CT and MRI in pregnancy and lactation Fergus































- Slides: 56

CT and MRI in pregnancy and lactation Fergus Coakley MD, Professor of Radiology and Urology, Vice Chair for Clinical Services, Chief of Abdominal Imaging, UCSF

Learning objectives u Detail the safety issues related to CT and MRI during pregnancy/lactation u Describe the problematic and newer applications of CT and MRI in pregnancy u Advise clinicians on appropriate use of imaging in pregnancy/lactation

Context u Growing demand radiation awareness: – 121% more tests over 10 years u Doctors poorly informed: – Superficial ACOG guidelines – 5% would suggest TOP after CT u Radiologists need to take the lead RSNA program 2007; 436 AJR 2004; 182: 1107 -1109 Medline hits for “CT radiation dose”

Safety of CT - Safety of MRI - Indications for CT and MRI Safety of CT

Risks of CT u Teratogenesis – Stochastic (threshold) u Carcinogenesis – Non-stochastic (no threshold) u Iodinated contrast

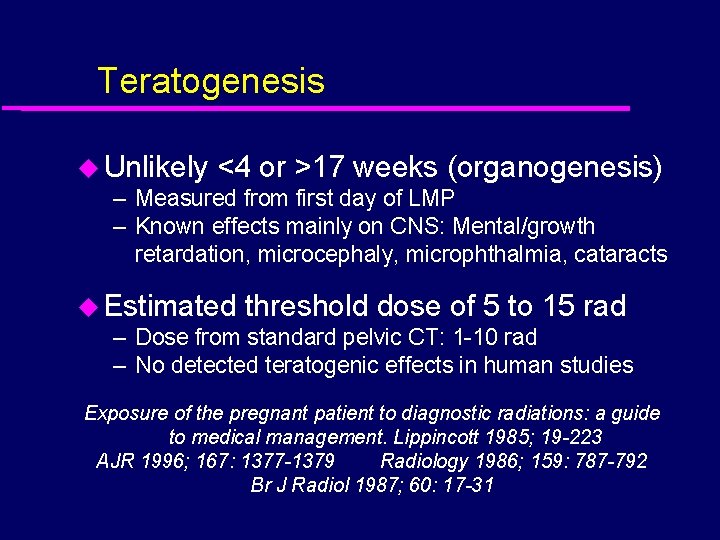
Teratogenesis u Unlikely <4 or >17 weeks (organogenesis) – Measured from first day of LMP – Known effects mainly on CNS: Mental/growth retardation, microcephaly, microphthalmia, cataracts u Estimated threshold dose of 5 to 15 rad – Dose from standard pelvic CT: 1 -10 rad – No detected teratogenic effects in human studies Exposure of the pregnant patient to diagnostic radiations: a guide to medical management. Lippincott 1985; 19 -223 AJR 1996; 167: 1377 -1379 Radiology 1986; 159: 787 -792 Br J Radiol 1987; 60: 17 -31

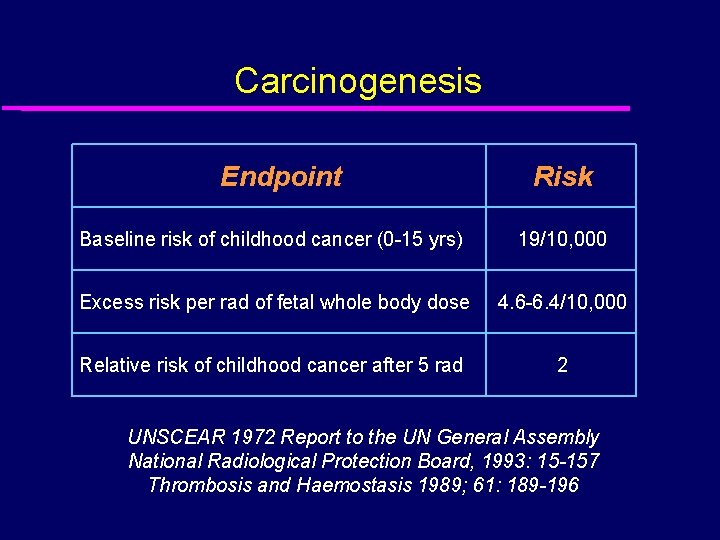
Carcinogenesis Endpoint Risk Baseline risk of childhood cancer (0 -15 yrs) 19/10, 000 Excess risk per rad of fetal whole body dose 4. 6 -6. 4/10, 000 Relative risk of childhood cancer after 5 rad 2 UNSCEAR 1972 Report to the UN General Assembly National Radiological Protection Board, 1993: 15 -157 Thrombosis and Haemostasis 1989; 61: 189 -196

Basis of risk estimates u Oxford Survey of Childhood Cancer u 547 case-control pairs (1953 -55): – Child (< 10) dying of cancer in England & Wales – Matched living control (age, sex, location) – Standard questionnaire to both mothers u OSCC subsequently extended: – 15, 276 case-control pairs by 1981 Lancet 1956; 2: 447 BJR Feb 1997; 130 -139

OSCC - Results Maternal radiation Control s Cases Risk To abdomen 43 85 2. 0 To other body part 55 58 1. 0 None 447 404 NA

Gestational age & carcinogenesis u Relative risk by trimester (OSCC data): First (< 10 weeks) First (All) Second Third 4. 6 3. 2 1. 3 J Radiol Prot 1988; 8: 3 -8

What should we do? u Only perform CT of the pregnant abdomen and pelvis if critical: – Clear clinical justification with benefit >> risk – No non-ionizing imaging options – CT of other body areas much smaller concern u Risks and benefits should be discussed with the patient/parents and documented: – Signed informed consent may be wise – Sample form at www. radiology. ucsf. edu

