CHEMISTRY OF MATCHES P 4 S 3 KCl





















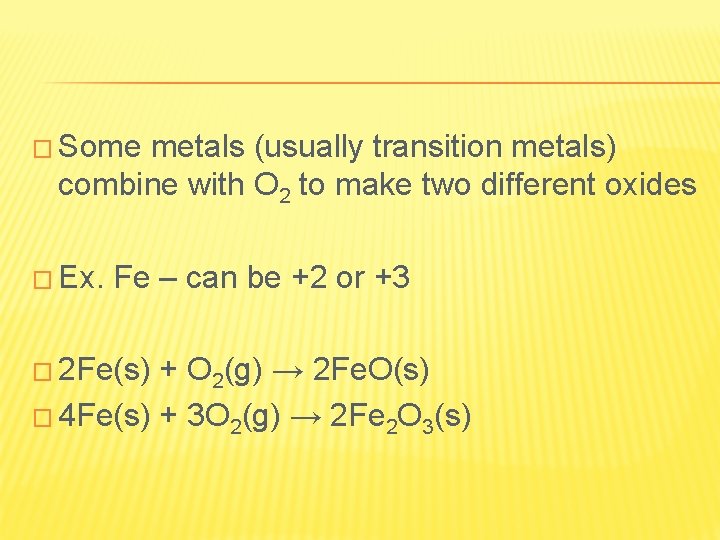



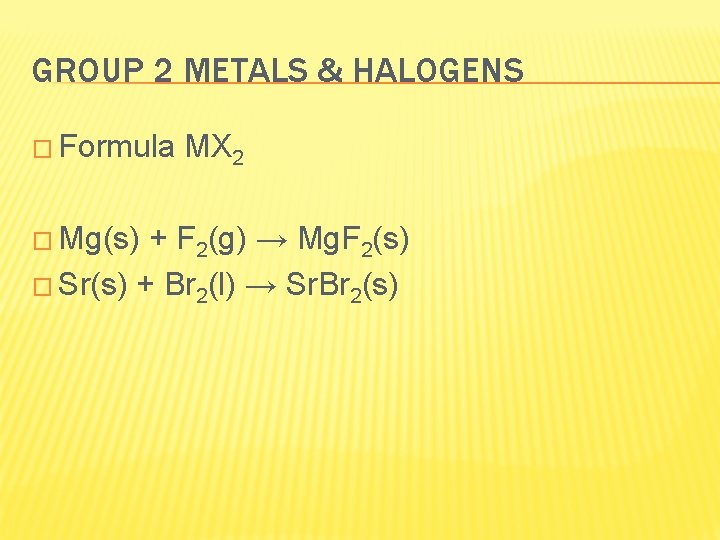













- Slides: 71

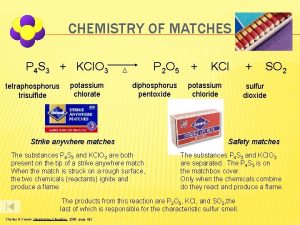
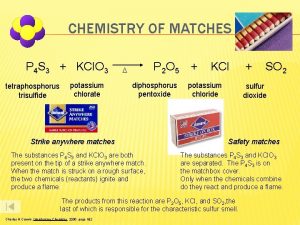
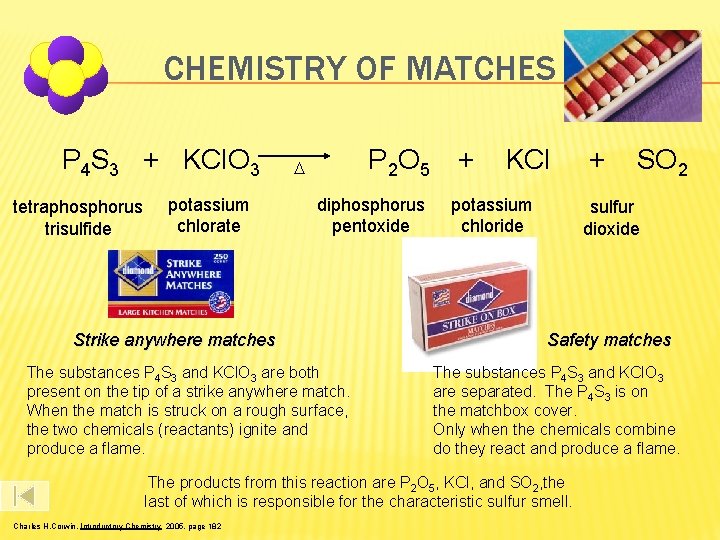
CHEMISTRY OF MATCHES P 4 S 3 + KCl. O 3 tetraphosphorus trisulfide potassium chlorate P 2 O 5 D diphosphorus pentoxide Strike anywhere matches The substances P 4 S 3 and KCl. O 3 are both present on the tip of a strike anywhere match. When the match is struck on a rough surface, the two chemicals (reactants) ignite and produce a flame. + KCl potassium chloride SO 2 sulfur dioxide Safety matches The substances P 4 S 3 and KCl. O 3 are separated. The P 4 S 3 is on the matchbox cover. Only when the chemicals combine do they react and produce a flame. The products from this reaction are P 2 O 5, KCl, and SO 2, the last of which is responsible for the characteristic sulfur smell. Charles H. Corwin, Introductory Chemistry 2005, page 182 +

Chemical Equations and Reactions CHAPTER 8

Describing Chemical Reactions SECTION 1

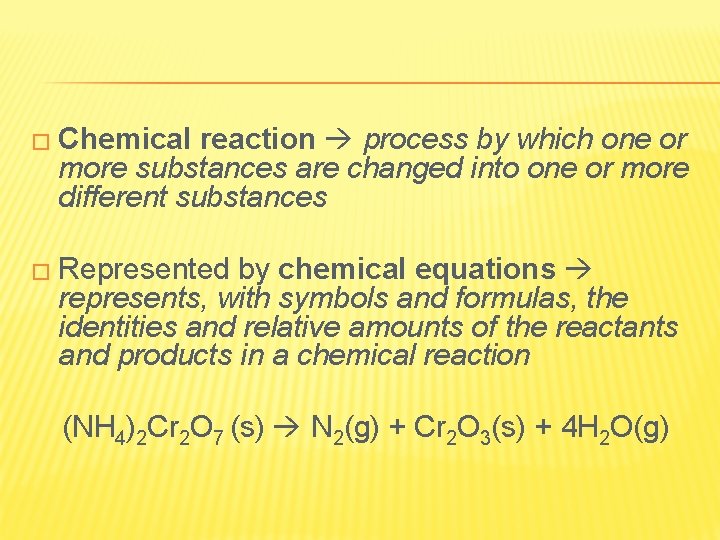
� Chemical reaction process by which one or more substances are changed into one or more different substances � Represented by chemical equations represents, with symbols and formulas, the identities and relative amounts of the reactants and products in a chemical reaction (NH 4)2 Cr 2 O 7 (s) N 2(g) + Cr 2 O 3(s) + 4 H 2 O(g)

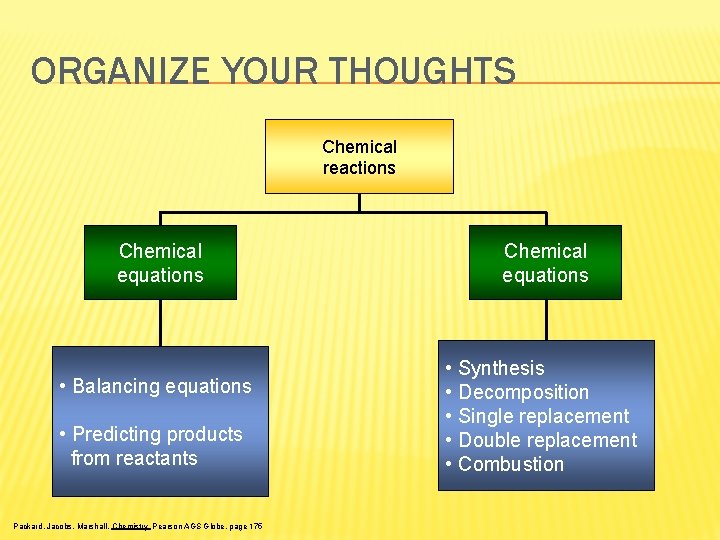
ORGANIZE YOUR THOUGHTS Chemical reactions Chemical equations • Balancing equations • Predicting products from reactants Packard, Jacobs, Marshall, Chemistry Pearson AGS Globe, page 175 Chemical equations • Synthesis • Decomposition • Single replacement • Double replacement • Combustion

