Lecture 14 Molecular structure o Rotational transitions o

- Slides: 17

Lecture 14: Molecular structure o Rotational transitions o Vibrational transitions o Electronic transitions PY 3 P 05

Bohn-Oppenheimer Approximation o Born-Oppenheimer Approximation is the assumption that the electronic motion and the nuclear motion in molecules can be separated. o This leads to molecular wavefunctions that are given in terms of the electron positions (ri) and the nuclear positions (Rj): o Involves the following assumptions: o Electronic wavefunction depends on nuclear positions but not upon their velocities, i. e. , the nuclear motion is so much slower than electron motion that they can be considered to be fixed. o The nuclear motion (e. g. , rotation, vibration) sees a smeared out potential from the fastmoving electrons. PY 3 P 05

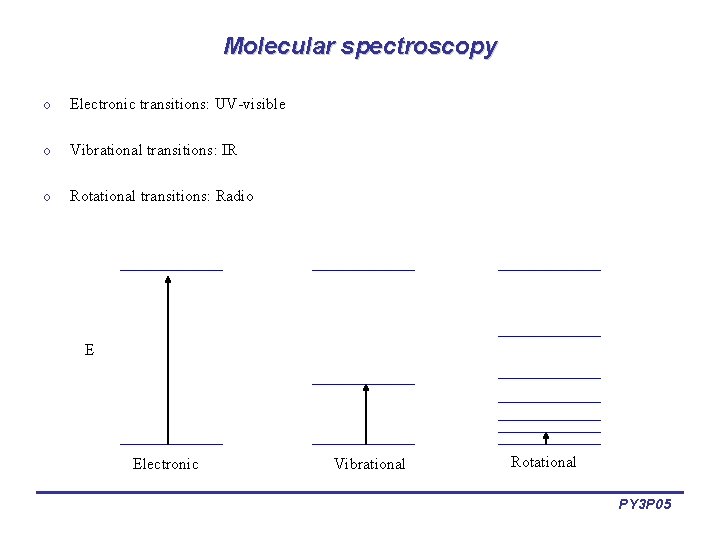
Molecular spectroscopy o Electronic transitions: UV-visible o Vibrational transitions: IR o Rotational transitions: Radio E Electronic Vibrational Rotational PY 3 P 05

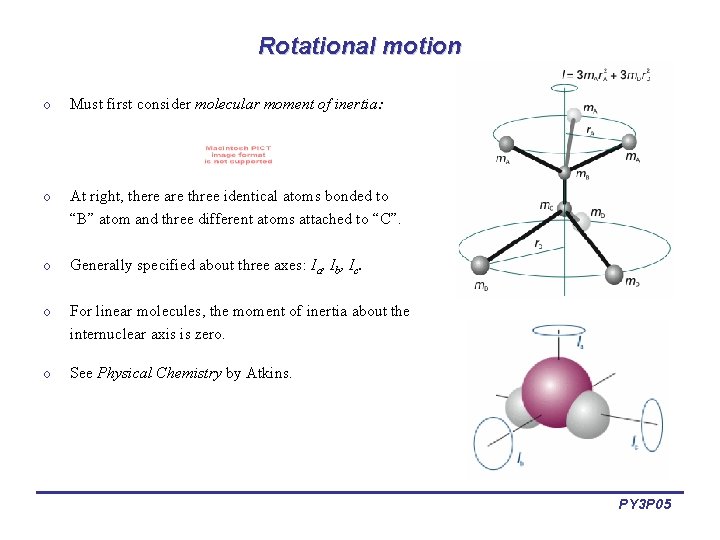
Rotational motion o Must first consider molecular moment of inertia: o At right, there are three identical atoms bonded to “B” atom and three different atoms attached to “C”. o Generally specified about three axes: Ia, Ib, Ic. o For linear molecules, the moment of inertia about the internuclear axis is zero. o See Physical Chemistry by Atkins. PY 3 P 05

Rotational motion o Rotation of molecules are considered to be rigid rotors. o Rigid rotors can be classified into four types: o Spherical rotors: have equal moments of intertia (e. g. , CH 4, SF 6). o Symmetric rotors: have two equal moments of inertial (e. g. , NH 3). o Linear rotors: have one moment of inertia equal to zero (e. g. , CO 2, HCl). o Asymmetric rotors: have three different moments of inertia (e. g. , H 2 O). PY 3 P 05