Parental counseling u Absolute risks: – Baseline risk of fatal childhood cancer = 1/2000 – Risk after fetal dose of 5 rads = 2/2000 u Practical comparisons for excess – Driving 20, 000 miles in a car – Living in New York City for 3 years risk: u ACOG guidelines are superficial: – Describe carcinogenic risk as "very small” – Conclude "abortion should not be recommended” – Do not discuss parental counseling/consent http: //www. physics. isu. edu/radinf/risk. htm Obstet Gynecol 2004; 104: 647 -651

Imaging fertile women u Varying historical approaches: – 10 day rule, 28 day rule, limited 10 day rule u Largely based on “all or nothing” concept of early risk, and ignores carcinogenesis u What are the regulatory and practical requirements? Statement from the 1983 Washington meeting of ICRP. Annals of International Commission on Radiological Protection 1984: 14 Board statement on diagnostic medical exposure to ionising radiation during pregnancy and estimates of late radiation risks to the UK population. Documents of the NRPB 1993; 4: 1 -14

Regulatory guidelines u No requirement for pregnancy testing u ACR: “Radiologists should be advised of known or possible pregnancy” u HHS: “A woman who is or thinks she is pregnant should be encouraged to give this information to the physician” Medical radiation: a guide to good practice. ACR 1985; 4 -8 DHSS publication no. HHS/FDA-86 -8254 AJR 1996; 167: 1377 -1379

Good practice u Pregnancy section on requisition forms u Prominent signage u Routine questioning by technologist

Good practice u No safe time during menstrual – Various “day rules” are obsolete cycle: u Any possibility of pregnancy: – Consult with clinician +/- perform pregnancy test u Earliest positive pregnancy test: – Serum h. CG - 7 days after conception – Urinary h. CG - first day of missed period u STALL!! – Request other opinions, e. g. surgical consult

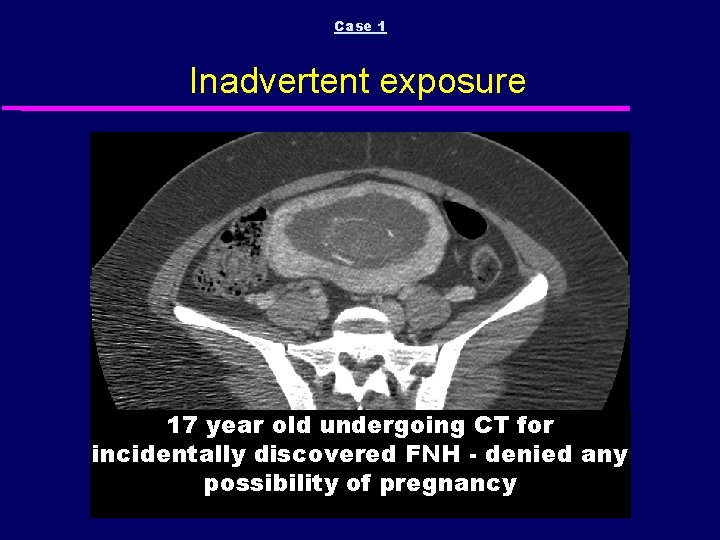
Case 1 Inadvertent exposure 17 year old undergoing CT for incidentally discovered FNH - denied any possibility of pregnancy

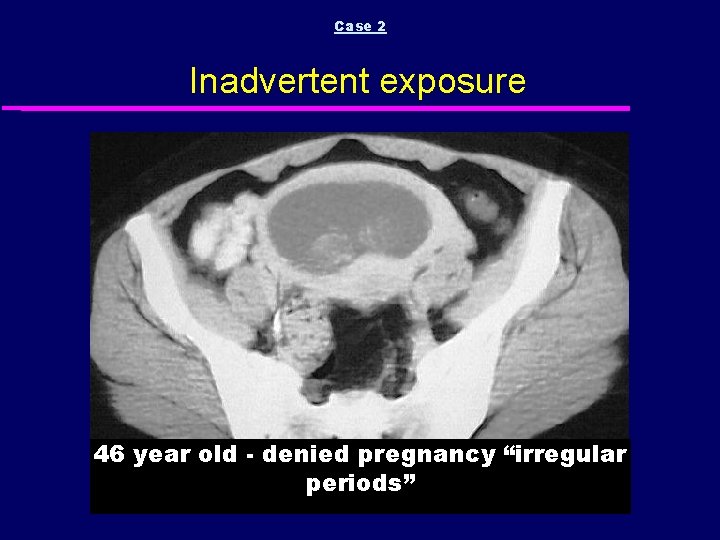
Case 2 Inadvertent exposure 46 year old - denied pregnancy “irregular periods”

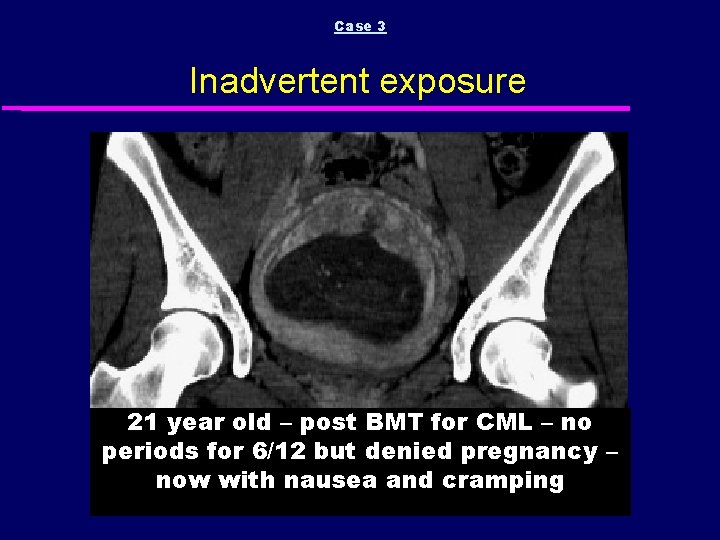
Case 3 Inadvertent exposure 21 year old – post BMT for CML – no periods for 6/12 but denied pregnancy – now with nausea and cramping