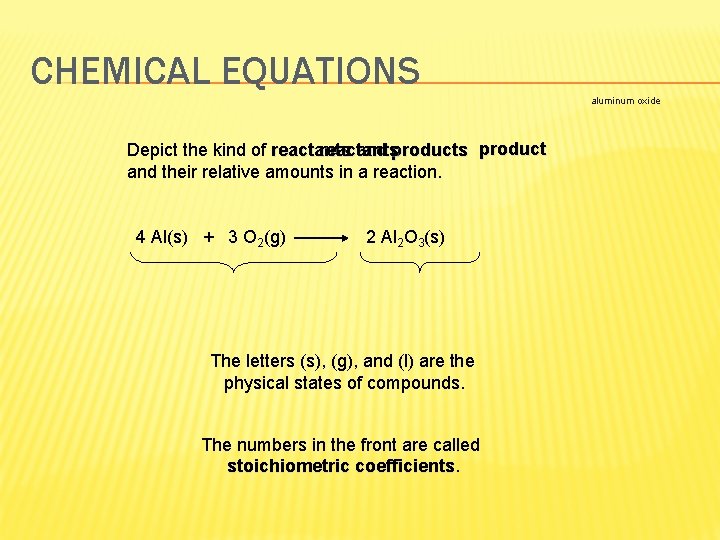
CHEMICAL EQUATIONS aluminum oxide reactants Depict the kind of reactants and products product and their relative amounts in a reaction. 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) The letters (s), (g), and (l) are the physical states of compounds. The numbers in the front are called stoichiometric coefficients

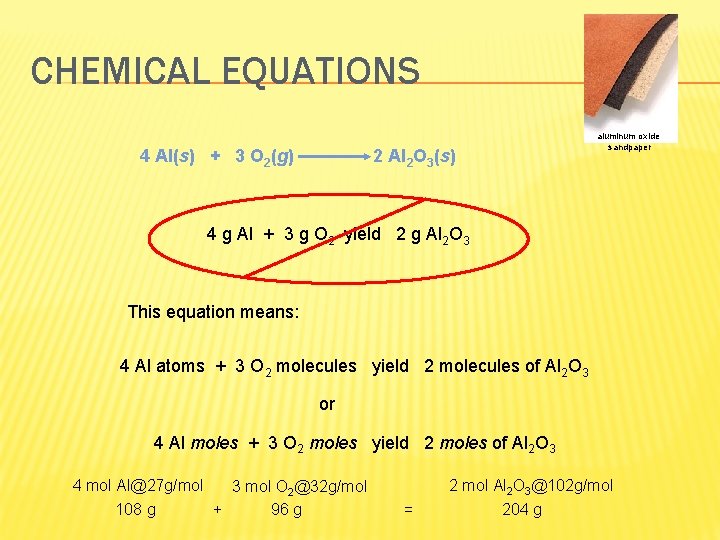
CHEMICAL EQUATIONS 4 Al(s) + 3 O 2(g) aluminum oxide sandpaper 2 Al 2 O 3(s) 4 g Al + 3 g O 2 yield 2 g Al 2 O 3 This equation means: 4 Al atoms + 3 O 2 molecules yield 2 molecules of Al 2 O 3 or 4 Al moles + 3 O 2 moles yield 2 moles of Al 2 O 3 4 mol Al@27 g/mol 3 mol O 2@32 g/mol 108 g + 96 g 2 mol Al 2 O 3@102 g/mol = 204 g

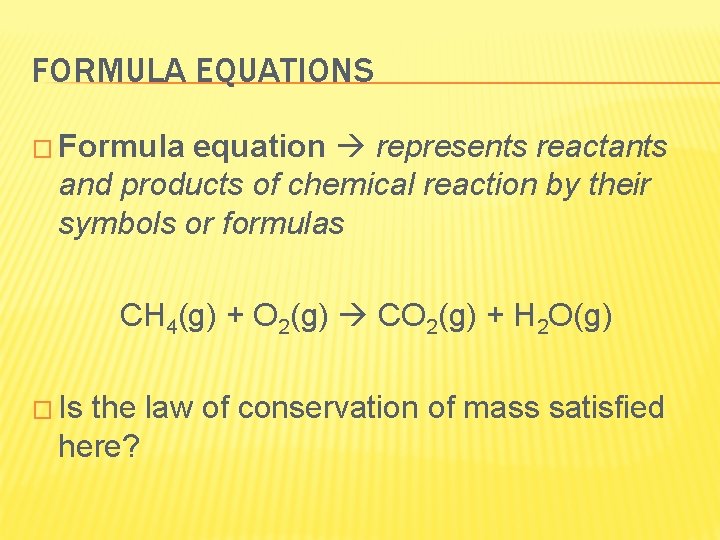
FORMULA EQUATIONS � Formula equation represents reactants and products of chemical reaction by their symbols or formulas CH 4(g) + O 2(g) CO 2(g) + H 2 O(g) � Is the law of conservation of mass satisfied here?

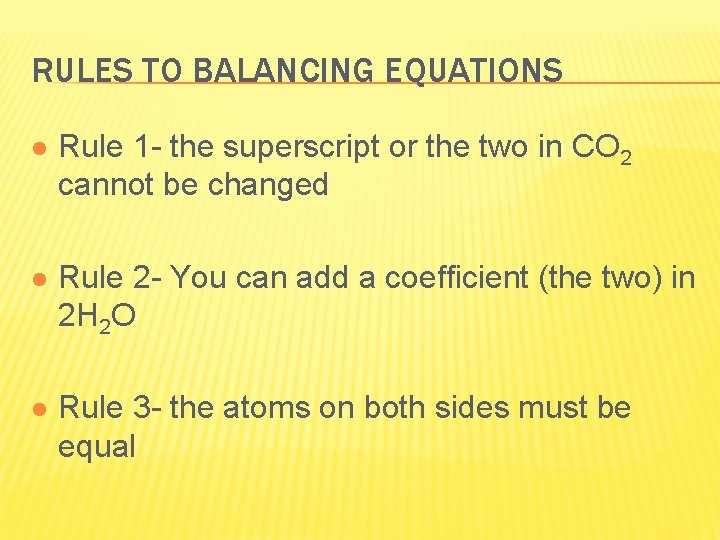
RULES TO BALANCING EQUATIONS l Rule 1 - the superscript or the two in CO 2 cannot be changed l Rule 2 - You can add a coefficient (the two) in 2 H 2 O l Rule 3 - the atoms on both sides must be equal

EXAMPLE

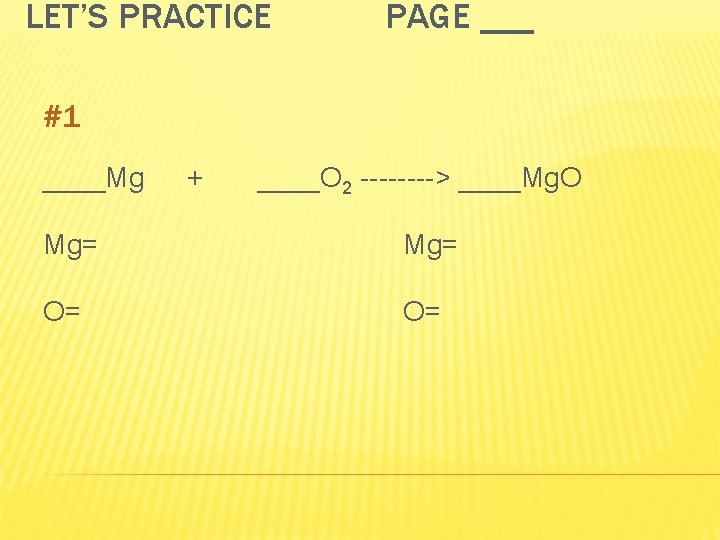
LET’S PRACTICE PAGE ___ #1 ____Mg + ____O 2 ----> ____Mg. O Mg= O= O=





