CHEMISTRY ATOMIC STRUCTURE TERMINOLOGY Chemistry study of composition











- Slides: 27

CHEMISTRY ATOMIC STRUCTURE

TERMINOLOGY Chemistry study of composition of matter and processes that build up and break down substances. Biochemistry study of chemical processes that help to sustain living things

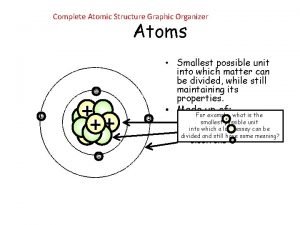
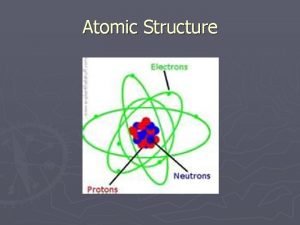
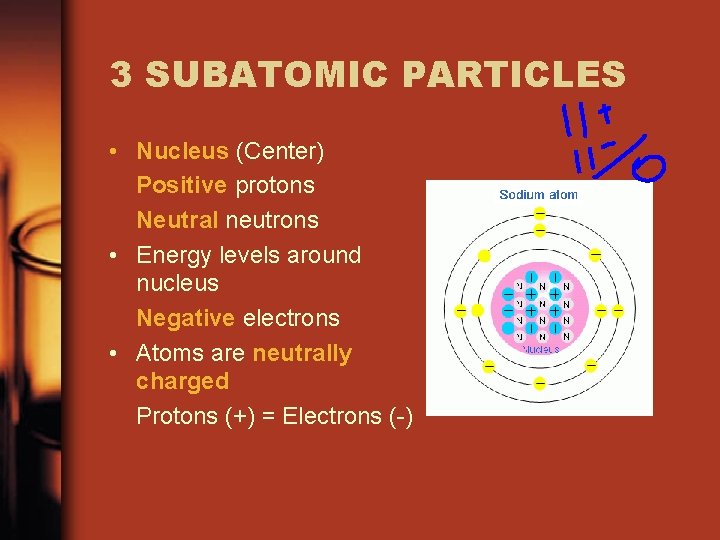
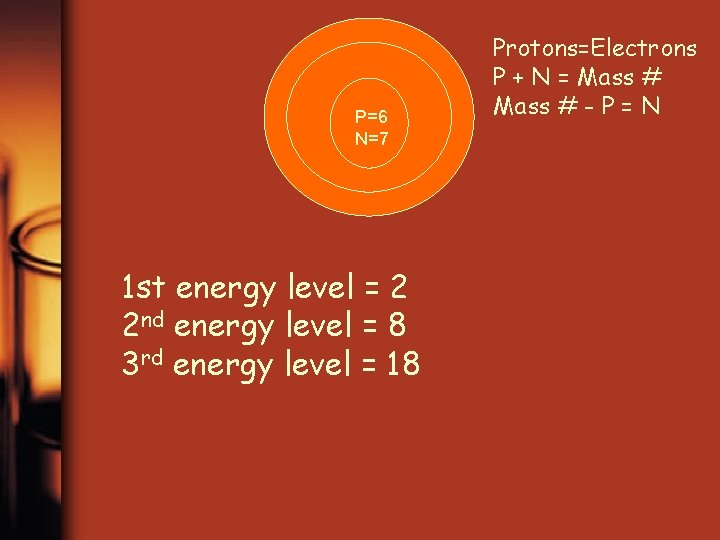
3 SUBATOMIC PARTICLES • Nucleus (Center) Positive protons Neutral neutrons • Energy levels around nucleus Negative electrons • Atoms are neutrally charged Protons (+) = Electrons (-)