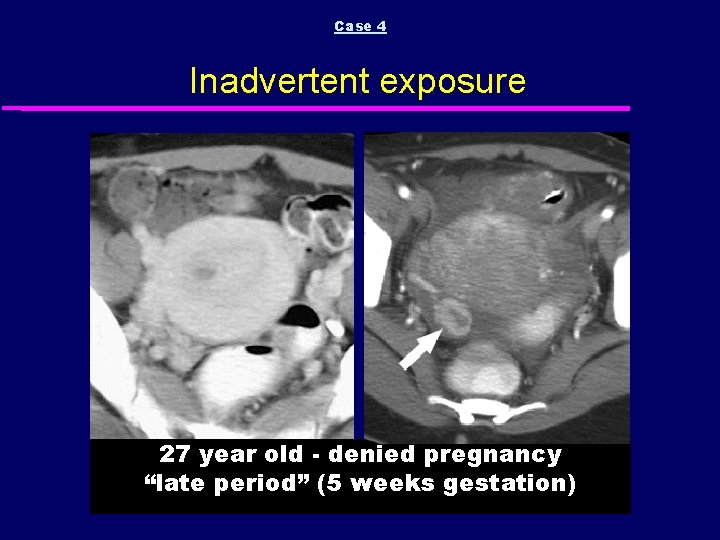
Case 4 Inadvertent exposure 27 year old - denied pregnancy “late period” (5 weeks gestation)

Case 4 Inadvertent exposure GESTATIONAL SAC DECIDUAL REACTION

Case 5 Inadvertent exposure PLACENTA CORPUS LUTEUM GESTATIONAL SAC 20 year old at 7 weeks gestation with RLQ pain

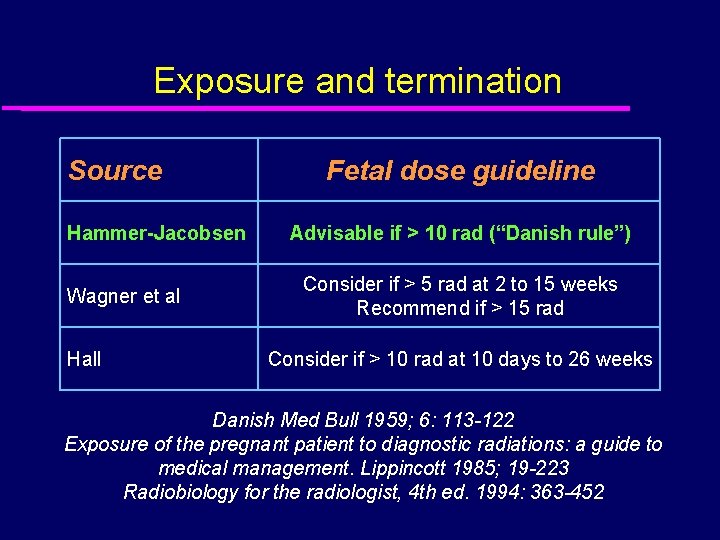
Exposure and termination Source Hammer-Jacobsen Wagner et al Hall Fetal dose guideline Advisable if > 10 rad (“Danish rule”) Consider if > 5 rad at 2 to 15 weeks Recommend if > 15 rad Consider if > 10 rad at 10 days to 26 weeks Danish Med Bull 1959; 6: 113 -122 Exposure of the pregnant patient to diagnostic radiations: a guide to medical management. Lippincott 1985; 19 -223 Radiobiology for the radiologist, 4 th ed. 1994: 363 -452

Fetal doses 1 rad 2 rad 3 rad Key point: Radiation dose from single CT of the pelvis is highly unlikely to justify termination Patel, S. J. et al. Radiographics 2007; 27: 1705 -1722 Copyright ©Radiological Society of North America, 2007

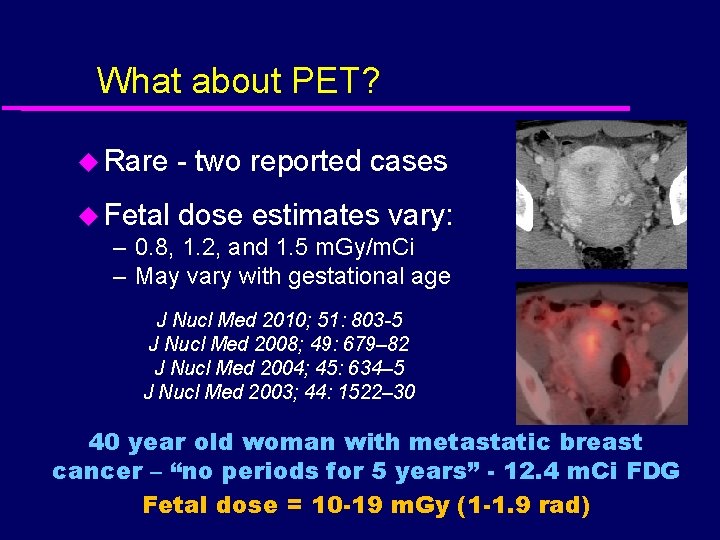
What about PET? u Rare - two reported cases u Fetal dose estimates vary: – 0. 8, 1. 2, and 1. 5 m. Gy/m. Ci – May vary with gestational age J Nucl Med 2010; 51: 803 -5 J Nucl Med 2008; 49: 679– 82 J Nucl Med 2004; 45: 634– 5 J Nucl Med 2003; 44: 1522– 30 40 year old woman with metastatic breast cancer – “no periods for 5 years” - 12. 4 m. Ci FDG Fetal dose = 10 -19 m. Gy (1 -1. 9 rad)

