Chemical Reactions Energy I Energy Stored in Chemical














- Slides: 49

Chemical Reactions (Energy)

I. Energy – Stored in Chemical ______, especially (__-__) bonds.

I. Energy – Stored in Chemical Bonds, especially (C-H) bonds. • Different forms of energy: A. ____ energy (sunlight) B. ____ energy (chemical bonds) C. ____ energy (movement)

I. Energy – Stored in Chemical Bonds, especially (C-H) bonds. • Different forms of energy: A. Radiant/Solar energy (sunlight) B. Chemical energy (chemical bonds) C. Kinetic/thermal energy (movement & heat)

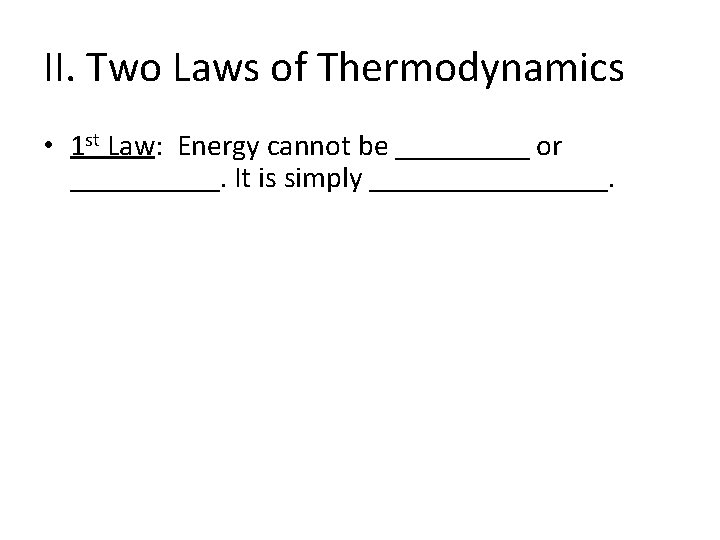
II. Two Laws of Thermodynamics • 1 st Law: Energy cannot be _____ or _____. It is simply ________.

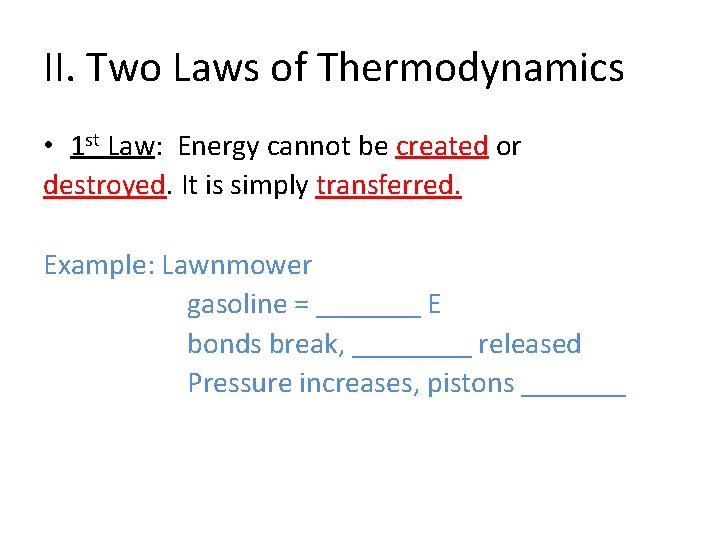
II. Two Laws of Thermodynamics • 1 st Law: Energy cannot be created or destroyed. It is simply transferred. Example: Lawnmower gasoline = _______ E bonds break, ____ released Pressure increases, pistons _______

