CMRR Human Research Training 2015 Siemens Video MRIs
























- Slides: 37

CMRR Human Research Training 2015

Siemens Video

MRI’s Capabilities 1. Anatomical imaging (water content or PD) 2. Imaging based on the different dynamical features of tissue water molecules, also known as “relaxation” (relaxation time constants: T 1, T 2*, T 1 r, T 2 r, TRAFF) 3. Imaging based on differences in diffusion coefficient (ADC) 4. Imaging of blood flow (tissue perfusion at the capillary level) 5. Contrast-enhanced MRI (contrast agents, Ktrans, angiography) 6. Imaging based on differences in magnetic susceptibility (phase contrast, blood oxygenation, blood volume, micro-calcifications) 7. Imaging to track molecules and cells labeled with iron-oxide nanoparticles (SPIONs) 8. MR Spectroscopy (metabolism)

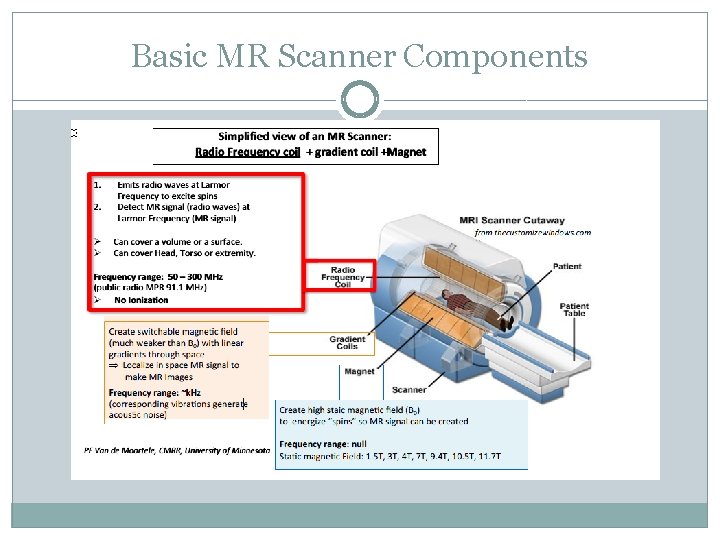
Basic MR Scanner Components

General Magnet Safety

Active versus Passive Shielding �Difference is how the static magnetic fringe field is managed. Active with reverse energized shielding coils � Fringe field is very limited, but the gradient is steep Tends to be more dangerous as you often won’t recognize that you are within the fringe field until it is too late Passive with iron sheets surrounding magnet � Not as efficient as active shielding, so magnet fringe field tends to extend much further from magnet

Superconducting Magnets �All CMRR MR systems are superconducting magnets. Electromagnets made from coiled wire that is cooled with liquid Helium (-270 degrees C) Does not require external power once energized Difficult and expensive to ramp up and down Magnet is ALWAYS on!

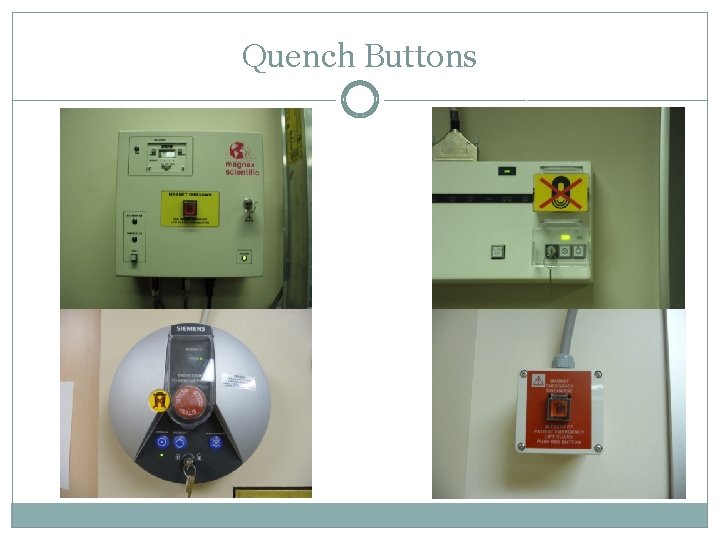
Magnet Quench • A quench is the ONLY way to remove the magnetic field in an emergency situation • The magnets should only be quenched under 2 circumstances • • In a life-threatening emergency, where someone is pinned against the magnet by a ferrous object In a major fire in the magnet room • In most cases the decision to quench should be left up to the fire department • Steps to take in the event a quench is deemed necessary • • Dial 911 Clear the room of all personnel if possible • • Open the magnet room door Press the magnet quench button • Notify Peter or Gregor and the Safety Officer of the quench • Quench results in rapid release of gaseous helium which can result in displaced oxygen or liquefied oxygen in the magnet room • There are 1 or 2 buttons for each magnet, before beginning a study on an unfamiliar magnet take a moment to locate the buttons

