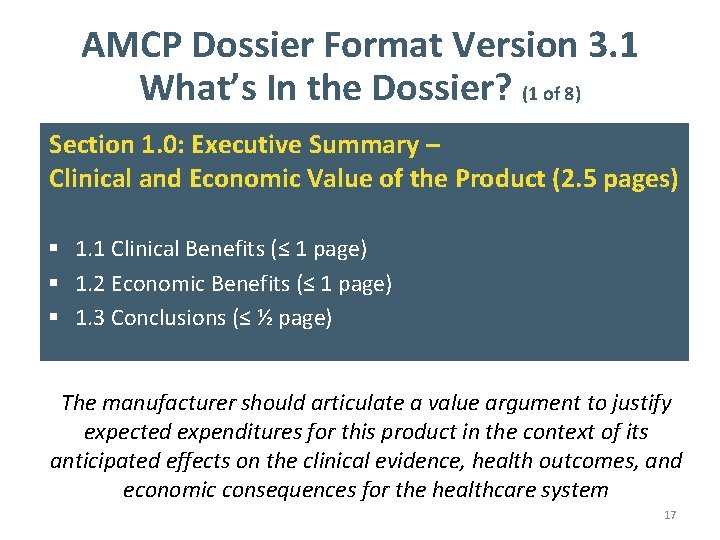
AMCP Dossier Format Version 3. 1 What’s In the Dossier? (1 of 8) Section 1. 0: Executive Summary – Clinical and Economic Value of the Product (2. 5 pages) § 1. 1 Clinical Benefits (≤ 1 page) § 1. 2 Economic Benefits (≤ 1 page) § 1. 3 Conclusions (≤ ½ page) The manufacturer should articulate a value argument to justify expected expenditures for this product in the context of its anticipated effects on the clinical evidence, health outcomes, and economic consequences for the healthcare system 17

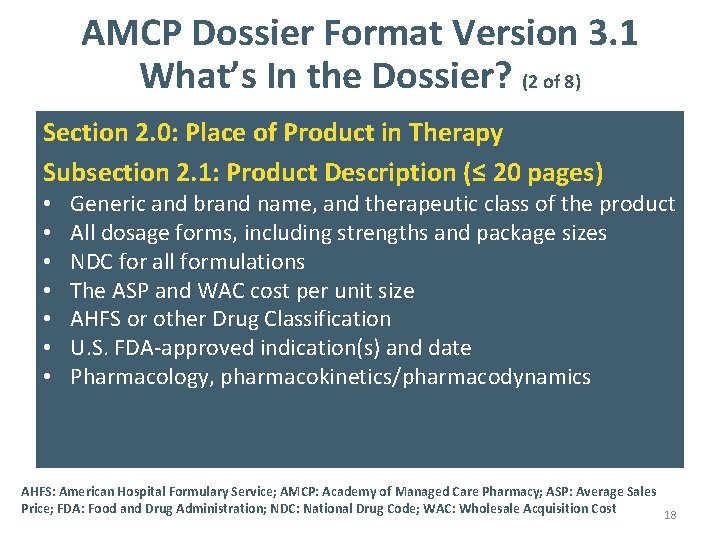
AMCP Dossier Format Version 3. 1 What’s In the Dossier? (2 of 8) Section 2. 0: Place of Product in Therapy Subsection 2. 1: Product Description (≤ 20 pages) • • Generic and brand name, and therapeutic class of the product All dosage forms, including strengths and package sizes NDC for all formulations The ASP and WAC cost per unit size AHFS or other Drug Classification U. S. FDA-approved indication(s) and date Pharmacology, pharmacokinetics/pharmacodynamics AHFS: American Hospital Formulary Service; AMCP: Academy of Managed Care Pharmacy; ASP: Average Sales Price; FDA: Food and Drug Administration; NDC: National Drug Code; WAC: Wholesale Acquisition Cost 18

AMCP Dossier Format Version 3. 1 What’s In the Dossier? (3 of 8) Section 2. 0: Place of Product in Therapy Subsection 2. 1: Product Description (≤ 20 pages, Cont’d) • Contraindications/warnings/precautions/adverse effects • Interactions(drug/drug, drug/food, drug/disease) • Dosing and administration • Access, e. g. restrictions on distribution, supply; prescribing restrictions • Co-prescribed/concomitant therapies • Big Table: Concise comparison of prescribing information of target product and primary comparator products in same therapeutic area AHFS: American Hospital Formulary Service; AMCP: Academy of Managed Care Pharmacy; ASP: Average Sales Price; FDA: Food and Drug Administration; NDC: National Drug Code; WAC: Wholesale Acquisition Cost 19

AMCP Dossier Format Version 3. 1 What’s In the Dossier? (4 of 8) Section 2. 0: Place of Product In Therapy Subsections 2. 2. 1, 2. 2. 2, 2. 2. 3, and 2. 3 • 2. 2. 1 Disease Description (≤ 2 pages)* – Format as bullets and tables • 2. 2. 2 Approaches To Treatment (≤ 2 pages)* – Format as bullets and tables • 2. 2. 3 Relevant Treatment Guidelines and Consensus Statements • 2. 3 Evidence for Pharmacogenomic Tests and Drugs * Can be expanded to 3 pages for Specialty Pharmaceuticals AMCP: Academy of Managed Care Pharmacy 20