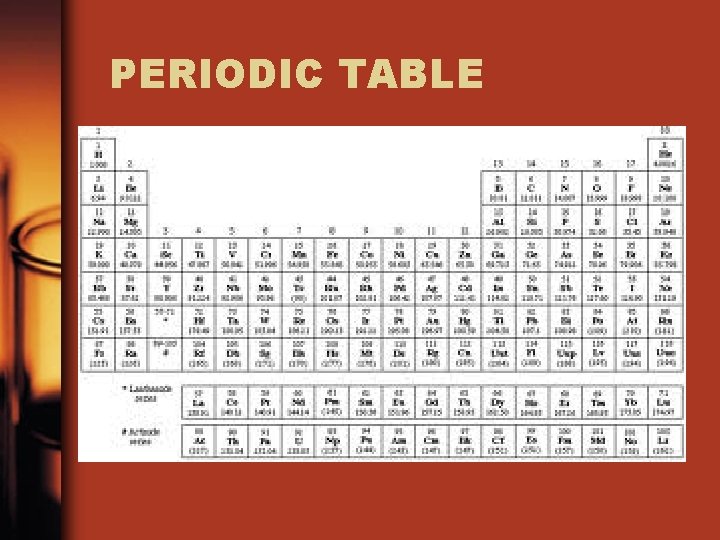
PERIODIC TABLE

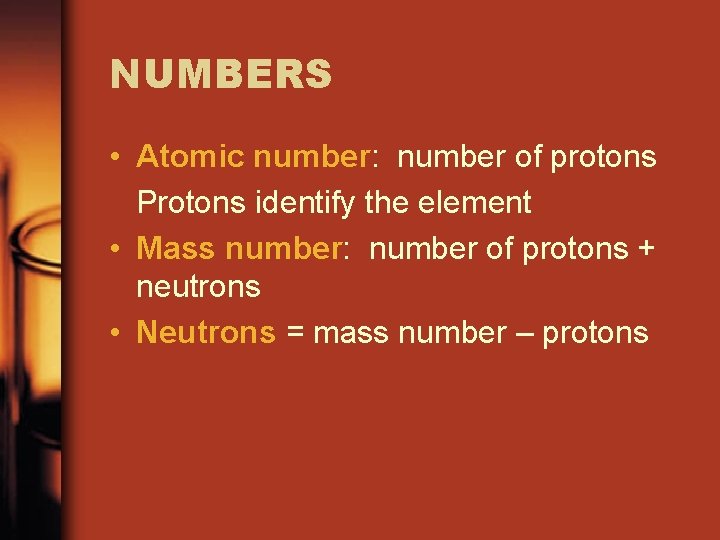
NUMBERS • Atomic number: number of protons Protons identify the element • Mass number: number of protons + neutrons • Neutrons = mass number – protons

P=6 N=7 1 st energy level = 2 2 nd energy level = 8 3 rd energy level = 18 Protons=Electrons P + N = Mass # - P = N

CALCULATIONS At. # 8 __ 2 P N ___ 12 ___ 8 13 ___ E Mass # ____ ___ 5

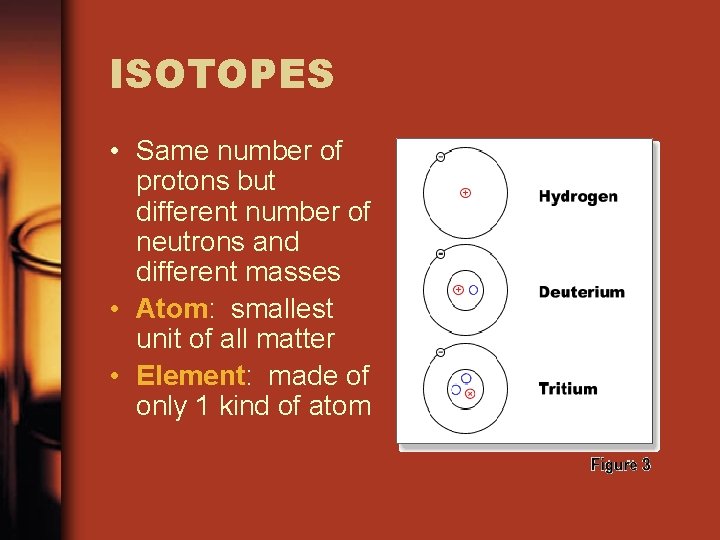
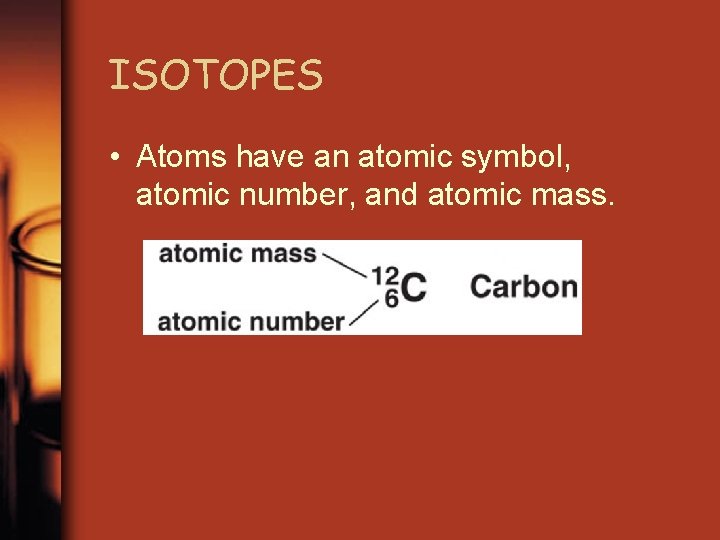
ISOTOPES • Same number of protons but different number of neutrons and different masses • Atom: smallest unit of all matter • Element: made of only 1 kind of atom