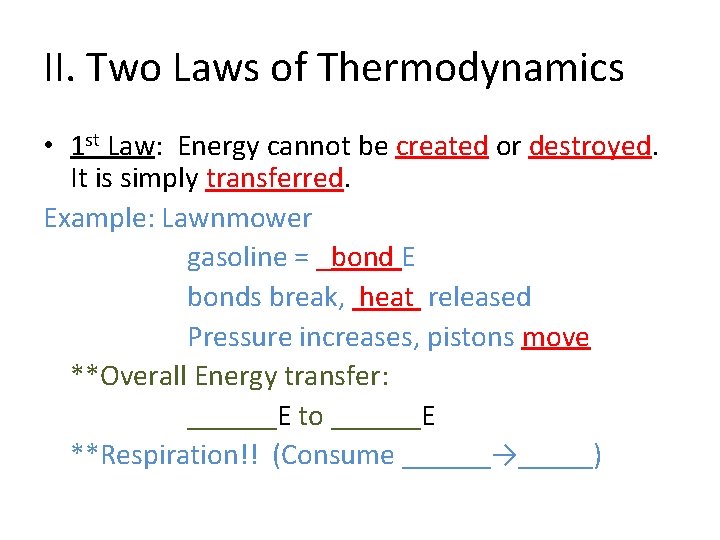
II. Two Laws of Thermodynamics • 1 st Law: Energy cannot be created or destroyed. It is simply transferred. Example: Lawnmower gasoline = _bond E bonds break, heat released Pressure increases, pistons move **Overall Energy transfer: ______E to ______E **Respiration!! (Consume ______→_____)

II. Two Laws of Thermodynamics • 1 st Law: Energy cannot be created or destroyed. It is simply transferred. Example: Lawnmower gasoline = bond E bonds break, heat released Pressure increases, pistons move **Overall Energy transfer: bond E to kinetic E **Respiration!! (Consume ______→_____)

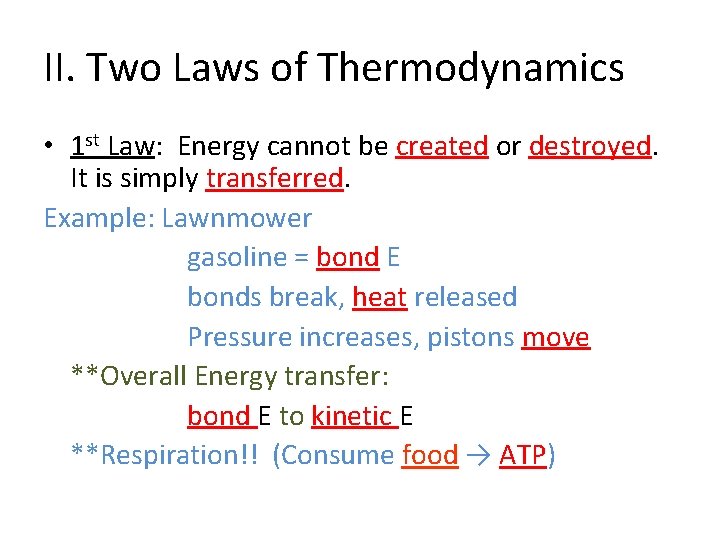
II. Two Laws of Thermodynamics • 1 st Law: Energy cannot be created or destroyed. It is simply transferred. Example: Lawnmower gasoline = bond E bonds break, heat released Pressure increases, pistons move **Overall Energy transfer: bond E to kinetic E **Respiration!! (Consume food → ATP)