Iodinated contrast in pregnancy u Iodinated contrast should be avoided: – Amniography can cause hypothyroidism – Mutagenic to human cells in vitro u NOT teratogenic in animals u Better than rescanning? Invest Radiol 1982; 17: 183 -185 Eur J Radiol 1994; 18 (Suppl 1): 21 -31 Invest Radiol 1989; 24 (Suppl 1): 16 -22 Am J Obstet Gynecol 1976; 126: 723 -726

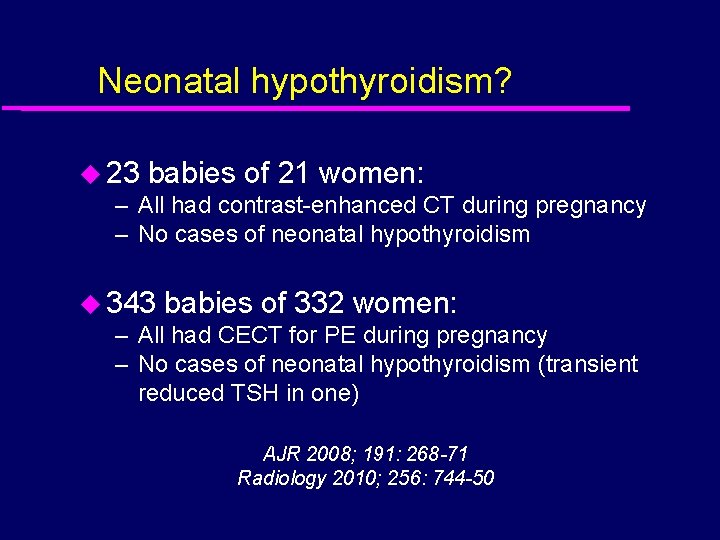
Neonatal hypothyroidism? u 23 babies of 21 women: – All had contrast-enhanced CT during pregnancy – No cases of neonatal hypothyroidism u 343 babies of 332 women: – All had CECT for PE during pregnancy – No cases of neonatal hypothyroidism (transient reduced TSH in one) AJR 2008; 191: 268 -71 Radiology 2010; 256: 744 -50

Iodinated contrast and lactation u Standard recommendation: – Stop breast-feeding for 24 hours u Weak rationale: – Minimal passage of IV contrast into breast milk – Minimal absorption of oral iodinated contrast – No thyroid dysfunction after neonatal IV contrast u Recommendation recently questioned: – Personal approach - continue breast-feeding Eur J Radiol 1992; 12: 22 -25 Acta Radiol Suppl. 1980; 362: 87 -92 Eur Radiol 2005; 15: 1234 -1240

Safety of CT - Safety of MRI - Indications for CT and MRI Safety of MRI

Risks of MRI u Teratogenesis u Acoustic damage u Gadolinium toxicity

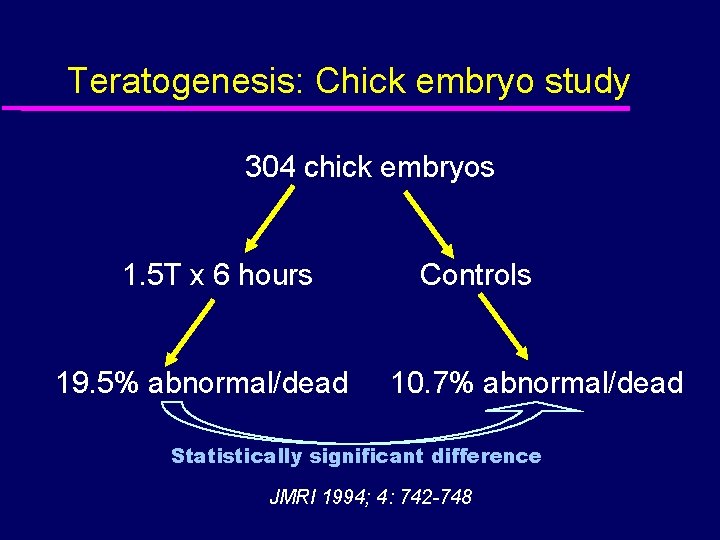
Teratogenesis: Chick embryo study 304 chick embryos 1. 5 T x 6 hours 19. 5% abnormal/dead Controls 10. 7% abnormal/dead Statistically significant difference JMRI 1994; 4: 742 -748

Acoustic damage u Follow-up of 20 children after fetal EPI: – 16/18 passed hearing test at 8/12 (16. 7 expected) u Intragastric sound intensity measurement: – Fetal exposure < maternal Am J Obstet Gynecol 1994; 170: 32 -33 Br J Radiol 1995; 68: 1090 -1094

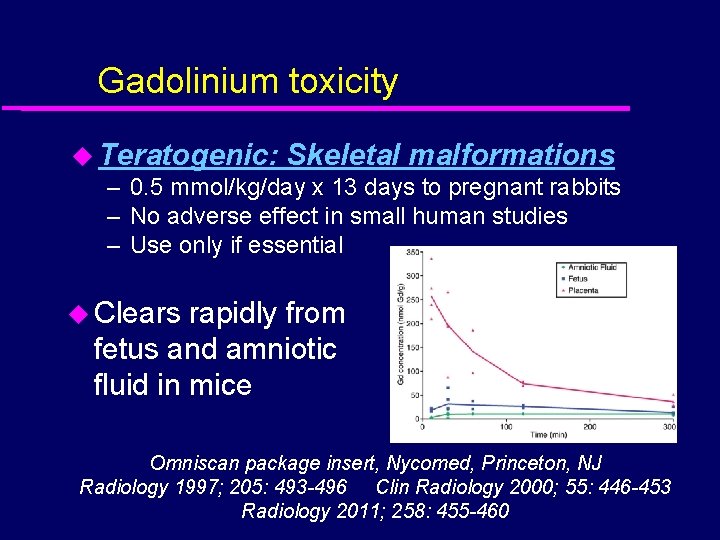
Gadolinium toxicity u Teratogenic: Skeletal malformations – 0. 5 mmol/kg/day x 13 days to pregnant rabbits – No adverse effect in small human studies – Use only if essential u Clears rapidly from fetus and amniotic fluid in mice Omniscan package insert, Nycomed, Princeton, NJ Radiology 1997; 205: 493 -496 Clin Radiology 2000; 55: 446 -453 Radiology 2011; 258: 455 -460