ISOTOPES • Atoms have an atomic symbol, atomic number, and atomic mass.

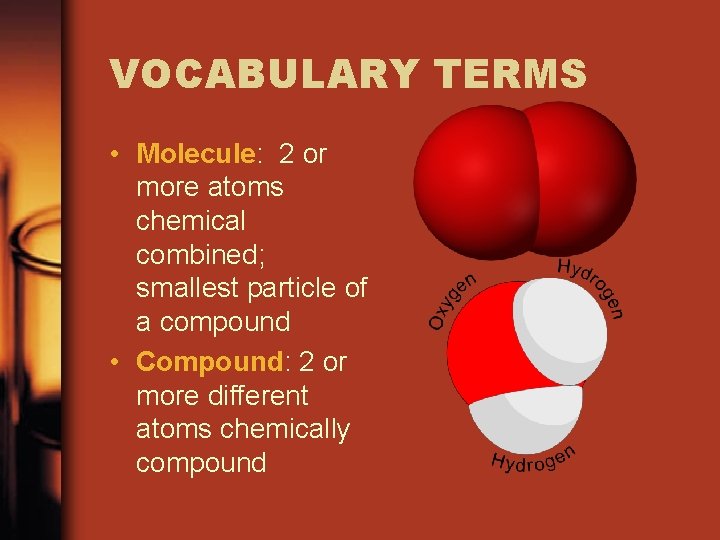
VOCABULARY TERMS • Molecule: 2 or more atoms chemical combined; smallest particle of a compound • Compound: 2 or more different atoms chemically compound

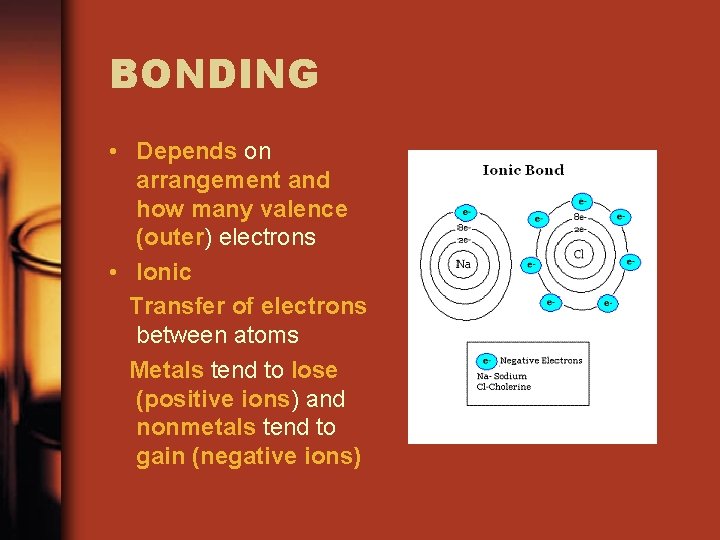
BONDING • Depends on arrangement and how many valence (outer) electrons • Ionic Transfer of electrons between atoms Metals tend to lose (positive ions) and nonmetals tend to gain (negative ions)