Quantized rotational energy levels o The classical expression for the energy of a rotating body is: where a is the angular velocity in radians/sec. o For rotation about three axes: o In terms of angular momentum (J = I ): o We know from QM that AM is quantized: , J = 0, 1, 2, … o Therefore, , J = 0, 1, 2, … PY 3 P 05

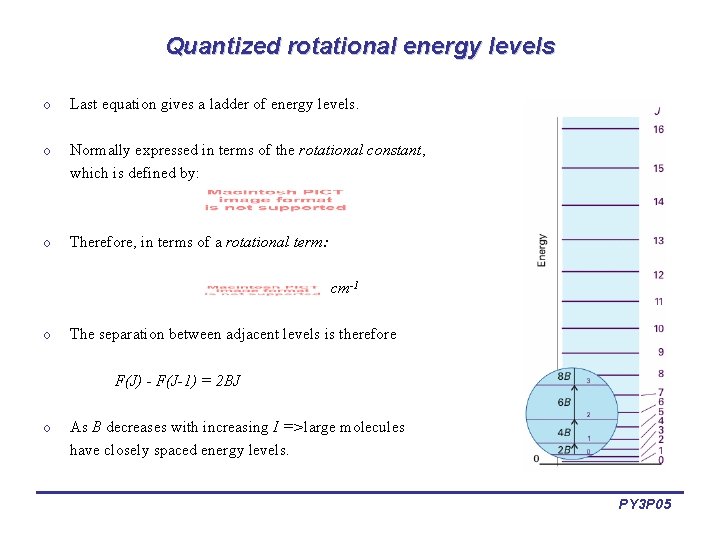
Quantized rotational energy levels o Last equation gives a ladder of energy levels. o Normally expressed in terms of the rotational constant, which is defined by: o Therefore, in terms of a rotational term: cm-1 o The separation between adjacent levels is therefore F(J) - F(J-1) = 2 BJ o As B decreases with increasing I =>large molecules have closely spaced energy levels. PY 3 P 05

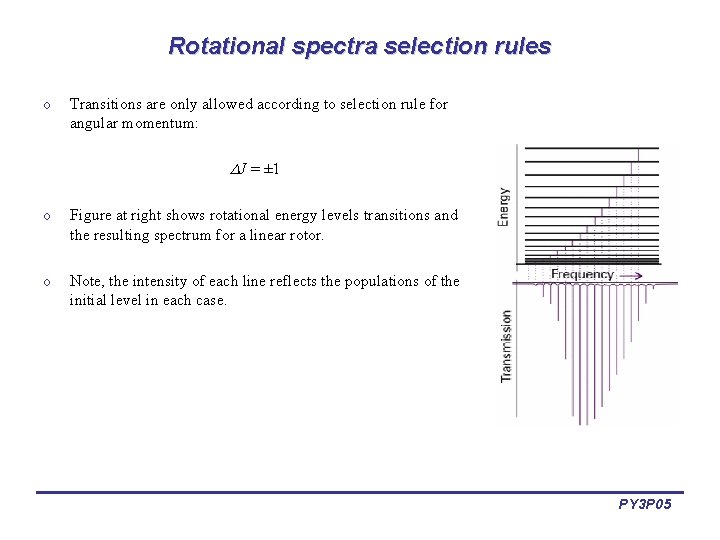
Rotational spectra selection rules o Transitions are only allowed according to selection rule for angular momentum: J = ± 1 o Figure at right shows rotational energy levels transitions and the resulting spectrum for a linear rotor. o Note, the intensity of each line reflects the populations of the initial level in each case. PY 3 P 05

Molecular vibrations o Consider simple case of a vibrating diatomic molecule, where restoring force is proportional to displacement (F = -kx). Potential energy is therefore V = 1/2 kx 2 o Can write the corresponding Schrodinger equation as where o The SE results in allowed energies v = 0, 1, 2, … PY 3 P 05