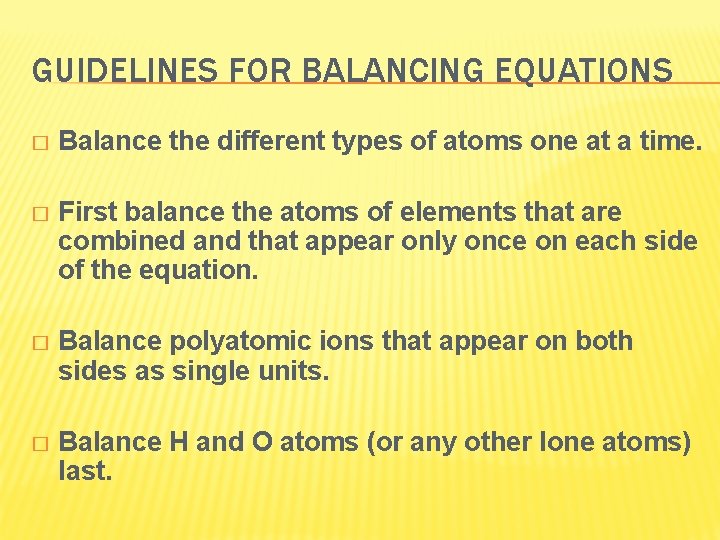
GUIDELINES FOR BALANCING EQUATIONS � Balance the different types of atoms one at a time. � First balance the atoms of elements that are combined and that appear only once on each side of the equation. � Balance polyatomic ions that appear on both sides as single units. � Balance H and O atoms (or any other lone atoms) last.

DO NOT!. . . � DO NOT WRITE INCORRECT FORMULAS, THIS WILL MESS UP YOUR BALANCING � DO NOT CHANGE SUBSCRIPTS IN FORMULAS TO BALANCE THE EQUATION!

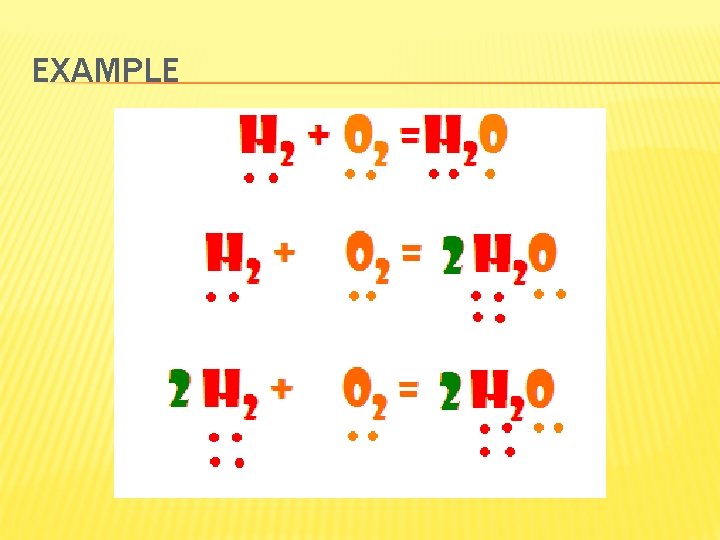
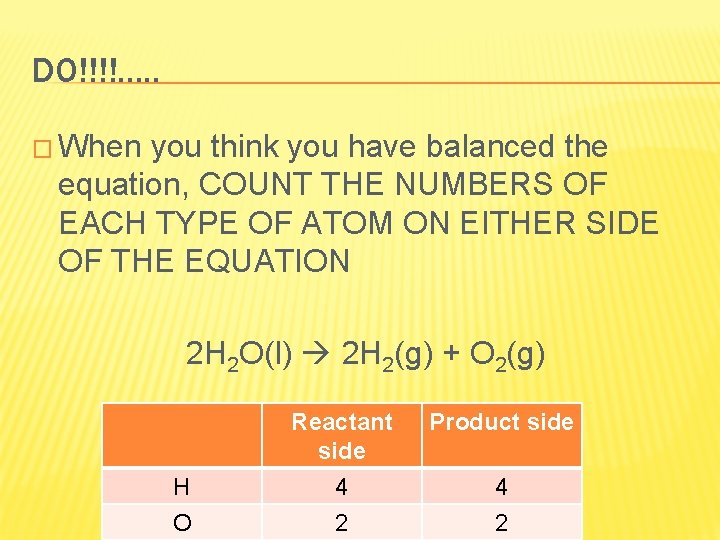
DO!!!!. . . � When you think you have balanced the equation, COUNT THE NUMBERS OF EACH TYPE OF ATOM ON EITHER SIDE OF THE EQUATION 2 H 2 O(l) 2 H 2(g) + O 2(g) H O Reactant side Product side 4 2

SAMPLE PROBLEM � The reaction of zinc with aqueous hydrochloric acid produces a solution of zinc chloride and hydrogen gas. Write a balanced equation for the reaction.

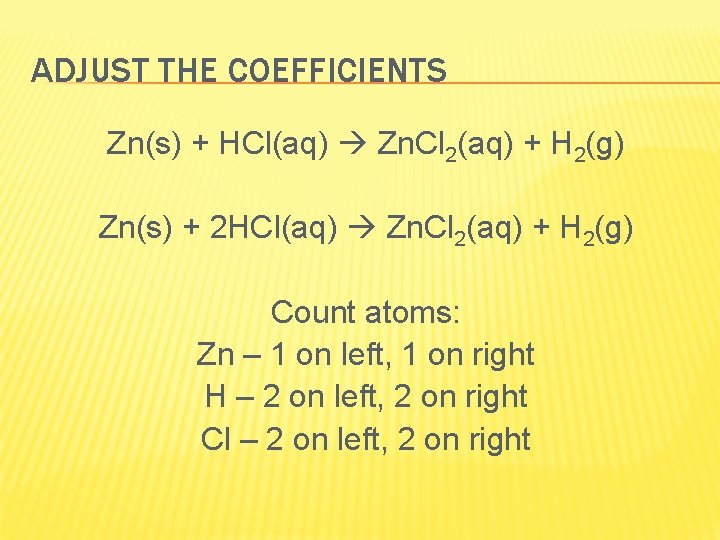
1. Write the word equation Zinc + hydrochloric acid zinc chloride + hydrogen 2. Write the formula equation Zn(s) + HCl(aq) Zn. Cl 2(aq) + H 2(g)

ADJUST THE COEFFICIENTS Zn(s) + HCl(aq) Zn. Cl 2(aq) + H 2(g) Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) Count atoms: Zn – 1 on left, 1 on right H – 2 on left, 2 on right Cl – 2 on left, 2 on right

PRACTICE PROBLEM 1 A � Write word, formula, and balanced chemical equations for magnesium and hydrochloric acid react to produce magnesium chloride and hydrogen. � Magnesium + hydrochloric acid magnesium chloride + hydrogen � Mg + HCl Mg. Cl 2 + H 2 � Mg + 2 HCl Mg. Cl 2 + H 2

PRACTICE PROBLEM 1 B � Write word, formula, and balanced chemical equations for silicon dioxide and hydrofluoric acid reacting to produce silicon tetrafluoride and water. � Silicon dioxide+ hydrofluoric acid silicon tetrafluoride + water � Si. O 2+ HF Si. F 4 + H 2 O � Si. O 2+ 4 HF Si. F 4 + 2 H 2 O