FDA and drugs in pregnancy Categor y Fetal dose (rads) A Controlled studies in women fail to demonstrate a risk to the fetus – remote possibility of fetal harm B Animal studies show no risks, but there are no controlled human studies Adverse effects in animals, but not in well-controlled human studies Use in pregnancy considered. GADOLINIUM probably safe (e. g. acetaminophen) C Studies in animals have revealed adverse effects on the fetus and no controlled studies in women, or studies in women and animals not available Only use if benefit justifies the potential risk (most prescribed medications) D Positive evidence of human fetal risk Benefits may be acceptable if the risk is high (life-threatening situation or serious disease with no other options, e. g. , most chemotherapy drugs) X IODINATED CONTRAST Studies in animals or women have demonstrated fetal abnormalities

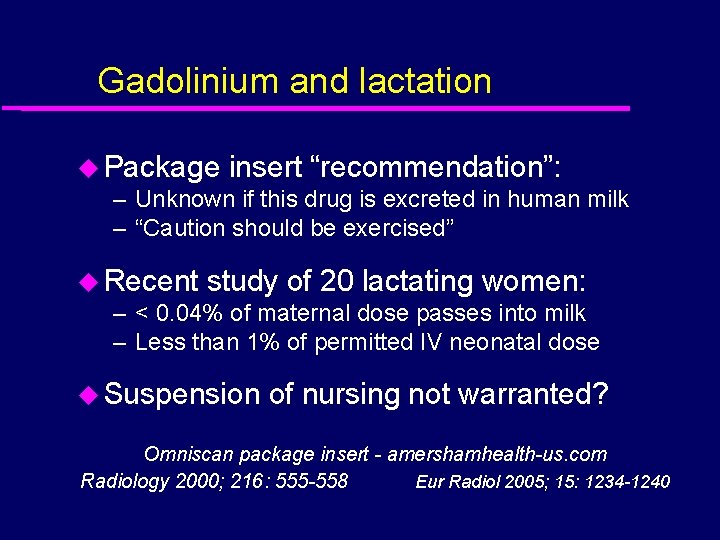
Gadolinium and lactation u Package insert “recommendation”: – Unknown if this drug is excreted in human milk – “Caution should be exercised” u Recent study of 20 lactating women: – < 0. 04% of maternal dose passes into milk – Less than 1% of permitted IV neonatal dose u Suspension of nursing not warranted? Omniscan package insert - amershamhealth-us. com Radiology 2000; 216: 555 -558 Eur Radiol 2005; 15: 1234 -1240

Safety of CT - Safety of MRI - Indications for CT and MRI

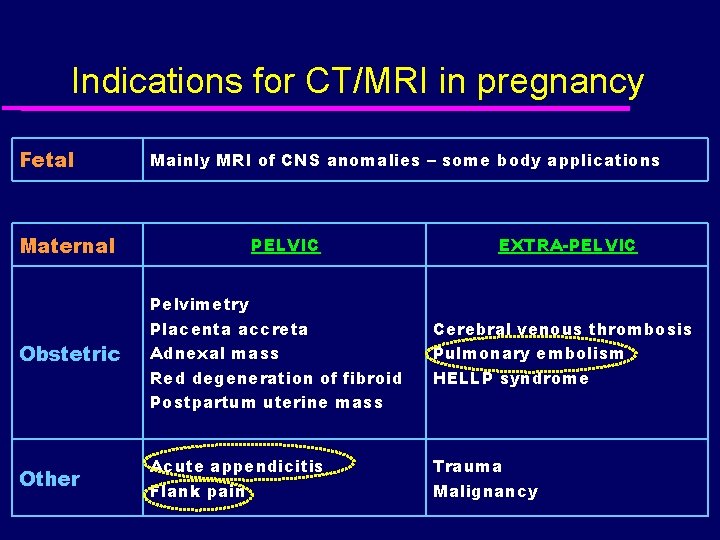
Indications for CT/MRI in pregnancy Fetal Maternal Mainly MRI of CNS anomalies – some body applications PELVIC EXTRA-PELVIC Obstetric Pelvimetry Placenta accreta Adnexal mass Red degeneration of fibroid Postpartum uterine mass Cerebral venous thrombosis Pulmonary embolism HELLP syndrome Other Acute appendicitis Flank pain Trauma Malignancy

Pulmonary embolism u PE rate = 0. 7 per 1000 pregnancies: – 50% occur after Cesarean section u Imaging options: – V/Q scan, helical CT, pulmonary angiography – No comparative studies in pregnancy – 25% of V/Q scans nondiagnostic in pregnancy (v. 7% in nonpregnant patients) Angiology 2002; 53: 429 -434 Obstet Gynecol 1999; 94: 730 -734 Arch Intern Med 2002; 162: 1170 -1175

Radiation doses from PE studies Test Fetal dose 3 -130 micro. Gy Helical CT Rises from first to third trimester V/Q scan 100 -370 micro. Gy Assumes reduced dose of Tc 99 m (37 -74 MBq) Pulmonary 500 micro. Gy angiogram Assumes brachial approach Radiology 2002; 224: 487 -492