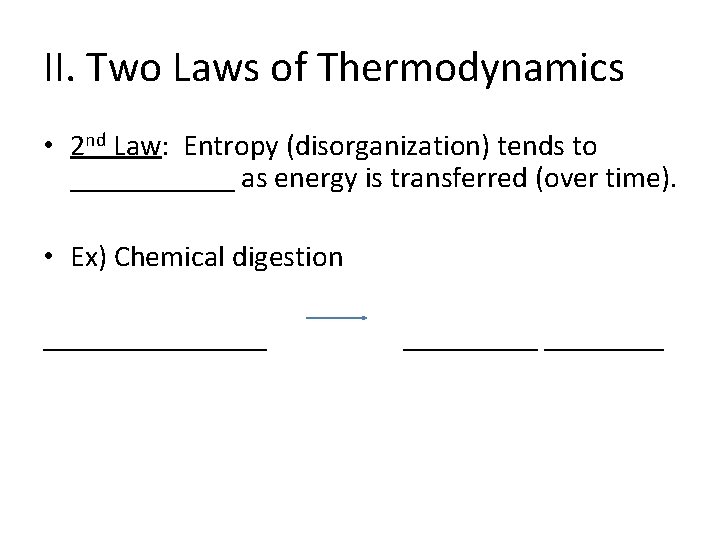
II. Two Laws of Thermodynamics • 2 nd Law: Entropy (disorganization) tends to ______ as energy is transferred (over time). • Ex) Chemical digestion ________

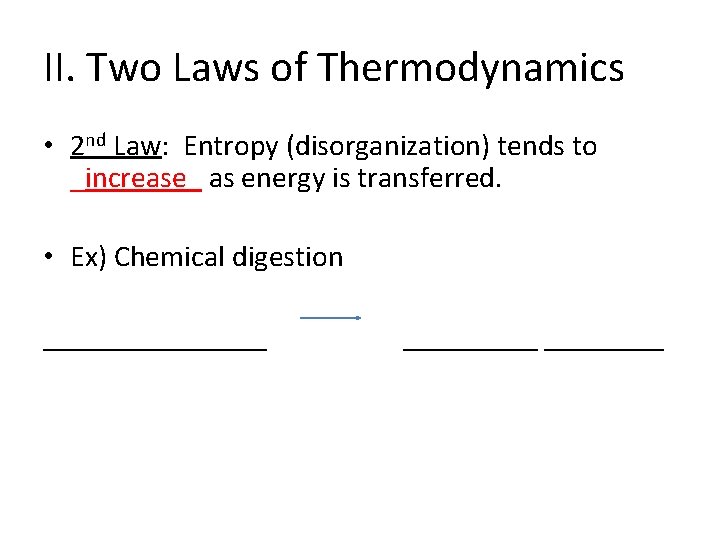
II. Two Laws of Thermodynamics • 2 nd Law: Entropy (disorganization) tends to _increase_ as energy is transferred. • Ex) Chemical digestion ________

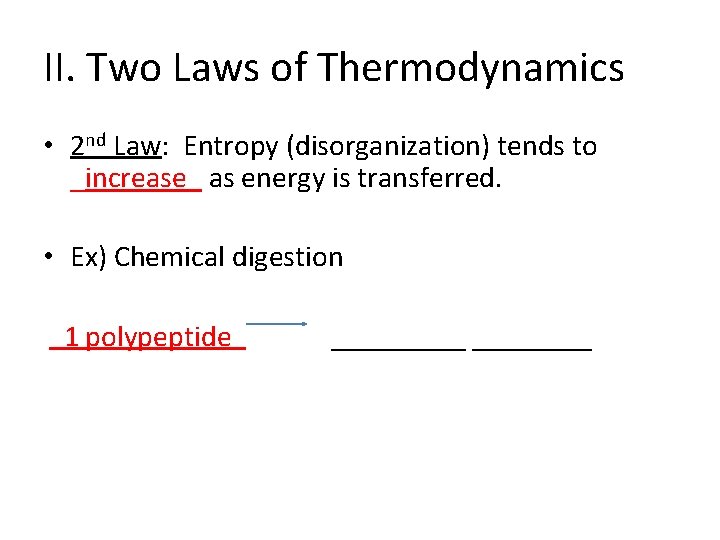
II. Two Laws of Thermodynamics • 2 nd Law: Entropy (disorganization) tends to _increase_ as energy is transferred. • Ex) Chemical digestion 1 polypeptide _____