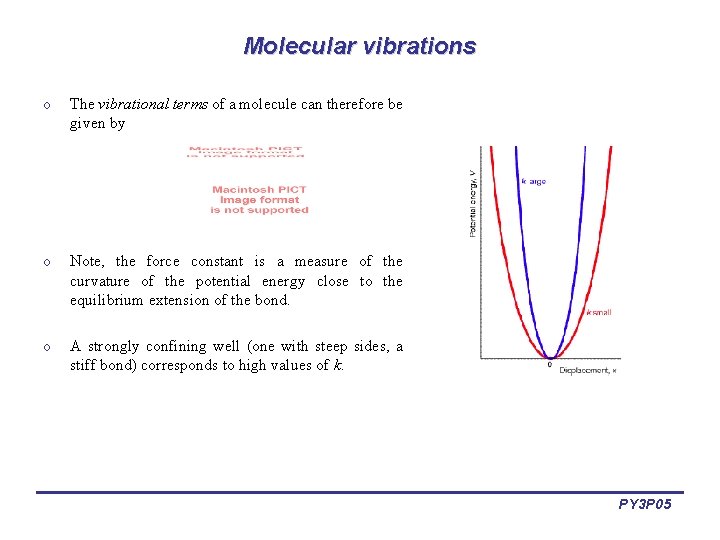
Molecular vibrations o The vibrational terms of a molecule can therefore be given by o Note, the force constant is a measure of the curvature of the potential energy close to the equilibrium extension of the bond. o A strongly confining well (one with steep sides, a stiff bond) corresponds to high values of k. PY 3 P 05

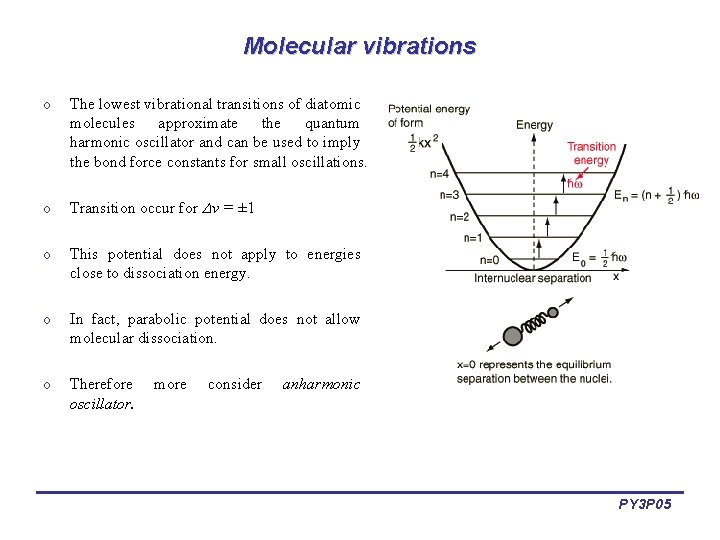
Molecular vibrations o The lowest vibrational transitions of diatomic molecules approximate the quantum harmonic oscillator and can be used to imply the bond force constants for small oscillations. o Transition occur for v = ± 1 o This potential does not apply to energies close to dissociation energy. o In fact, parabolic potential does not allow molecular dissociation. o Therefore oscillator. more consider anharmonic PY 3 P 05

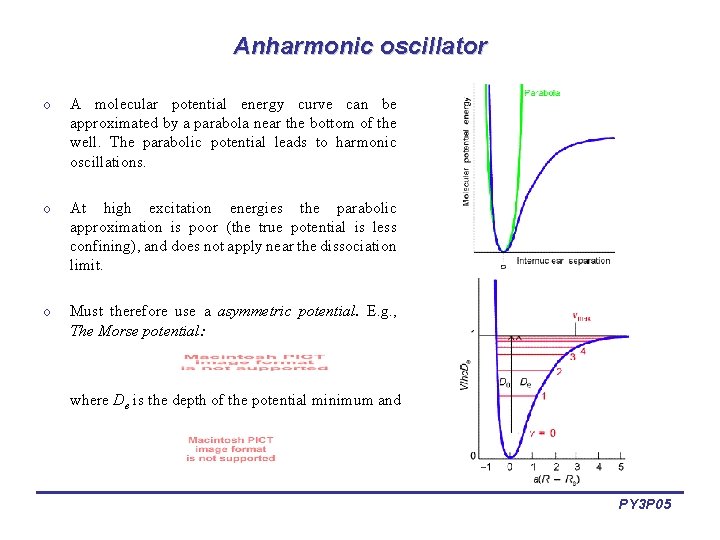
Anharmonic oscillator o A molecular potential energy curve can be approximated by a parabola near the bottom of the well. The parabolic potential leads to harmonic oscillations. o At high excitation energies the parabolic approximation is poor (the true potential is less confining), and does not apply near the dissociation limit. o Must therefore use a asymmetric potential. E. g. , The Morse potential: where De is the depth of the potential minimum and PY 3 P 05