PRACTICE PROBLEM 2 � Write word, formula and balanced equations for aqueous nitric acid reacts with solid magnesium hydroxide to produce aqueous magnesium nitrate and water. � Nitric acid + magnesium hydroxide magnesium nitrate + water � HNO 3(aq) + Mg(OH)2(s) Mg(NO 3)2(aq) + H 2 O(l) � 2 HNO 3(aq) + Mg(OH)2(s) Mg(NO 3)2(aq) + 2 H 2 O(l)

PRACTICE PROBLEM 3 � Ammonium sulfate crystals are made by treating ammonia gas, often a byproduct from coke-ovens, with aqueous sulfuric acid: � 2 NH 3(g) + H 2 SO 4(aq) → (NH 4)2 SO 4(s)


PRACTICE PROBLEM 4 � Aluminum sulfate and calcium hydroxide are used in a water-purification process. When added to water, they dissolve and react to produce 2 insoluble products, aluminum hydroxide and calcium sulfate. Write a balanced equation for this reaction. Al 2(SO 4)3(aq) + 3 Ca(OH)2(aq) 2 Al(OH)3(s) + 3 Ca. SO 4(s)

PRACTICE PROBLEM 5 � Write balanced chemical equations for the following reaction: Solid sodium combines with chlorine gas to produce solid sodium chloride. � 2 Na(s) + Cl 2(g) → 2 Na. Cl(s)

PRACTICE PROBLEM 6 � When solid copper reacts with aqueous silver nitrate, the products are aqueous copper(II) nitrate and solid silver. Cu(s) + 2 Ag. NO 3(aq) → Cu(NO 3)2(aq) + 2 Ag(s)

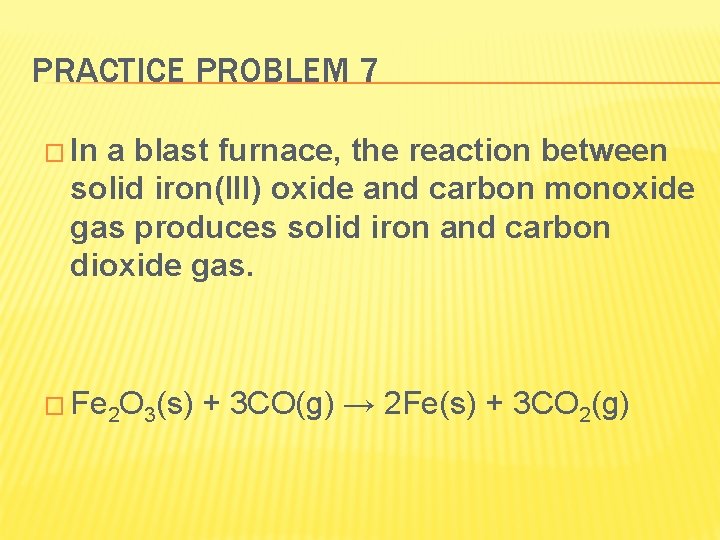
PRACTICE PROBLEM 7 � In a blast furnace, the reaction between solid iron(III) oxide and carbon monoxide gas produces solid iron and carbon dioxide gas. � Fe 2 O 3(s) + 3 CO(g) → 2 Fe(s) + 3 CO 2(g)

Types of Chemical Reactions SECTION 2

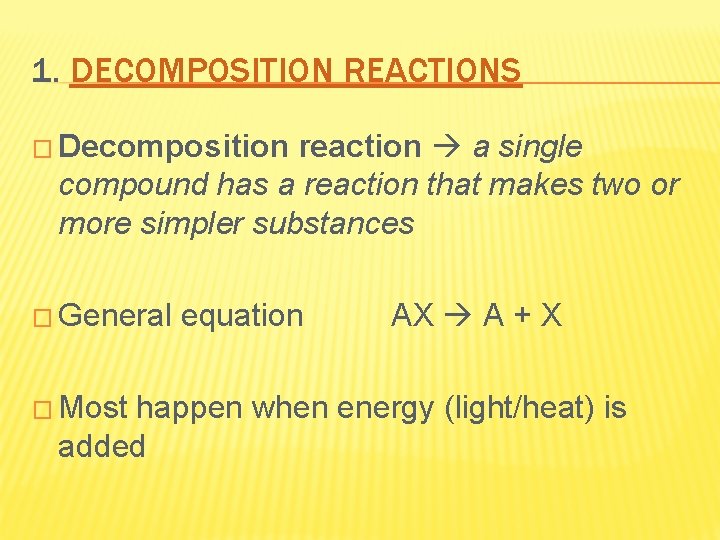
1. DECOMPOSITION REACTIONS � Decomposition reaction a single compound has a reaction that makes two or more simpler substances � General � Most equation AX A + X happen when energy (light/heat) is added

2. SYNTHESIS REACTIONS � Synthesis (composition) reaction two or more substances combine to form a new compound � General equation A + X AX

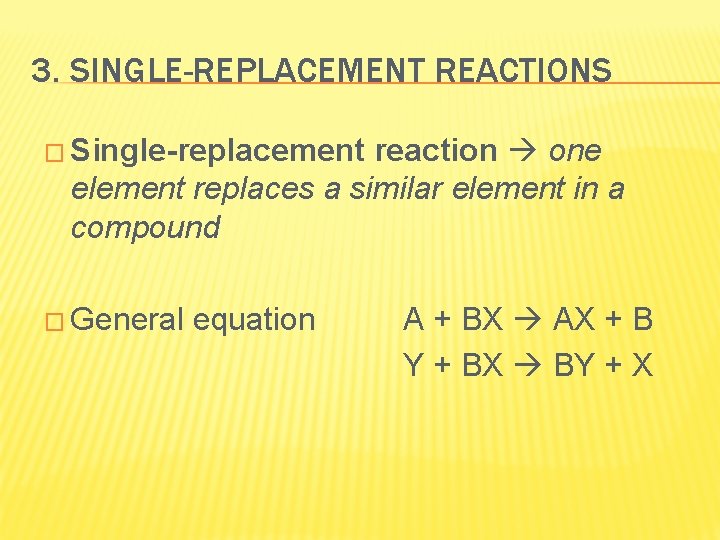
3. SINGLE-REPLACEMENT REACTIONS � Single-replacement reaction one element replaces a similar element in a compound � General equation A + BX AX + B Y + BX BY + X

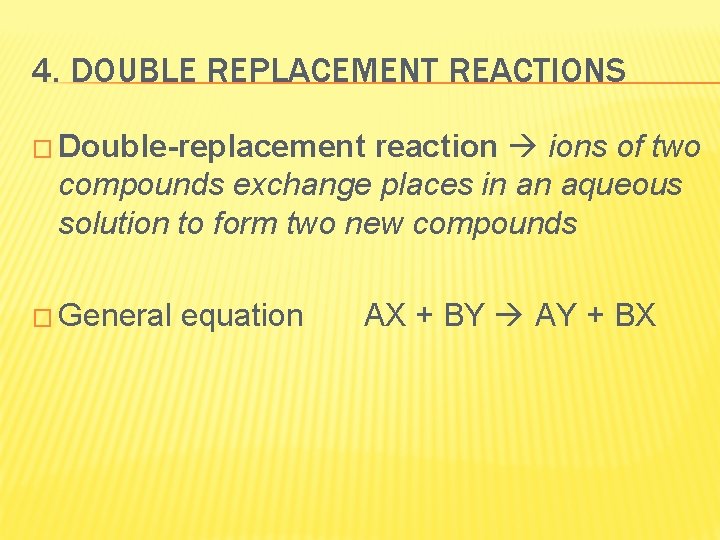
4. DOUBLE REPLACEMENT REACTIONS � Double-replacement reaction ions of two compounds exchange places in an aqueous solution to form two new compounds � General equation AX + BY AY + BX