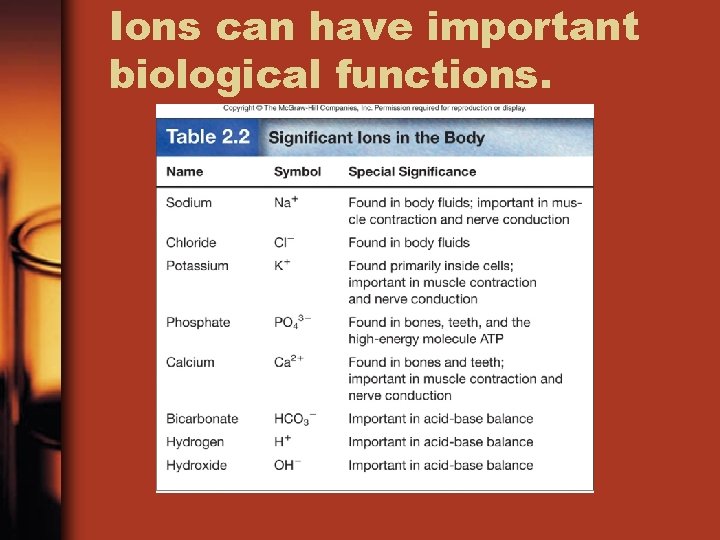
Ions can have important biological functions.

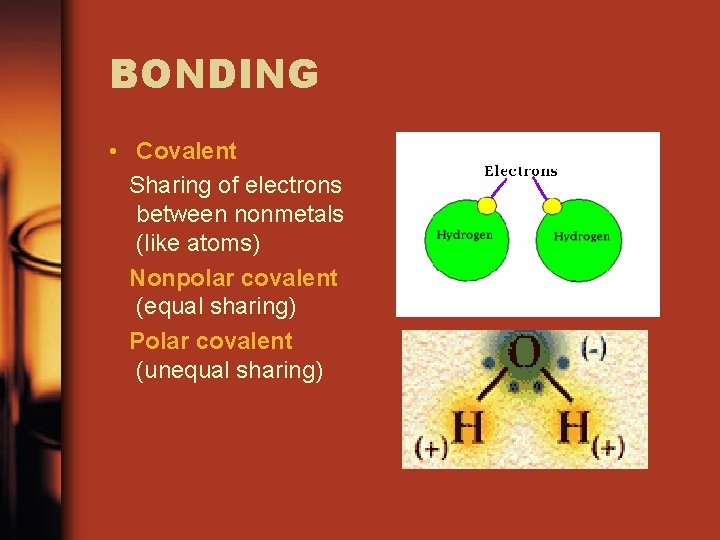
BONDING • Covalent Sharing of electrons between nonmetals (like atoms) Nonpolar covalent (equal sharing) Polar covalent (unequal sharing)

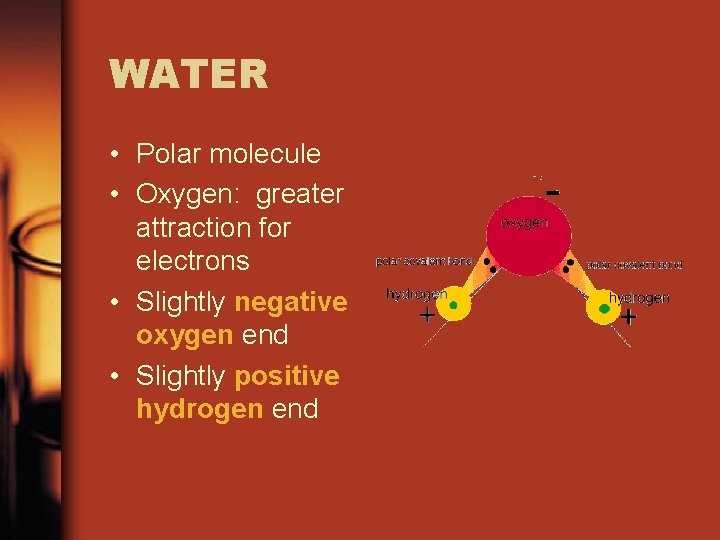
WATER • Polar molecule • Oxygen: greater attraction for electrons • Slightly negative oxygen end • Slightly positive hydrogen end