Perfusion only scan? Dose CTPA Q scan Maternal 2. 0 Sv 0. 6 Sv Breast 10. 0 m. Gy 0. 28 m. Gy Fetus 0. 01 m. Gy 0. 12 m. Gy British Medical Journal 2005; 331: 350

Acute appendicitis in pregnancy u Major indication for surgery in pregnancy: – 1 in 1500 pregnancies – Diagnosis clinically difficult, 25% perforation rate u Limited data on role of imaging: – CT 100% accurate (n = 2 of 7) – US 100% sensitive & 96% specific (n = 15 of 42) – US could not be performed in 3 (all > 35 weeks) Mil Med. 1999; 164: 671 -674 Am J Obstet Gynecol 2001; 184: 954 -957 AJR 1992; 159: 539 -542

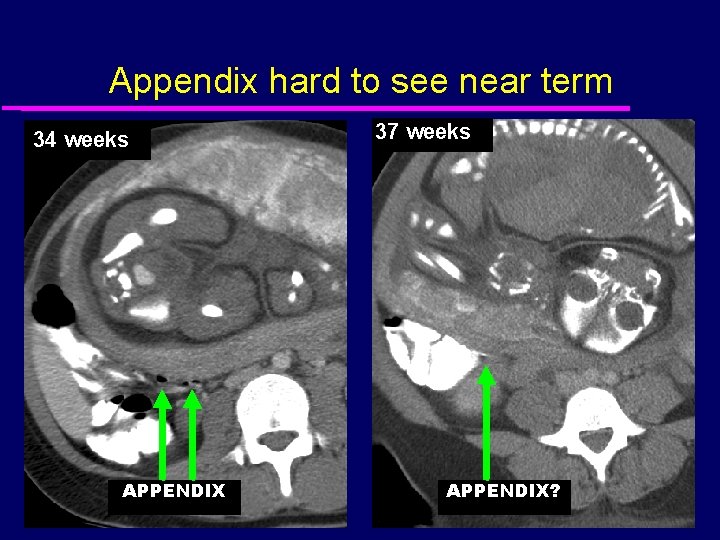
Appendix hard to see near term 34 weeks APPENDIX 37 weeks APPENDIX?

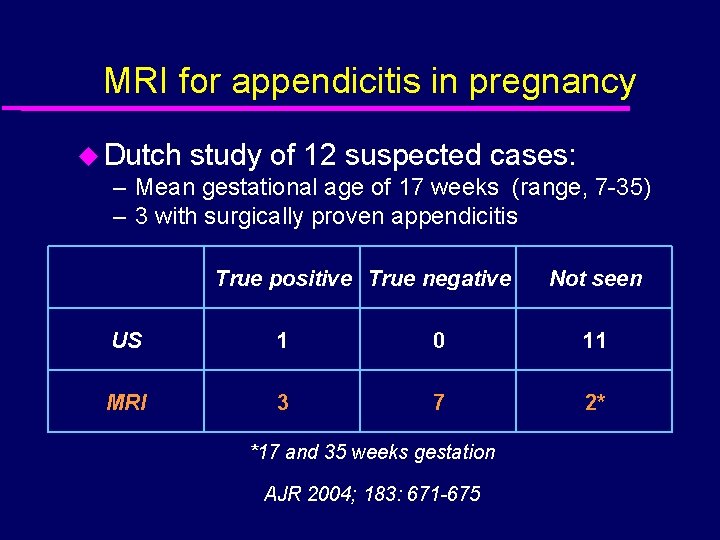
MRI for appendicitis in pregnancy u Dutch study of 12 suspected cases: – Mean gestational age of 17 weeks (range, 7 -35) – 3 with surgically proven appendicitis True positive True negative Not seen US 1 0 11 MRI 3 7 2* *17 and 35 weeks gestation AJR 2004; 183: 671 -675

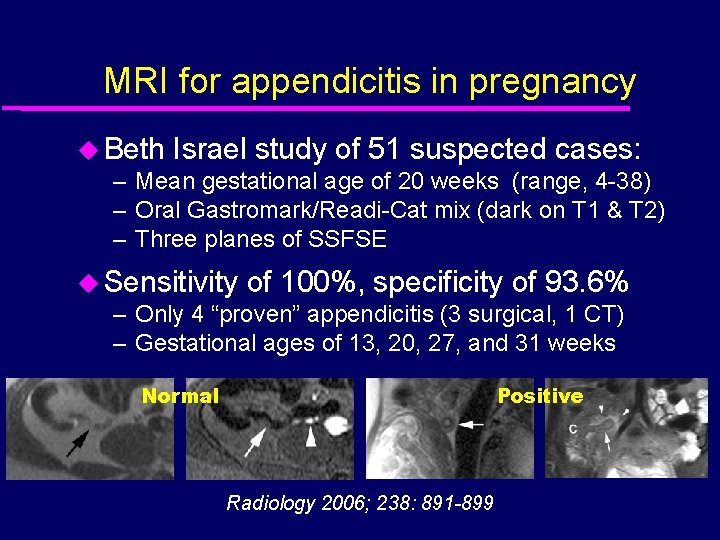
MRI for appendicitis in pregnancy u Beth Israel study of 51 suspected cases: – Mean gestational age of 20 weeks (range, 4 -38) – Oral Gastromark/Readi-Cat mix (dark on T 1 & T 2) – Three planes of SSFSE u Sensitivity of 100%, specificity of 93. 6% – Only 4 “proven” appendicitis (3 surgical, 1 CT) – Gestational ages of 13, 20, 27, and 31 weeks Normal Positive Radiology 2006; 238: 891 -899