AMCP Dossier Format Version 3. 1 What’s In the Dossier? (5 of 8) Section 3. 0: Supporting Clinical Evidence Subsection 3. 1: Summaries of Key Clinical Studies Supporting Labeled Indications (≤ 2 pages/study) a. b. c. d. e. f. g. h. i. j. k. l. m. Name of the clinical trial or study and publication citation(s) Study objective, location and date(s) Trial design, randomization, and blinding procedures Setting, inclusion, and exclusion criteria Patient characteristics Dropout rates and procedures for handling dropouts Treatment, including dosage regimens, washout period Clinical outcome(s) measured (delineate primary vs. secondary endpoints) Other outcomes measured (e. g. , patient-reported outcomes) and principal findings Statistical significance of outcomes and power calculations Validation of outcomes instruments (if applicable) Generalizability of the population treated Study limitations, as stated by the authors AMCP: Academy of Managed Care Pharmacy 21

AMCP Dossier Format Version 3. 1 What’s In the Dossier? (6 of 8) Section 3. 0: Supporting Clinical Evidence Subsections 3. 1. 1. , 3. 1. 2. , 3. 1. 3, 3. 1. 4 § 3. 1. 1 All Other Relevant Published and Unpublished Clinical Studies Supporting Labeled Indications § 3. 1. 2 All Published and Unpublished Data and Clinical Studies Supporting Off-Label Indications § Additional items: a) U. S. FDA CDER Office of Drug Safety; b) Access information for ongoing clinical trials (at clinicaltrials. gov). § 3. 1. 3 Clinical Evidence Spreadsheets of all Published and Unpublished Trials § 3. 1. 4 Summary of Evidence From Secondary Sources (Cochrane reviews, systematic reviews, AHRQ evidence summaries, HTAs) AHRQ: Agency for Healthcare Research and Quality; AMCP: Academy of Managed Care Pharmacy; ; CDER: Center 22 for Drug Evaluation and Research; FDA: Food and Drug Administration; HTA: Health Technology Assessment

AMCP Dossier Format Version 3. 1 What’s In the Dossier? (7 of 8) Section 4. 0: Economic Value and Modeling Report (≤ 20 pages) • • 4. 1 Abstract 4. 2. Introduction / Background 4. 3 Methods 4. 4 Results 4. 5 Limitations 4. 6 Discussion Minimum 2 Figures, 3 Tables (detailed instructions provided) 4. 7 Interactive Model AMCP: Academy of Managed Care Pharmacy 23

AMCP Dossier Format Version 3. 1 What’s In the Dossier? (8 of 8) Section 5. 0: Other Supporting Evidence § 5. 1 Summaries of Other Relevant Evidence (≤ 2 pages/study) § Includes retrospective studies, comparative observational studies, studies of patient adherence/persistence § 5. 1. 1. Summarize relevant economic studies not covered elsewhere Section 6. 0: Supporting Information § 6. 1 References § 6. 2 Appendices: Prescribing information, CER addendum, REMS program documents, etc. AMCP: Academy of Managed Care Pharmacy; CER: comparative effectiveness research REMS: Risk Evaluation and Mitigation Strategy 24

Economic Models In the AMCP Dossier • Section 4 of the AMCP Dossier Format requests a descriptive narrative and an interactive model (typically Excel-based) – Allows healthcare systems to apply and evaluate internal data (ie, membership, prevalence, cost estimates) • Most common model types: – Cost-effectiveness (assesses overall clinical risk-benefit and economic value of drug in relation to other available drugs/treatments) – Financial model (minimal approach: financial impact on pharmacy budget only) – Budget impact model AMCP: Academy of Managed Care Pharmacy 25

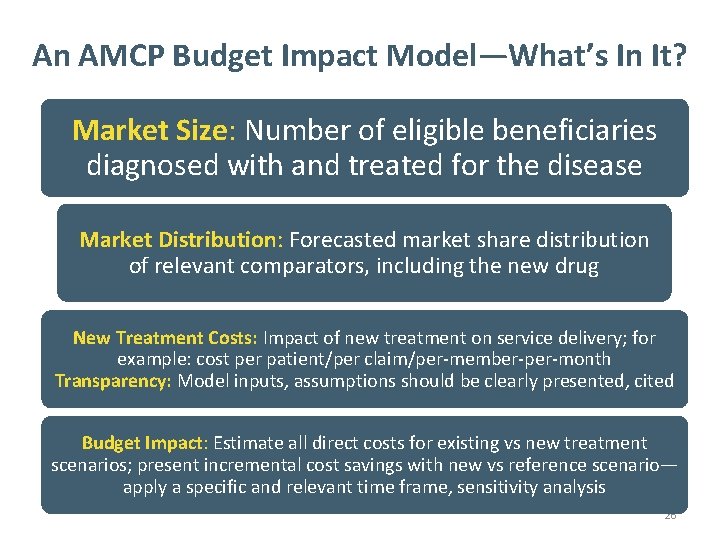
An AMCP Budget Impact Model—What’s In It? Market Size: Number of eligible beneficiaries diagnosed with and treated for the disease Market Distribution: Forecasted market share distribution of relevant comparators, including the new drug New Treatment Costs: Impact of new treatment on service delivery; for example: cost per patient/per claim/per-member-per-month Transparency: Model inputs, assumptions should be clearly presented, cited Budget Impact: Estimate all direct costs for existing vs new treatment scenarios; present incremental cost savings with new vs reference scenario— apply a specific and relevant time frame, sensitivity analysis 26