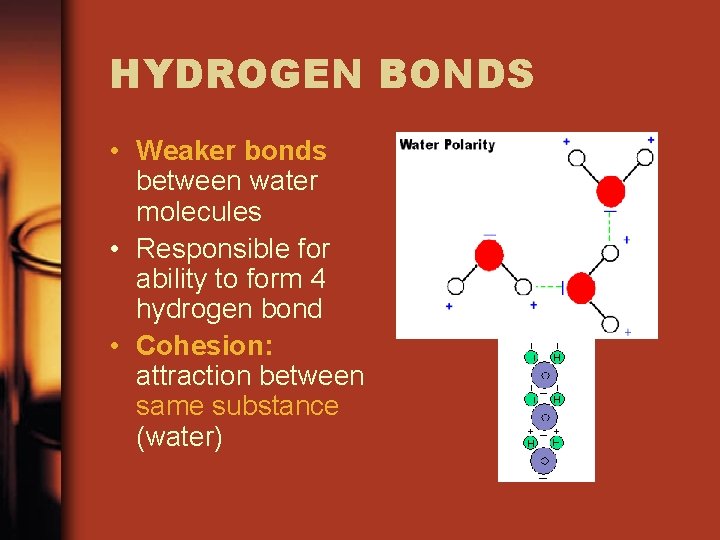
HYDROGEN BONDS • Weaker bonds between water molecules • Responsible for ability to form 4 hydrogen bond • Cohesion: attraction between same substance (water)

PROPERTIES OF WATER DUE TO COVALENT BOND • Surface tension: adhesion (different molecules) and cohesion (like molecules) • Capillary action: movement of water up roots • http: //www. youtube. com/watch? feature=pla yer_detailpage&v=VHn. FMPxte. Go

CHARACTERISTICS OF WATER • High specific heat: water warms and cools very slowly Biological importance: aquatic organisms are able to adjust slowly to the changing environment. • Freezing: bonds expand ice becomes less dense and will float Biological importance: top frozen layer helps insulate lake/pond

FREEZING • Ice is less dense as a solid than as a liquid (ice floats) • Frozen water forms a crystal-like lattice with molecules set at fixed distances.

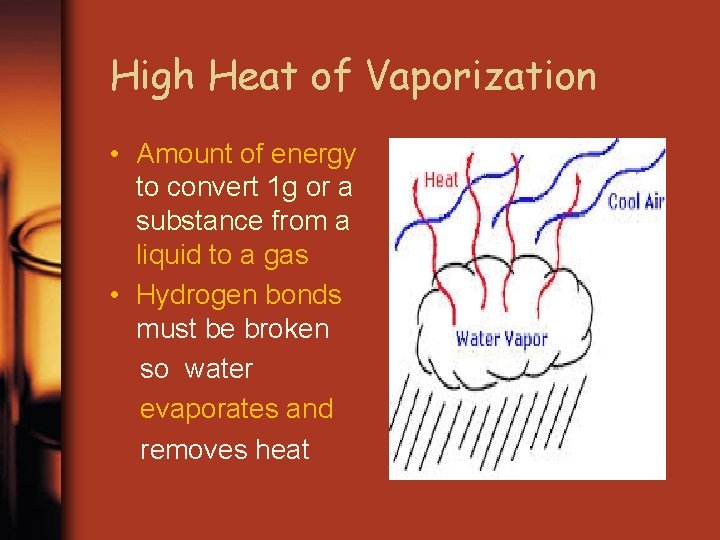
High Heat of Vaporization • Amount of energy to convert 1 g or a substance from a liquid to a gas • Hydrogen bonds must be broken so water evaporates and removes heat

Solutions & Suspensions • Many things dissolve in water--the Universal Solvent • Usually part of a mixture. • Two types of mixtures: Solutions Suspensions Defn: material composed of 2 or more substances physically combined

SOLUTIONS • Evenly distributed substances • Solute: material dissolved • Solvent: dissolving material • Solute: salt Solvent: water

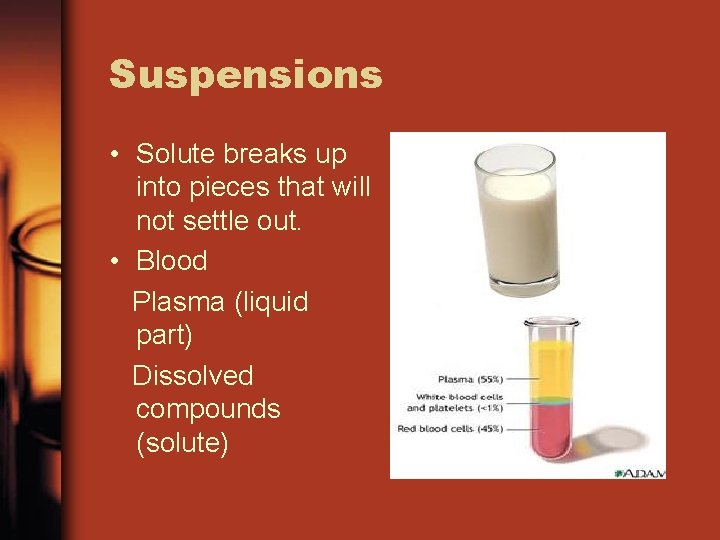
Suspensions • Solute breaks up into pieces that will not settle out. • Blood Plasma (liquid part) Dissolved compounds (solute)