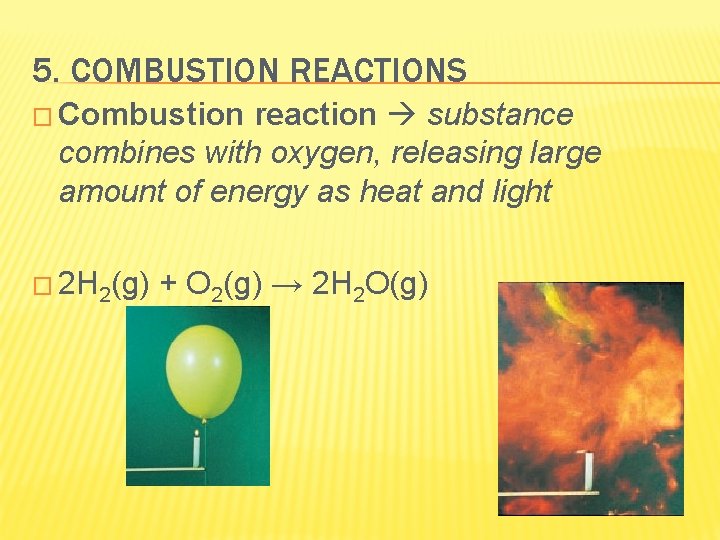
5. COMBUSTION REACTIONS � Combustion reaction substance combines with oxygen, releasing large amount of energy as heat and light � 2 H 2(g) + O 2(g) → 2 H 2 O(g)

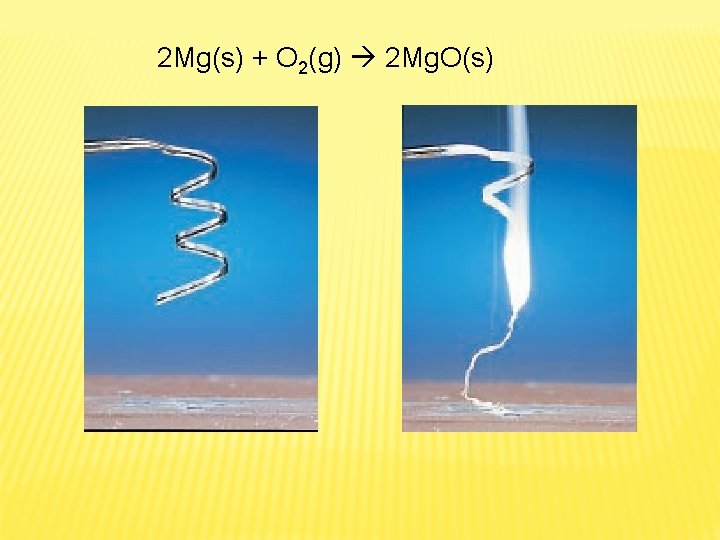
REACTIONS OF ELEMENTS WITH OXYGEN AND SULFUR � One simple type of synthesis reaction is combination of element with oxygen to form an oxide of the element � Almost all metals react with oxygen to form oxides � Ex. Magnesium burned magnesium oxide

2 Mg(s) + O 2(g) 2 Mg. O(s)

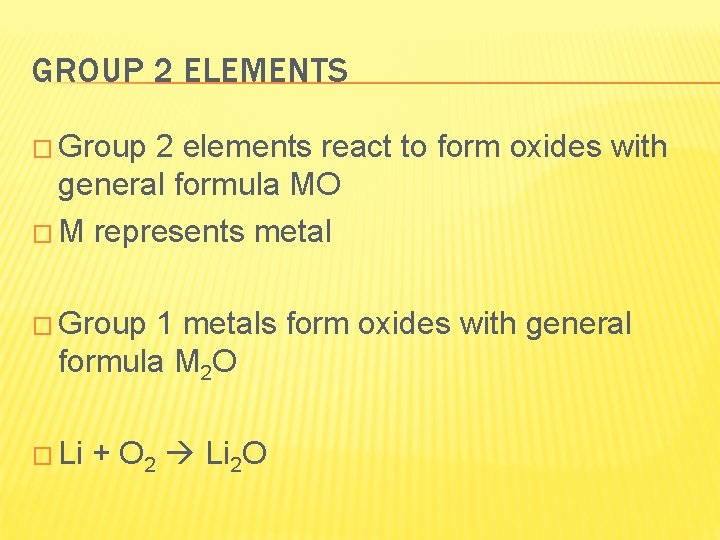
GROUP 2 ELEMENTS � Group 2 elements react to form oxides with general formula MO � M represents metal � Group 1 metals form oxides with general formula M 2 O � Li + O 2 Li 2 O

REACTIONS WITH SULFUR � Groups 1 and 2 react with sulfur to make sulfides of the element � Group 1 M 2 S � Group 2 MS � 16 Rb(s) + S 8(s) → 8 Rb 2 S(s) � 8 Ba(s) + S 8(s) → 8 Ba. S(s)
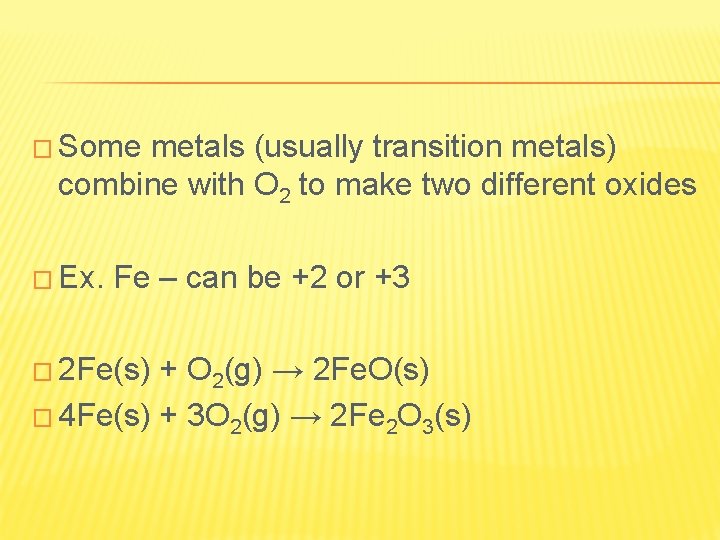
� Some metals (usually transition metals) combine with O 2 to make two different oxides � Ex. Fe – can be +2 or +3 � 2 Fe(s) + O 2(g) → 2 Fe. O(s) � 4 Fe(s) + 3 O 2(g) → 2 Fe 2 O 3(s)

NONMETALS � Nonmetals also react with oxygen to make oxides � Sulfur reacts with oxygen to make sulfur dioxide � When carbon is burned, it makes carbon dioxide � S 8(s) + 8 O 2(g) → 8 SO 2(g) � C(s) + O 2(g) → CO 2(g)

� Hydrogen reacts with oxygen to make dihydrogen monoxide � 2 H 2(g) + O 2(g) → 2 H 2 O(g)

� Most metals react with halogens to make either ionic or covalent compounds � Ex. Group 1 reacts with halogens to form ionic compounds with formula MX � M = metal, X = halogen � 2 Na(s) + Cl 2(g) → 2 Na. Cl(s) � 2 K(s) + I 2(g) → 2 KI(s) REACTIONS OF METALS & HALOGENS
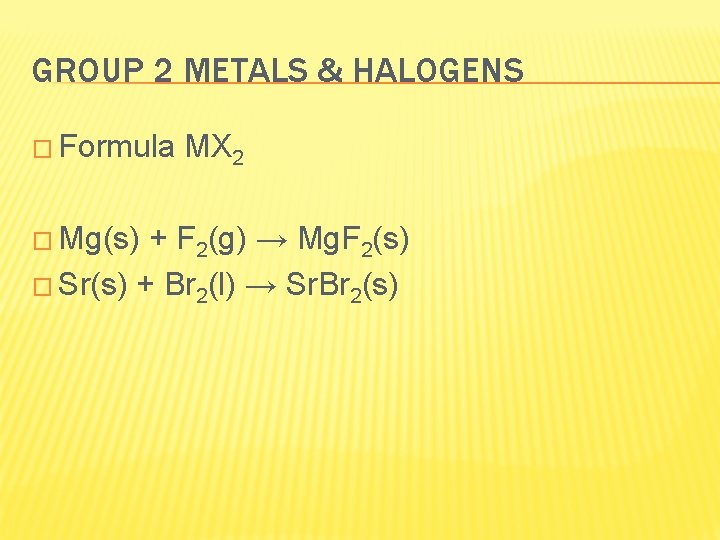
GROUP 2 METALS & HALOGENS � Formula � Mg(s) MX 2 + F 2(g) → Mg. F 2(s) � Sr(s) + Br 2(l) → Sr. Br 2(s)