MRI for appendicitis in pregnancy Normal Positive

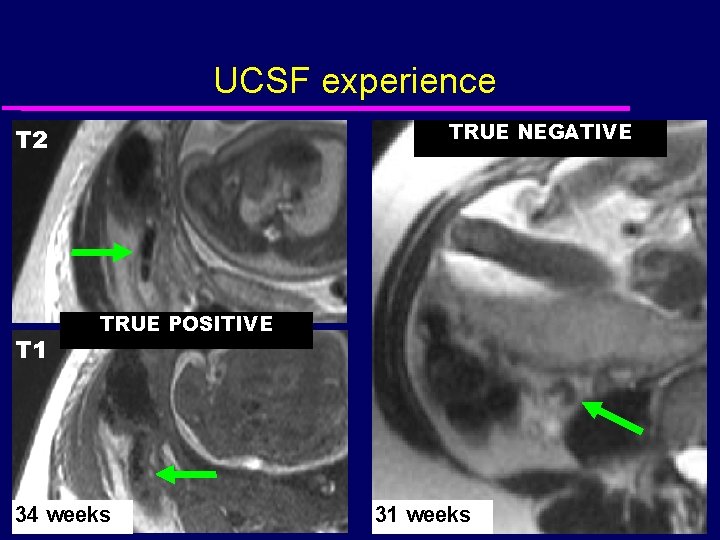
UCSF experience TRUE NEGATIVE T 2 T 1 TRUE POSITIVE 34 weeks 31 weeks

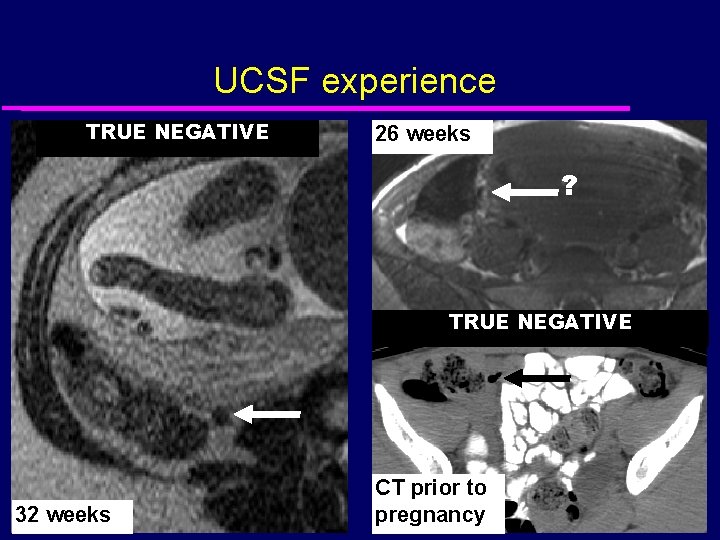
UCSF experience TRUE NEGATIVE 26 weeks ? TRUE NEGATIVE 32 weeks CT prior to pregnancy

UCSF experience SMALL BOWEL OBSTRUCTION 18 weeks FORNICEAL RUPTURE 14 weeks

Flank pain u Hydronephrosis common in pregnancy: – Probably mechanical – Consider stones, etc if symptomatic u Imaging options: – US, NECT, IVP, isotope renography, MRU – No established optimal approach

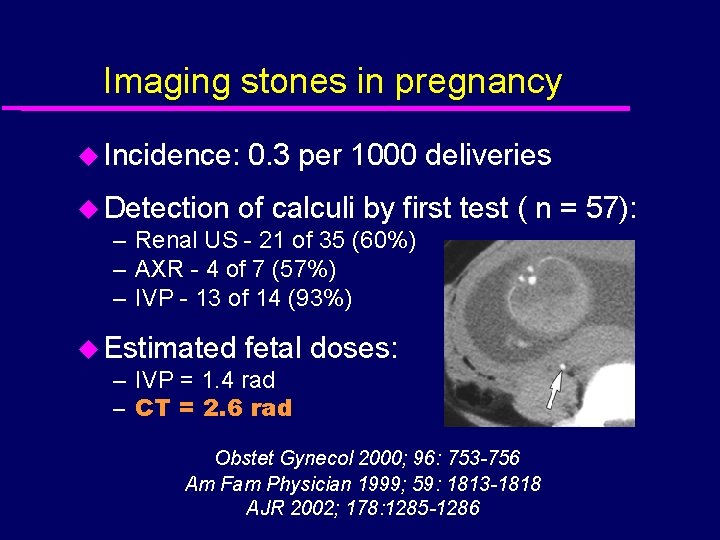
Imaging stones in pregnancy u Incidence: 0. 3 per 1000 deliveries u Detection of calculi by first – Renal US - 21 of 35 (60%) – AXR - 4 of 7 (57%) – IVP - 13 of 14 (93%) u Estimated fetal – IVP = 1. 4 rad – CT = 2. 6 rad test ( n = 57): doses: Obstet Gynecol 2000; 96: 753 -756 Am Fam Physician 1999; 59: 1813 -1818 AJR 2002; 178: 1285 -1286

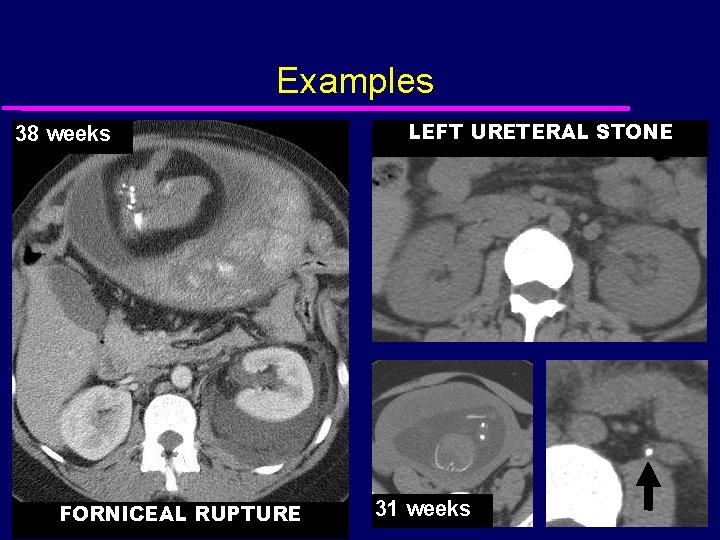
Examples 38 weeks FORNICEAL RUPTURE LEFT URETERAL STONE 31 weeks