Quench Buttons

Cryogen Safety � 3 Main hazards associated with the cryogens (Liquid Helium and liquid Nitrogen) used to cool magnets during/after a quench Frostbite and/or hypothermia � Do not touch surfaces that have been exposed to helium gas and appear “frosty” Asphyxiation � Oxygen can be displaced or liquefied during a quench especially if the quench vent is blocked Explosions � If the quench vent system is blocked the helium gas may be released into the scanner room pressurizing the room creating an “explosion” possibility.

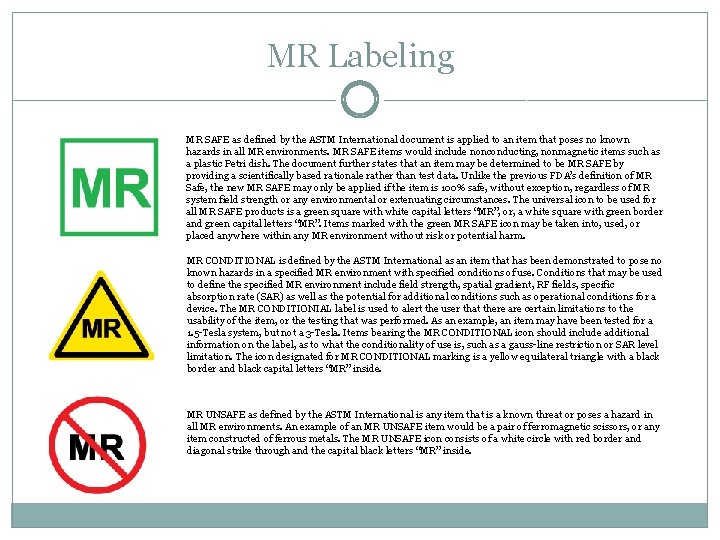
MR Labeling MR SAFE as defined by the ASTM International document is applied to an item that poses no known hazards in all MR environments. MR SAFE items would include nonconducting, nonmagnetic items such as a plastic Petri dish. The document further states that an item may be determined to be MR SAFE by providing a scientifically based rationale rather than test data. Unlike the previous FDA’s definition of MR Safe, the new MR SAFE may only be applied if the item is 100% safe, without exception, regardless of MR system field strength or any environmental or extenuating circumstances. The universal icon to be used for all MR SAFE products is a green square with white capital letters “MR”, or, a white square with green border and green capital letters “MR”. Items marked with the green MR SAFE icon may be taken into, used, or placed anywhere within any MR environment without risk or potential harm. MR CONDITIONAL is defined by the ASTM International as an item that has been demonstrated to pose no known hazards in a specified MR environment with specified conditions of use. Conditions that may be used to define the specified MR environment include field strength, spatial gradient, RF fields, specific absorption rate (SAR) as well as the potential for additional conditions such as operational conditions for a device. The MR CONDITIONIAL label is used to alert the user that there are certain limitations to the usability of the item, or the testing that was performed. As an example, an item may have been tested for a 1. 5 -Tesla system, but not a 3 -Tesla. Items bearing the MR CONDITIONAL icon should include additional information on the label, as to what the conditionality of use is, such as a gauss-line restriction or SAR level limitation. The icon designated for MR CONDITIONAL marking is a yellow equilateral triangle with a black border and black capital letters “MR” inside. MR UNSAFE as defined by the ASTM International is any item that is a known threat or poses a hazard in all MR environments. An example of an MR UNSAFE item would be a pair of ferromagnetic scissors, or any item constructed of ferrous metals. The MR UNSAFE icon consists of a white circle with red border and diagonal strike through and the capital black letters “MR” inside.