� Active metals highly reactive metals � Oxides of active metals react with water to make metal hydroxides � Ca. O(s) � Ca. O + H 2 O(l) → Ca(OH)2(s) = lime � Ca(OH)2 is important in setting cement SYNTHESIS REACTIONS WITH OXIDES

OXYACIDS � Many oxides of nonmetals react with water to make oxyacids � SO 2(g) + H 2 O(l) → H 2 SO 3(aq) � In air polluted with SO 2, reacts with oxygen in air to form sulfuric acid (acid rain) � 2 H 2 SO 3(aq) + O 2(g) → 2 H 2 SO 4(aq)

PRACTICE PROBLEMS � Identify the products in each of the following reactions: � a. hydrogen burned in oxygen � H 2 O � b. H 2(g) + N 2(g) � NH 3 � c. Ca. O(s) + H 2 O(l) � Ca(OH)2(aq)

DECOMPOSITION OF BINARY COMPOUNDS � Simplest kind of decomposition reaction is binary compound into its elements � Ex. Passing electricity through water � 2 H 2 O(l) electricity 2 H 2(g) + O 2(g)

� Electrolysis decomposition of a substance by electricity

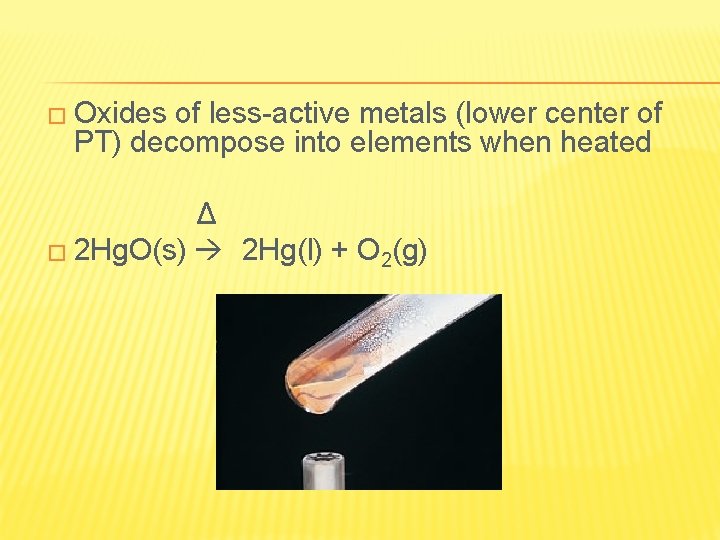
� Oxides of less-active metals (lower center of PT) decompose into elements when heated Δ � 2 Hg. O(s) 2 Hg(l) + O 2(g)

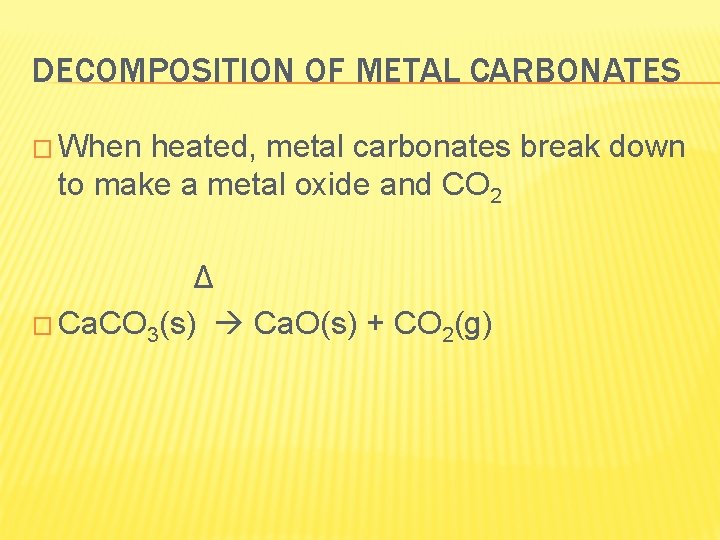
DECOMPOSITION OF METAL CARBONATES � When heated, metal carbonates break down to make a metal oxide and CO 2 Δ � Ca. CO 3(s) Ca. O(s) + CO 2(g)

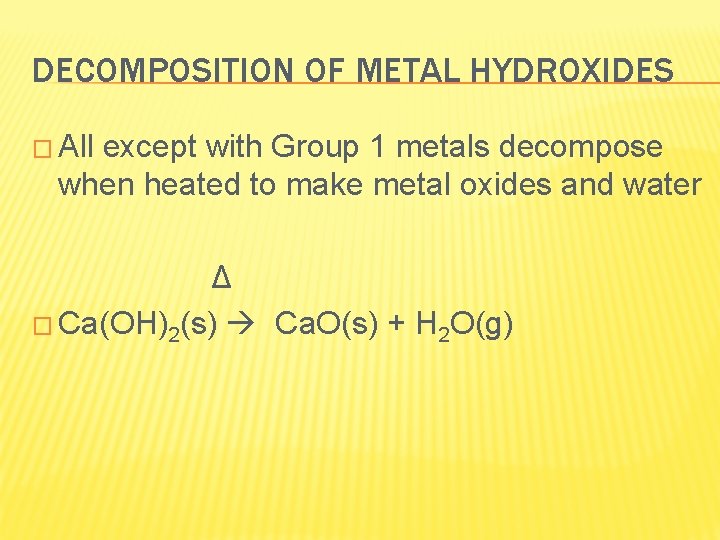
DECOMPOSITION OF METAL HYDROXIDES � All except with Group 1 metals decompose when heated to make metal oxides and water Δ � Ca(OH)2(s) Ca. O(s) + H 2 O(g)

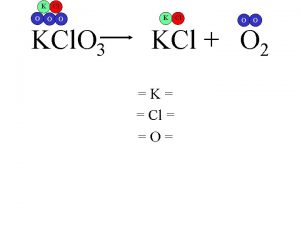
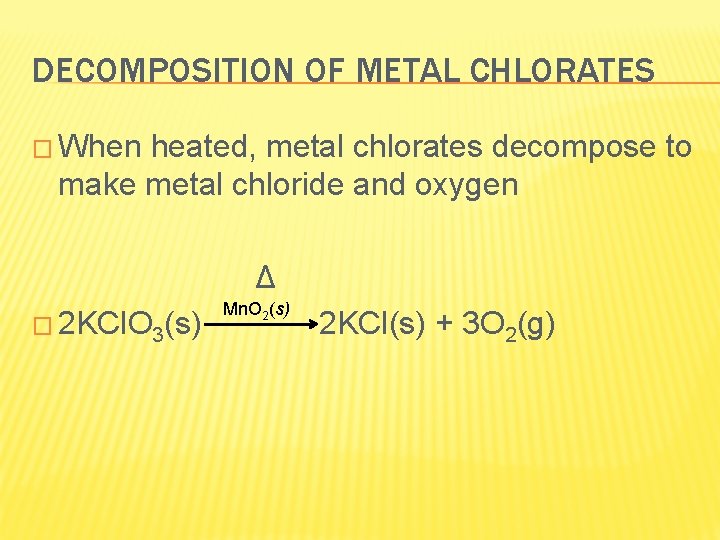
DECOMPOSITION OF METAL CHLORATES � When heated, metal chlorates decompose to make metal chloride and oxygen Δ � 2 KCl. O 3(s) Mn. O 2(s) 2 KCl(s) + 3 O 2(g)