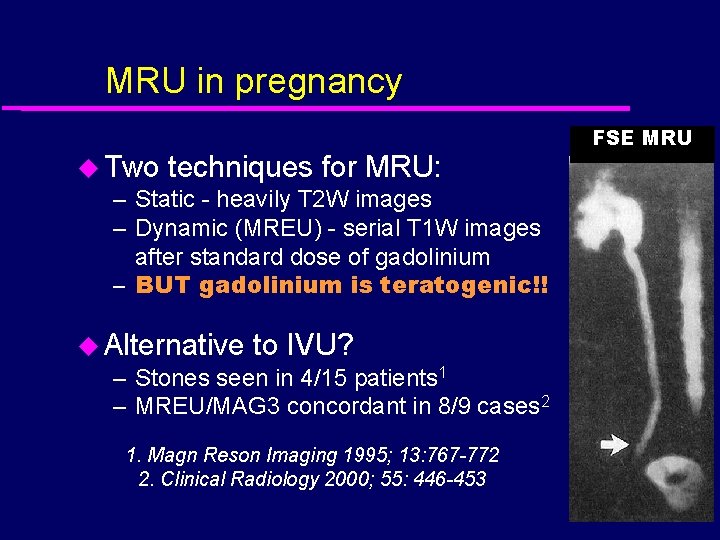
MRU in pregnancy u Two techniques for MRU: – Static - heavily T 2 W images – Dynamic (MREU) - serial T 1 W images after standard dose of gadolinium – BUT gadolinium is teratogenic!! u Alternative to IVU? – Stones seen in 4/15 patients 1 – MREU/MAG 3 concordant in 8/9 cases 2 1. Magn Reson Imaging 1995; 13: 767 -772 2. Clinical Radiology 2000; 55: 446 -453 FSE MRU

Take home points u CT and pregnancy: – Teratogenesis unlikely at diagnostic doses – Carcinogenesis is a real risk – Document risk/benefit discussion, or signed consent u MRI and pregnancy: – No proven risk, but avoid first trimester studies u Contrast and pregnancy/lactation: – Iodinated contrast is (probably) safe – Gadolinium is (relatively) contraindicated – No need to stop breast-feeding

Take home points u Suspected PE in pregnancy: – CT preferred to V/Q scans throughout pregnancy u Suspected appendicitis in pregnancy: – All modalities limited near term - US worth trying – MRI may help if US inconclusive u Flank pain in pregnancy: – US first – but may be indeterminate – Manage symptomatically versus limited IVP? – Remember forniceal rupture u Obstet & Gynecol 2008; 112: 333 -340

Case study u 20 year with SEVERE flare of known Crohn’s disease at 19 weeks gestation u “Must rule out abscess” - GI attending CONTRAST-ENHANCED CT OR GAD-ENHANCED MRI?

“We’ve created a safe, nonjudgmental environment that will leave your child ill prepared for real life”
 Leydig cells histology
Leydig cells histology Pregnancy mri
Pregnancy mri Early pregnancy pictures of spotting during pregnancy
Early pregnancy pictures of spotting during pregnancy Densenet number of parameters
Densenet number of parameters Drugs in lactation
Drugs in lactation Lactation physiology
Lactation physiology United states lactation consultant association
United states lactation consultant association Ps12q
Ps12q Parturition
Parturition Lactation
Lactation Pictures of pyometra in dogs
Pictures of pyometra in dogs Singulair 10 mg buy online
Singulair 10 mg buy online Teat meatus
Teat meatus Lactation tetany
Lactation tetany Rob fergus
Rob fergus Crystal dental fergus
Crystal dental fergus Gus social fergus
Gus social fergus Rob fergus
Rob fergus Picme2.0
Picme2.0 Free surfer
Free surfer Gp mri indications
Gp mri indications Serebral pedinkül
Serebral pedinkül Image formation in mri
Image formation in mri Is type 1 acromion serious?
Is type 1 acromion serious? How mri works
How mri works Mri hydrogen atoms
Mri hydrogen atoms Mri fourier transform
Mri fourier transform Translate
Translate Hoglund brain imaging center
Hoglund brain imaging center Head of caudate blood supply
Head of caudate blood supply Geraldine tran
Geraldine tran Haghighat mri center
Haghighat mri center Valuenomics
Valuenomics Amegdala
Amegdala First mri image 1973
First mri image 1973 Mri hydrogen atoms
Mri hydrogen atoms Gracilis muscle mri
Gracilis muscle mri Lesion ap psychology
Lesion ap psychology Lauterbur
Lauterbur Vertical mri
Vertical mri Midbrain ct
Midbrain ct Entry slice phenomenon
Entry slice phenomenon Foramen rotundum ct
Foramen rotundum ct Mri k space
Mri k space Brain stem on mri
Brain stem on mri Angular momentum mri
Angular momentum mri Atrial clip procedure
Atrial clip procedure Mri scanner
Mri scanner Moire artifact mri
Moire artifact mri Jt spencer death
Jt spencer death Gp mri indications
Gp mri indications Disadvantage of mri
Disadvantage of mri Nmr kolena
Nmr kolena Mri scanner
Mri scanner Fgatir ge mri
Fgatir ge mri Mri question
Mri question Siemens mri safety video
Siemens mri safety video