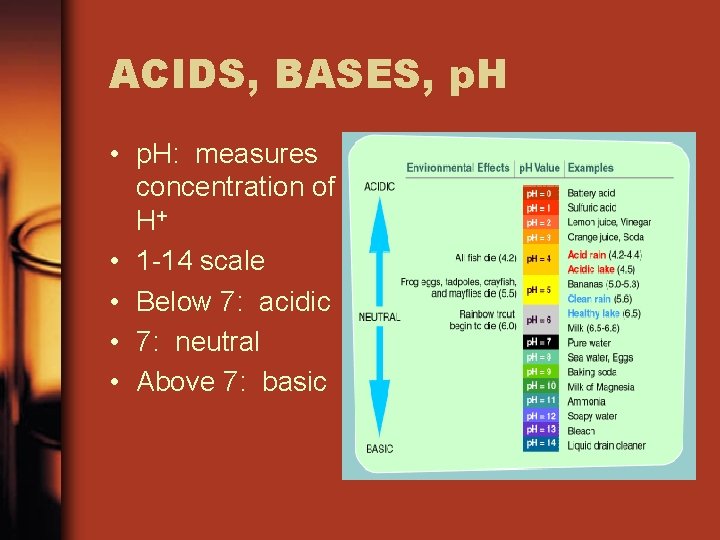
ACIDS, BASES, p. H • p. H: measures concentration of H+ • 1 -14 scale • Below 7: acidic • 7: neutral • Above 7: basic

ACIDS/BASES • Acid: form H+ in solution • Bases: form OHin solution

REVIEW • Which bond is formed by the transfer of electrons between atoms? (Ionic) • Which bond is the weakest and forms between water molecules? (hydrogen) • Acids form ____ ions in solution. (H+ --hydrogen ions) • What is the solute? (material dissolved in solution)

WATER CHARACTERISTICS • http: //www. youtube. com/watch? feat ure=player_detailpage&v=0 e. NSnj 4 Z f. Z 8

REVIEW • What is adhesion? (attraction between molecules of different substances) • What is the importance of capillary action? (pulls water into roots) • Name the bond formed from unequal sharing of electrons. (polar covalent bond)
 Atomic structure and properties ap chemistry
Atomic structure and properties ap chemistry First ionization energy of calcium
First ionization energy of calcium Ap chemistry atomic structure and periodicity
Ap chemistry atomic structure and periodicity Ap chemistry chapter 7 atomic structure and periodicity
Ap chemistry chapter 7 atomic structure and periodicity What is calcium ionization energy
What is calcium ionization energy The study of composition structure and properties of matter
The study of composition structure and properties of matter Relative formula mass of hcl
Relative formula mass of hcl Periyodik tablo periodic trends
Periyodik tablo periodic trends Periodic table with properties
Periodic table with properties How do you calculate atomic mass
How do you calculate atomic mass Atomic mass and atomic number difference
Atomic mass and atomic number difference Atomic number vs atomic radius
Atomic number vs atomic radius Percent composition examples
Percent composition examples Basic atomic structure worksheet
Basic atomic structure worksheet 460+370
460+370 Rectification formula
Rectification formula Atom graphic organizer
Atom graphic organizer Aristotle atomic
Aristotle atomic What is z in atomic structure
What is z in atomic structure Tungsten atomic structure
Tungsten atomic structure Democritus atom model
Democritus atom model Atomic structure poster project
Atomic structure poster project Atomic theory graphic organizer
Atomic theory graphic organizer Timeline of atomic structure
Timeline of atomic structure Unit 4 atomic structure
Unit 4 atomic structure History of the atom webquest
History of the atom webquest Atom and its structure
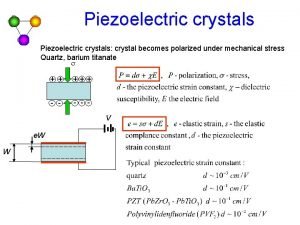
Atom and its structure Piezoelectric crystal atomic structure
Piezoelectric crystal atomic structure