Fire Extinguishers � The red metallic fire extinguishers should never be brought into the magnet room under any circumstance. The blue and white (non-metallic) fire extinguishers are located in the RF rooms and can be used to extinguish fires in the magnet rooms. If there is a fire in the magnet room you have two options: Remove all personnel, close the door, and call the fire department Use a blue and white (non-metallic) fire extinguisher to attempt to extinguish the fire. Magnetic Non-Magnetic

Subject Safety

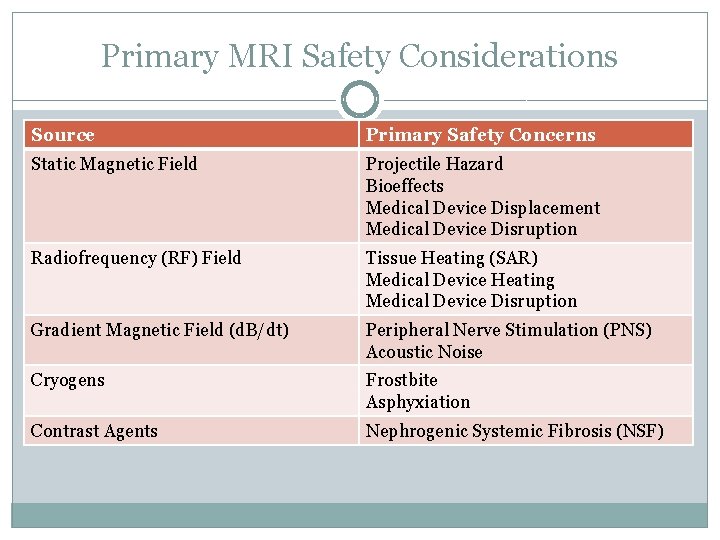
Primary MRI Safety Considerations Source Primary Safety Concerns Static Magnetic Field Projectile Hazard Bioeffects Medical Device Displacement Medical Device Disruption Radiofrequency (RF) Field Tissue Heating (SAR) Medical Device Heating Medical Device Disruption Gradient Magnetic Field (d. B/dt) Peripheral Nerve Stimulation (PNS) Acoustic Noise Cryogens Frostbite Asphyxiation Contrast Agents Nephrogenic Systemic Fibrosis (NSF)

Static Magnetic Field (B 0) �Spatial gradient �Translational forces �Torque/Rotational forces �Lenz’s forces �Magnetohydrodynamic effect �Flow potentials (if ECG triggering/gating is required)

Radiofrequency Issues �Local Heating �Global Heating �Implant Heating �Subject Positioning Skin-to-Skin Contact Skin-to-Bore Contact Avoiding Loops �Cable Heating

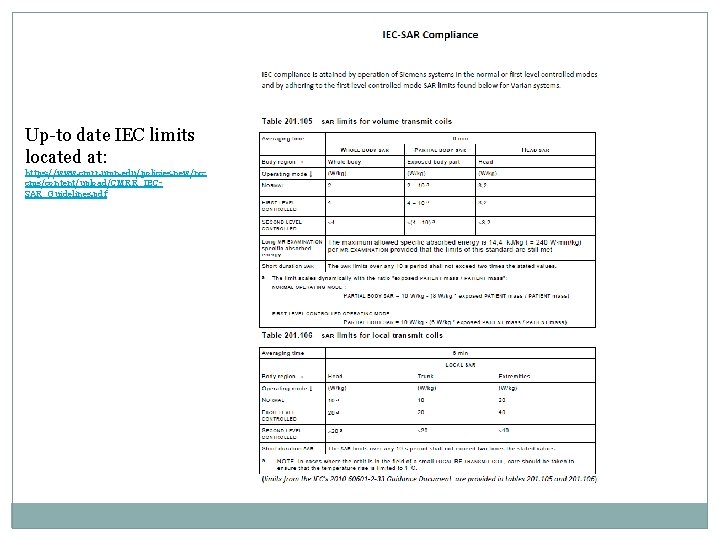
Up-to date IEC limits located at: https: //www. cmrr. umn. edu/policies. new/nccms/content/upload/CMRR_IECSAR_Guidelines. pdf

Scanner Operating Modes �Normal Operating Mode None of the outputs have a value that could cause physiological stress �First Level Mode One or more output may reach a value that cause physiological stress �Second Level Mode One or more output reach a value that may produce significant risk � FDA Approval is Required