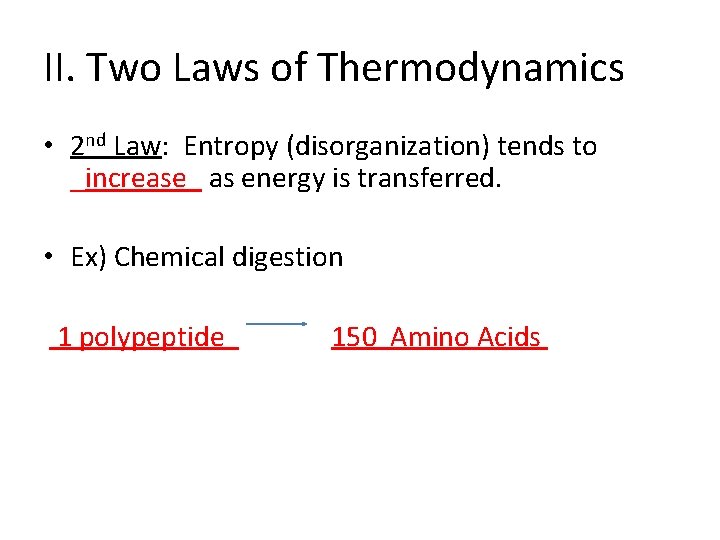
II. Two Laws of Thermodynamics • 2 nd Law: Entropy (disorganization) tends to _increase_ as energy is transferred. • Ex) Chemical digestion 1 polypeptide 150 Amino Acids

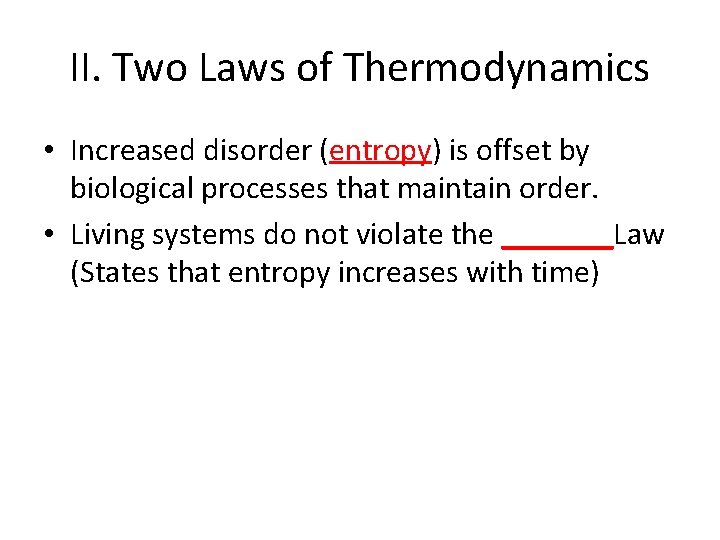
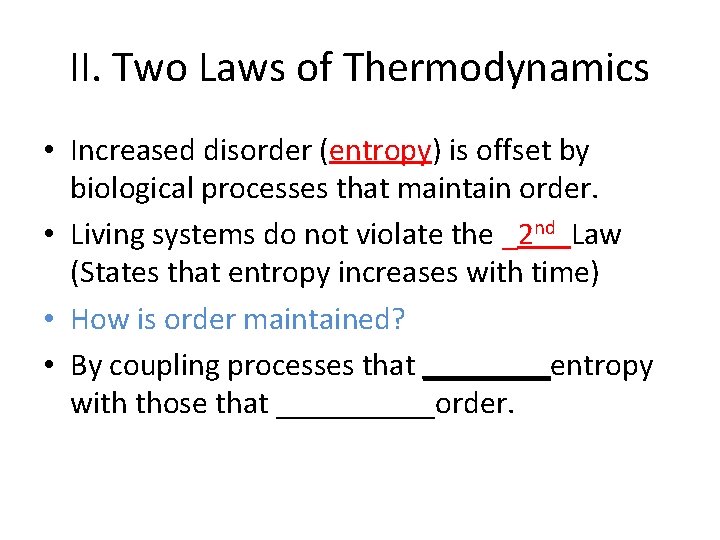
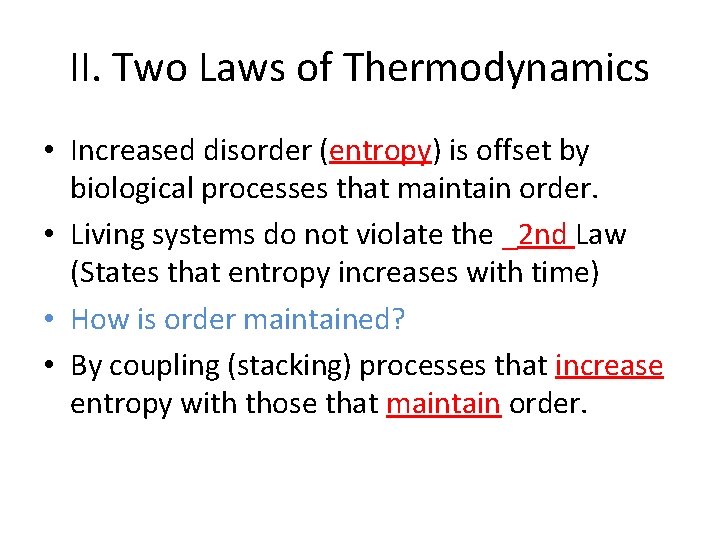
II. Two Laws of Thermodynamics • Increased disorder (entropy) is offset by biological processes that maintain order.