DECOMPOSITION OF ACIDS � Certain acids decompose into nonmetal oxides and water � H 2 CO 3(aq) → CO 2(g) + H 2 O(l) Δ � H 2 SO 4(aq) SO 3(g) + H 2 O(l)

PRACTICE PROBLEMS � Predict the products for these decomposition reactions � a. sodium chlorate Sodium chloride + oxygen � b. calcium carbonate Calcium oxide + carbon dioxide � c. potassium bromide Potassium + bromine

REPLACEMENT OF A METAL IN A COMPOUND BY ANOTHER METAL � Aluminum is more active than lead � When solid aluminum is placed in aqueous lead(II) nitrate, the aluminum replaces the lead 2 Al(s) + 3 Pb(NO 3)2(aq) → 3 Pb(s) + 2 Al(NO 3)3(aq) � Based on activity series of metals


REPLACEMENT OF HYDROGEN IN WATER BY METAL � Most-active metals (Group 1) react strongly with water to make metal hydroxides and hydrogen � 2 Na(s) + 2 H 2 O(l) → 2 Na. OH(aq) + H 2(g) � Less-active metals react with steam or other form of energy

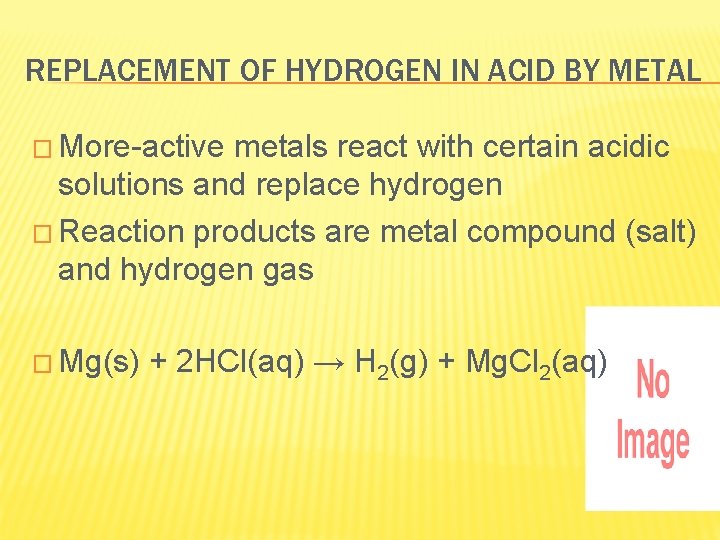
REPLACEMENT OF HYDROGEN IN ACID BY METAL � More-active metals react with certain acidic solutions and replace hydrogen � Reaction products are metal compound (salt) and hydrogen gas � Mg(s) + 2 HCl(aq) → H 2(g) + Mg. Cl 2(aq)

REPLACEMENT OF HALOGENS � One halogen replaces another � Fluorine is most reactive � Can replace any other halogen � Cl 2(g) + 2 KBr(aq) → 2 KCl(aq) + Br 2(l) � F 2(g) + 2 Na. Cl(aq) → 2 Na. F(aq) + Cl 2(g) � Br 2(l) + KCl(aq) → no reaction

PRACTICE PROBLEMS � For the following equations, predict what the products will be: � a. Ag + KNO 3 → No reaction � b. Zn + Ag. NO 3 → Zn(NO 3)2 + Ag � c. Cl 2 + KI → I 2 + 2 KCl

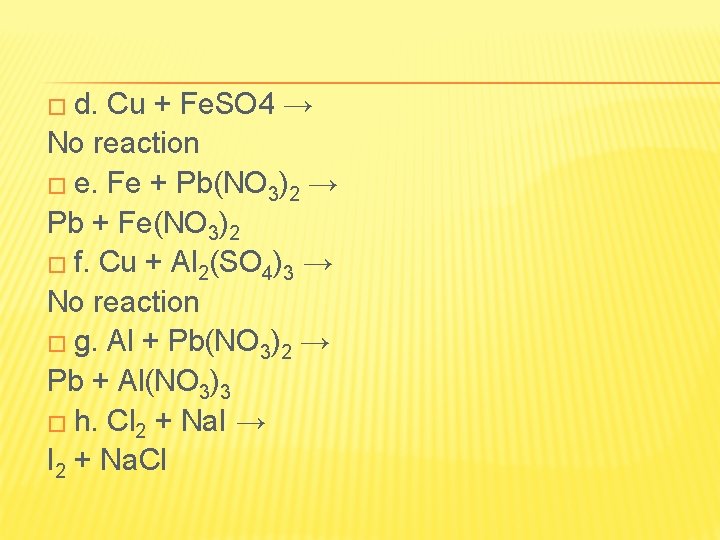
� d. Cu + Fe. SO 4 → No reaction � e. Fe + Pb(NO 3)2 → Pb + Fe(NO 3)2 � f. Cu + Al 2(SO 4)3 → No reaction � g. Al + Pb(NO 3)2 → Pb + Al(NO 3)3 � h. Cl 2 + Na. I → I 2 + Na. Cl

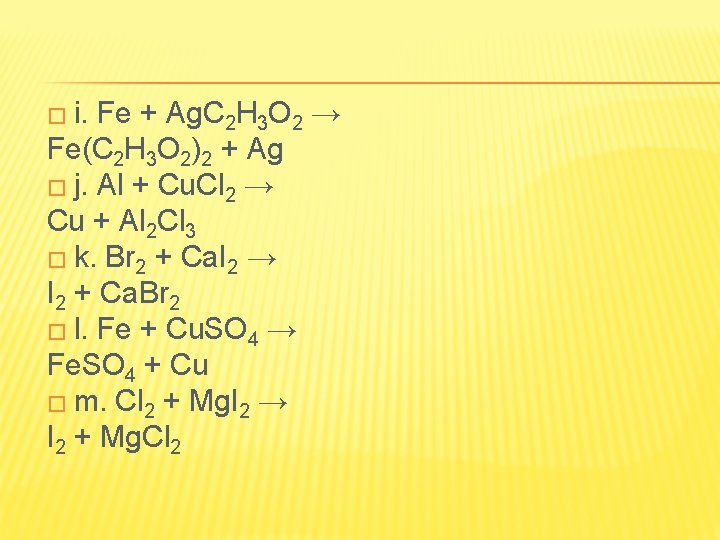
� i. Fe + Ag. C 2 H 3 O 2 → Fe(C 2 H 3 O 2)2 + Ag � j. Al + Cu. Cl 2 → Cu + Al 2 Cl 3 � k. Br 2 + Ca. I 2 → I 2 + Ca. Br 2 � l. Fe + Cu. SO 4 → Fe. SO 4 + Cu � m. Cl 2 + Mg. I 2 → I 2 + Mg. Cl 2

FORMATION OF A PRECIPITATE � Occurs when cations of one reactant combine with anions of another to form insoluble (or slightly soluble) compound � 2 KI(aq) + Pb(NO 3)2(aq) → Pb. I 2(s) + 2 KNO 3(aq)

FORMATION OF A GAS � In some D-R reactions, one product is insoluble gas that bubbles out of mixture � Fe. S(s) + 2 HCl(aq) → H 2 S(g) + Fe. Cl 2(aq)

FORMATION OF WATER � In some D-R reactions, water is one of the products � HCl(aq) + Na. OH(aq) → Na. Cl(aq) + H 2 O(l)