Gradient Magnetic Fields (d. B/dt) �d. B/dt is ratio between change in amplitude of magnetic field (d. B) and the time it takes to make the change (dt) Safety Concerns � Heating Minimal compared to RF induced heating � Peripheral Nerve Stimulation Induced unwanted electrical fields in body Some people are more susceptible than others Scanner is programmed to limit d. B/dt to levels that do not produce severe discomfort or painful nerve stimulation � Implanted Device Malfunction � Acoustic Noise Rapidly switching gradients creates acoustic noise that can cause hearing damage

Special Safety Considerations �Special considerations that should be taken into account when evaluating the potential for thermal injury Impaired Thermoregulatory System � Not able to dissipate RF generated heat efficiently Sedated Subjects � Unable to alert operator to potential problems Use/Presence of Materials that Inhibit Cooling � Use of padding or other materials that decrease or inhibit evaporative cooling such as padding or dielectric pads Physical Size of Subject � Skin to coil contact increases risk of burns, so if subject is too large to allow for the use of pads to limit skin to coil contact this increases the risk of thermal injury

Possible side effects �Please alert the individual being scanned of the following possible side effects: Dizziness Nausea Metallic taste Claustrophobia Peripheral Nerve Stimulation Vertigo

Subject Monitoring Systems �There a number of systems available for monitoring subjects Squeeze ball � All subjects should be given the squeeze bulb to use during all scans � The squeeze bulb should be tested every time the subject is moved to isocenter to ensure the pneumatic line isn’t compromised ECG, Pulse, Respiratory Monitoring � These devices must be properly placed and shielded to prevent risk of burns to the subject. � Note that the magnetic field alters the contours of the electrocardiogram. If a patient requires the use of a defibrillator (defibrillation should NEVER be performed in the scanner room), monitoring electrodes applied for use in the scanner should be removed first to avoid electrical burns.

Subject Screening Policy �Any person entering the MR scanner Room must complete a screening form that is reviewed by the investigator prior to entering the room. �A new screening form must be completed by subjects for each day they are scanned. If any doubts regarding the screening responses – do NOT allow the individual to enter the MR scanner. The fact that the individual has been scanned in an MR scanner before (even at CMRR) is never a sufficient basis upon which to conclude that the subject can enter the scanner room safely.

Explicit Contraindications �People with the following items/conditions can NOT be scanned: Pregnancy – The risk to the fetus has not been determined Pacemaker Aneurysm Clips Vagus Nerve Stimulation (VNS) Deep Brain Stimulators (DBS) Non-removable Insulin Pumps Abandoned Leads Neuro Stimulators Nerve Stimulators

General Scanning Policies

2 Person Rule � Solo scanning of human subjects is against CMRR policy. � It is the responsibility of the researcher to ensure that, in addition to the operator running the scanner, a second person is available during the entirety of every scanning session. The second person is not required to be in the scanner suite throughout the session, but must be in the building and available to respond to a Code Blue page at any time. � The recommended best practice is to schedule two people from your own research team for every experiment. � Under no circumstances may you leave a subject in the scanner without a qualified scanner operator in the console room. If there is a computer or equipment malfunction during a study, call your second research team member to help. If your second research team member is not in the room, do not use the paging system yourself during normal business hours – call the front desk and ask that your colleague be paged. If there is no answer at the front desk, then follow the instructions on the emergency contact sheet posted near the phone in the console room to use the paging system to call for assistance.

Hearing Protection �It is mandatory for the patient/volunteer and all others who will be present in the magnet room during the scan session to wear hearing protection in the form of earplugs, headphones, or a combination of earplugs and headphones that are provided by the facility. Earplugs and/or headphones are available at each scanner. Earplugs can be used alone. Avotec headphones can be used alone. Sensimetrics earbuds can be used alone. Siemens headphones must be used in conjunction with another form of hearing protection.

Researcher/Staff Pregnancy Policy �Based on the American College of Radiology Guidelines that state that “Pregnant health care practitioners are permitted to work in and around the MR environment throughout all stages of their pregnancy” CMRR employees, researchers, and anyone else with, or seeking, access to the CMRR can maintain their access or be granted new access while pregnant, but must ultimately make that decision for themselves.