II. Two Laws of Thermodynamics • Increased disorder (entropy) is offset by biological processes that maintain order. • Living systems do not violate the _______Law (States that entropy increases with time)

II. Two Laws of Thermodynamics • Increased disorder (entropy) is offset by biological processes that maintain order. • Living systems do not violate the _2 nd Law (States that entropy increases with time) • How is order maintained? • By coupling processes that ____entropy with those that _____order.

II. Two Laws of Thermodynamics • Increased disorder (entropy) is offset by biological processes that maintain order. • Living systems do not violate the _2 nd Law (States that entropy increases with time) • How is order maintained? • By coupling (stacking) processes that increase entropy with those that maintain order.

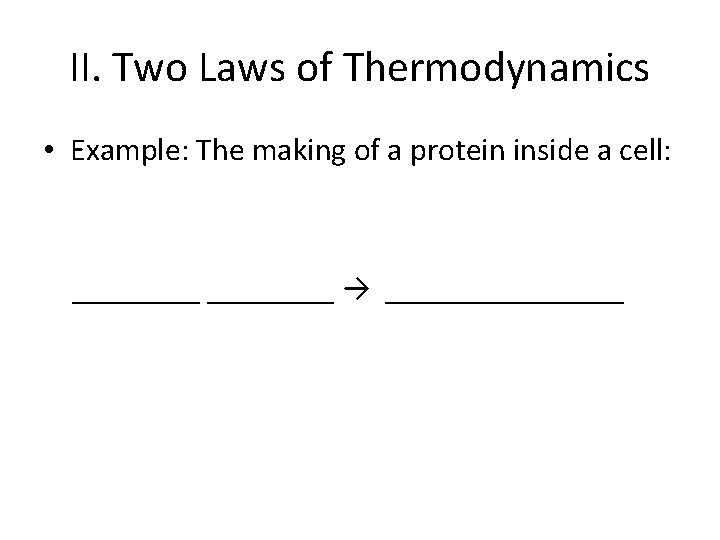
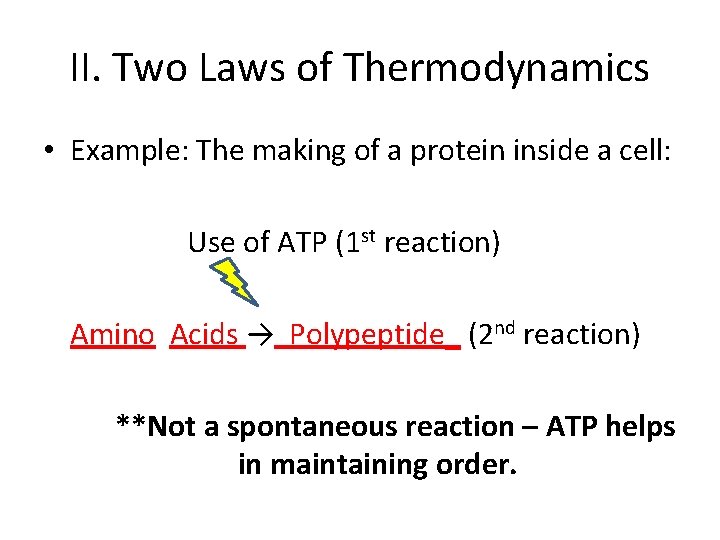
II. Two Laws of Thermodynamics • Example: The making of a protein inside a cell: ________ → ________