Anharmonic oscillator o The Schrödinger equation can be solved for the Morse potential, giving permitted energy levels: where xe is the anharmonicity constant: o The second term in the expression for G increases with v => levels converge at high quantum numbers. o The number of vibrational levels for a Morse oscillator is finite: v = 0, 1, 2, …, vmax PY 3 P 05

Vibrational-rotational spectroscopy o Molecules vibrate and rotate at the same time => S(v, J) = G(v) + F(J) o Selection rules obtained by combining rotational selection rule ΔJ = ± 1 with vibrational rule Δv = ± 1. o When vibrational transitions of the form v + 1 v occurs, ΔJ = ± 1. o Transitions with ΔJ = -1 are called the P branch: o Transitions with ΔJ = +1 are called the R branch: o Q branch are all transitions with ΔJ = 0 PY 3 P 05

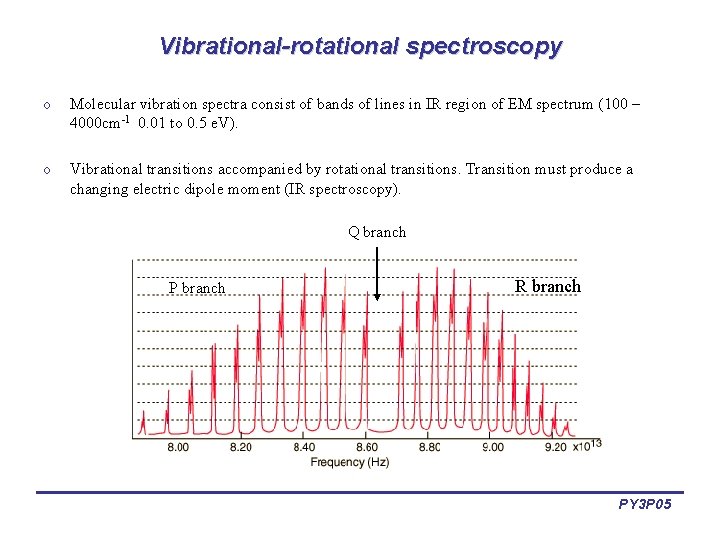
Vibrational-rotational spectroscopy o Molecular vibration spectra consist of bands of lines in IR region of EM spectrum (100 – 4000 cm-1 0. 01 to 0. 5 e. V). o Vibrational transitions accompanied by rotational transitions. Transition must produce a changing electric dipole moment (IR spectroscopy). Q branch P branch R branch PY 3 P 05

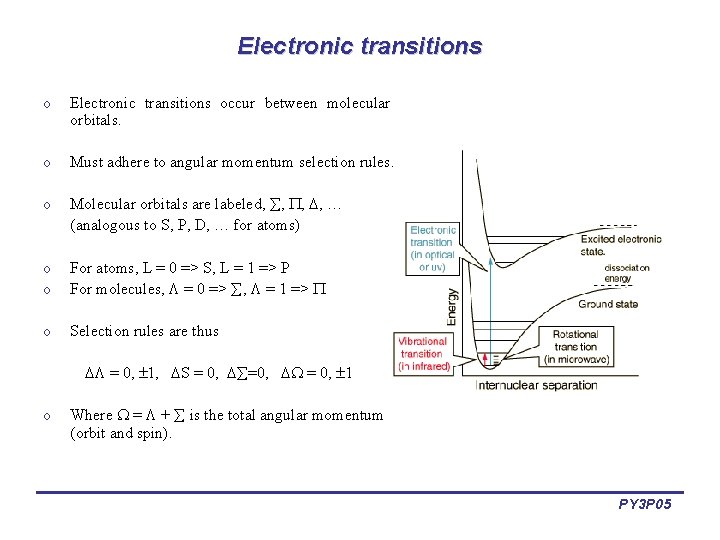
Electronic transitions occur between molecular orbitals. o Must adhere to angular momentum selection rules. o Molecular orbitals are labeled, , … (analogous to S, P, D, … for atoms) o o For atoms, L = 0 => S, L = 1 => P For molecules, = 0 => , = 1 => o Selection rules are thus = 0, 1, S = 0, =0, = 0, 1 o Where = + is the total angular momentum (orbit and spin). PY 3 P 05

The End! o All notes and tutorial set available from http: //www. physics. tcd. ie/people/peter. gallagher/lectures/py 3004/ o Questions? Contact: o peter. gallagher@tcd. ie o Room 3. 17 A in SNIAM PY 3 P 05