Gowning Policy � All research subjects/patients are required to wear CMRR-provided scrubs or gowns during MR scans unless given a policy exemption � Designated changing rooms are 1 -122, 1 -206 E, 1 -302 A. These rooms cannot be reserved and should only be used for changing. � Reservable consenting rooms are 1 -143, 1 -206 C, 1 -206 D, 1 -123. These rooms should not be reserved for changing purposes. � Restrooms and curtained areas near the 3 T’s can also be used as changing rooms as needed. � Scrubs sized Small-Xlarge are in each changing room. Child sized, Xxlarge and XXXlarge are located in the linen supply closet outside the 7 T/AS. � Exemptions are given on a study by study basis and must be requested through the Safety Committee https: //www. cmrr. umn. edu/policies/nccms/content/upload/CMRR_Scrub_Gown_Policy. pdf for CMRR policy information

MR Professionals Sign-off Process �It is the responsibility of all personnel who are involved in conducting human subject research at CMRR to screen ALL people entering the magnet room. �The screening form is broken into 3 sections. Affirmative answers in section 1 – can be signed off by the researcher. Section 2 – The signature of the PI or a Co-Investigator as listed on the IRB protocol, MR professional or CMRR MR Technologist is required prior to scanning Section 3 - The signature of MR Professional is required prior to scanning. Can be done in person, via phone or by email. �In order to minimize un-used scan time, obtain all necessary approvals prior to the day of the scan. https: //www. cmrr. umn. edu/policies/nccms/content/upload/CMRR_Affirmative_Answer_SOP. pdf

Incidental Findings �In the event that a researcher sees something questionable/abnormal on a scan please follow the information below: � 1. Include in your consent for the approved text found at CMRR’s Human Subjects Research Page � 2. There are two stages to submitting the scan for review Create a review request via the web-based interface at https: //www. cmrr. umn. edu/scanreviews 2/ After filling out the required information on the online form, from transfer the appropriate scans from the scanner console to the Radiology DICOM server

�The review should be complete within a few weeks. When the review is complete, you can see the results by clicking on the “Read Completed Reviews” in the User section of the Scan Review website. � 4) The project is charged $50 per reading. The complete policy can be reviewed at: http: //www. cmrr. umn. edu/internal/Human. Subjects. s html

Biohazardous Waste Disposal The proper procedure is: 1) 2) 3) 4) Bag your biohazardous waste in a red biohazard bag at the point of generation. At the conclusion of your study tie/tape the bag shut. Place the bag in one of the large red sharps containers in the refrigerator in the RAR garage. If there is no red sharps container in the refrigerator, if the container is full, or if it feels close to the maximum weight of 50 lbs a new container should be started. To start a new container place it in the refrigerator and line it with one of the large biohazard bags that can be found inside of the empty containers. Once the red sharps container is full the liner bag must be tied and the lid must be snapped on. Failure to do either of these items will result in the container not be picked up. �http: //www. dehs. umn. edu/bio_wastedisptble. htm

Standard Forms �The CMRR Safety screening form is required for ALL scans �Depending on the strength of the magnet and CMRR affiliation additional forms may be required. Subject information, Exit Questionnaire, CMRR Subject Reimbursement Form. �Consent Form Template includes Required Language �https: //www. cmrr. umn. edu/policies/irb. php

Contrast Injections �All contrast injections must be conducted with a radiologist available within CMRR. Contrast coverage must be arranged by contacting CCIR staff

Medical Emergencies � Step 1: Dial 911 � Step 2: Remove subject from scanner and/or magnet room if possible (MR Safe wheelchair and gurney located in CTSI patient prep area as well as 3 T/7 T RF room) If and Only If emergency involves a ferrous object pinning someone against the magnet initiate magnet quench � Step 3: Administer CPR if necessary (AED’s located near copier on “classic” side and outside of 3 T on the “new” side) � Step 4: Meet responding personnel at front door, escort them to subject, and communicate dangers of facility �It is extremely important that you Do not allow responding personnel to enter the magnet room unless they have been fully screened and briefed on the dangers.

Required Tracking �Personnel in the Center for Magnetic Resonance Research (CMRR), the site where you are participating in MRI research, maintain a list of the names and contact information of all participants included in research at this facility. This information is required and will be used by CMRR to notify participants of significant new information about the effects of MR on human health that develop over the course of MRI research. Participant’s identifying information is stored securely and it is maintained in a confidential manner by persons with oversight of research conducted at the CMRR.