PRACTICE PROBLEMS � Classify each of the following reactions as synthesis, decomposition, singlereplacement, double-replacement, or combustion: � a. N 2(g) + 3 H 2(g) → 2 NH 3(g) � synthesis � b. 2 Li(s) + 2 H 2 O(l ) → 2 Li. OH(aq) + H 2(g) � single-replacement � c. 2 Na. NO 3(s) → 2 Na. NO 2(s) + O 2(g) � decomposition

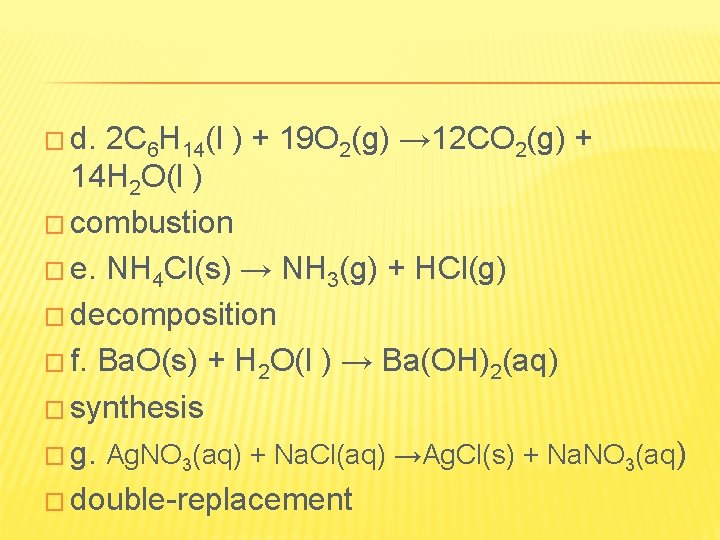
� d. 2 C 6 H 14(l ) + 19 O 2(g) → 12 CO 2(g) + 14 H 2 O(l ) � combustion � e. NH 4 Cl(s) → NH 3(g) + HCl(g) � decomposition � f. Ba. O(s) + H 2 O(l ) → Ba(OH)2(aq) � synthesis � g. Ag. NO 3(aq) + Na. Cl(aq) →Ag. Cl(s) + Na. NO 3(aq) � double-replacement

PRACTICE PROBLEM � For each of the following reactions, identify the missing substances, then balance the final equation. Each slot may be one OR MORE substances. � a. synthesis: _____ → Li 2 O � 4 Li + O 2 2 Li 2 O � b. decomposition: Mg(Cl. O 3)2 → _____ � Mg(Cl. O 3)2 Mg. Cl 2 + 3 O 2

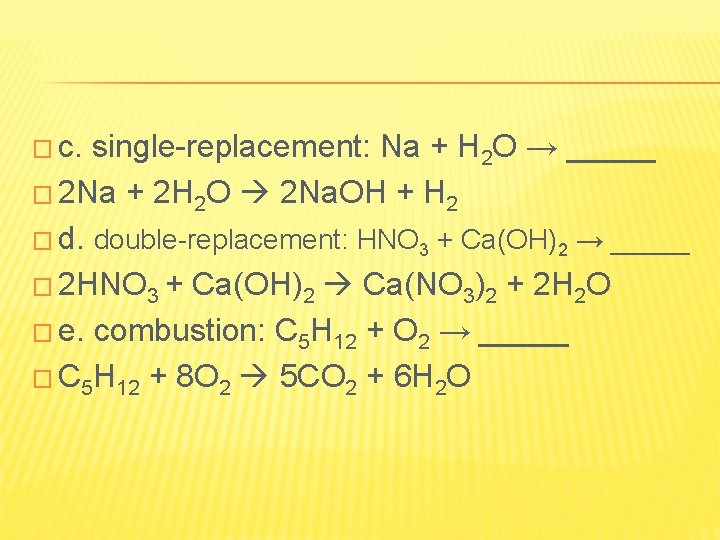
� c. single-replacement: Na + H 2 O → _____ � 2 Na + 2 H 2 O 2 Na. OH + H 2 � d. double-replacement: HNO 3 + Ca(OH)2 → _____ � 2 HNO 3 + Ca(OH)2 Ca(NO 3)2 + 2 H 2 O � e. combustion: C 5 H 12 + O 2 → _____ � C 5 H 12 + 8 O 2 5 CO 2 + 6 H 2 O
 Chemistry of matches
Chemistry of matches Kirchhoff's
Kirchhoff's Kcl law
Kcl law Mesh current method with current source
Mesh current method with current source Kvl loop
Kvl loop Kcl equation examples
Kcl equation examples Born haber cycle of kcl
Born haber cycle of kcl Tentukan tegangan jatuh di resistor ab
Tentukan tegangan jatuh di resistor ab Katoda i anoda elektroliza
Katoda i anoda elektroliza Which statement describe the solute
Which statement describe the solute Use ohm's law, kvl, and kcl to find vx.
Use ohm's law, kvl, and kcl to find vx. Kcl equation
Kcl equation Potassium dose calculation
Potassium dose calculation Kcl correction
Kcl correction Toonisuus
Toonisuus Kcl formula
Kcl formula Born haber cycle of kcl
Born haber cycle of kcl Thevenin and norton
Thevenin and norton Personal tutor dashboard kcl
Personal tutor dashboard kcl Kcl precipitate
Kcl precipitate Principles of portfolio assessment
Principles of portfolio assessment Which geometric construction matches the diagram below?
Which geometric construction matches the diagram below? Graph y=x^2-1
Graph y=x^2-1 Behavior that matches group expectations
Behavior that matches group expectations Blue marble matches answer key
Blue marble matches answer key Lesson 4: esta ropa spanish i b unit 5: en la tienda
Lesson 4: esta ropa spanish i b unit 5: en la tienda Three grey geese tongue twister
Three grey geese tongue twister Collective noun passage
Collective noun passage Ancient chinese matches
Ancient chinese matches Practiscore matches
Practiscore matches Identify the energy conversions in the illustrations below
Identify the energy conversions in the illustrations below Portfolio assessment matches assessment to teaching
Portfolio assessment matches assessment to teaching Blue marble matches answer key
Blue marble matches answer key Broccoli countable
Broccoli countable Behavior that matches group expectations
Behavior that matches group expectations Identify a contextual selector that matches any element
Identify a contextual selector that matches any element Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Whats a sigfig
Whats a sigfig A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis Significant figures in chemistry rules
Significant figures in chemistry rules Organic chemistry reaction pathways
Organic chemistry reaction pathways How to find one mole of a compound
How to find one mole of a compound Chemistry sol review
Chemistry sol review Nysedregents
Nysedregents Leo and ger
Leo and ger What is electrochemical
What is electrochemical Nbs reaction
Nbs reaction Molecularity of reaction
Molecularity of reaction Chandrakanta bandyopadhyay
Chandrakanta bandyopadhyay Bu organic chemistry
Bu organic chemistry Why is it important to never leave a heat source unattended
Why is it important to never leave a heat source unattended Ice table chemistry
Ice table chemistry Pressed fiber pad chemistry
Pressed fiber pad chemistry Table g chemistry
Table g chemistry Balancing equations chapter 8
Balancing equations chapter 8 Pfizer green chemistry
Pfizer green chemistry Chemistry food and drugs
Chemistry food and drugs Metric system chemistry
Metric system chemistry Chemistry element riddles
Chemistry element riddles Hexanoic acid structure formula
Hexanoic acid structure formula Spectroscopic data in chemistry
Spectroscopic data in chemistry Organic chemistry
Organic chemistry Types of reactions chemistry
Types of reactions chemistry Inert pair effect
Inert pair effect Chemistry
Chemistry Chapter 13 review ions in aqueous solutions
Chapter 13 review ions in aqueous solutions Jennie borders chemistry
Jennie borders chemistry Rotational motion chemistry
Rotational motion chemistry Chemistry unit 4 grade 11
Chemistry unit 4 grade 11 Ben franklin aphorisms
Ben franklin aphorisms Pf3 electron pair geometry
Pf3 electron pair geometry