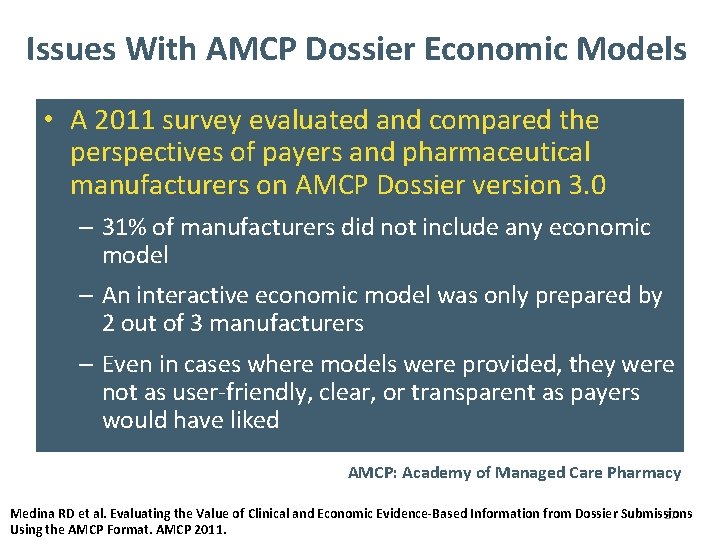
Issues With AMCP Dossier Economic Models • A 2011 survey evaluated and compared the perspectives of payers and pharmaceutical manufacturers on AMCP Dossier version 3. 0 – 31% of manufacturers did not include any economic model – An interactive economic model was only prepared by 2 out of 3 manufacturers – Even in cases where models were provided, they were not as user-friendly, clear, or transparent as payers would have liked AMCP: Academy of Managed Care Pharmacy Medina RD et al. Evaluating the Value of Clinical and Economic Evidence-Based Information from Dossier Submissions 27 Using the AMCP Format. AMCP 2011.

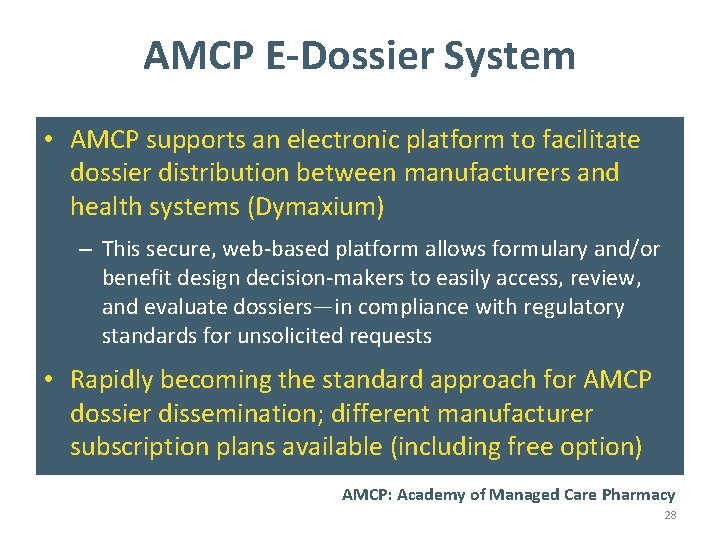
AMCP E-Dossier System • AMCP supports an electronic platform to facilitate dossier distribution between manufacturers and health systems (Dymaxium) – This secure, web-based platform allows formulary and/or benefit design decision-makers to easily access, review, and evaluate dossiers—in compliance with regulatory standards for unsolicited requests • Rapidly becoming the standard approach for AMCP dossier dissemination; different manufacturer subscription plans available (including free option) AMCP: Academy of Managed Care Pharmacy 28

Writing AMCP Dossiers: Practical Issues AMCP Dossiers are a mix of more traditional medical writing and health economics writing • Highly structured document, based on a defined format; plain formatting is a plus • Length depends on number of indications and body of available research, but typically ~50 -200 pages [goal <100 pages] • Generally a team project— challenging for a single freelancer (can be 400 -500 hours’ work on tight turnaround) • Writers likely to be assigned specific sections • Requires close project management AMCP: Academy of Managed Care Pharmacy 29

The Dossier Thank you! It’s time for Q & A 30