II. Two Laws of Thermodynamics • Example: The making of a protein inside a cell: Amino Acids → Polypeptide_

II. Two Laws of Thermodynamics • Example: The making of a protein inside a cell: Use of ATP (1 st reaction) Amino Acids → Polypeptide_ (2 nd reaction) **Not a spontaneous reaction – ATP helps in maintaining order.

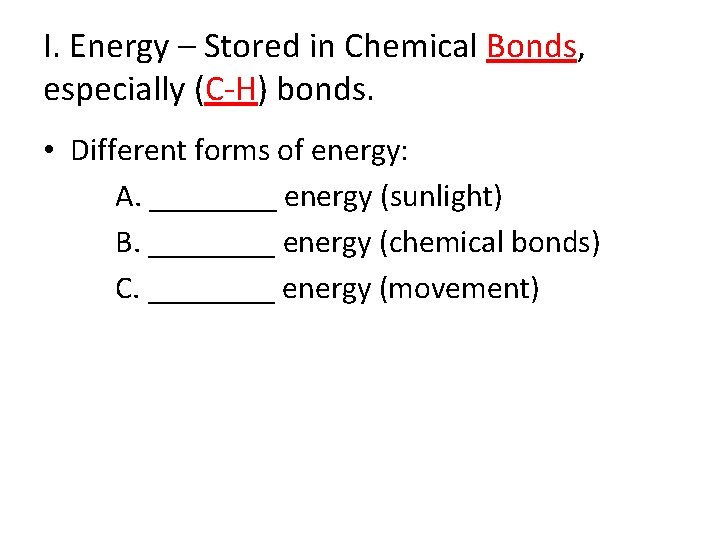
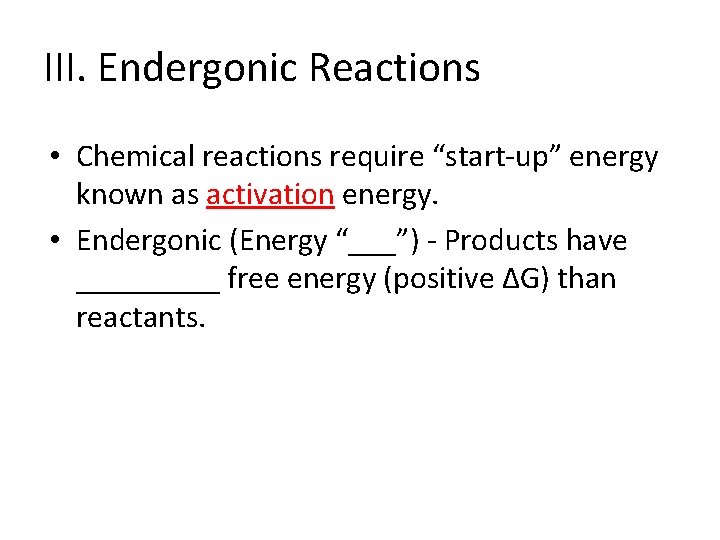
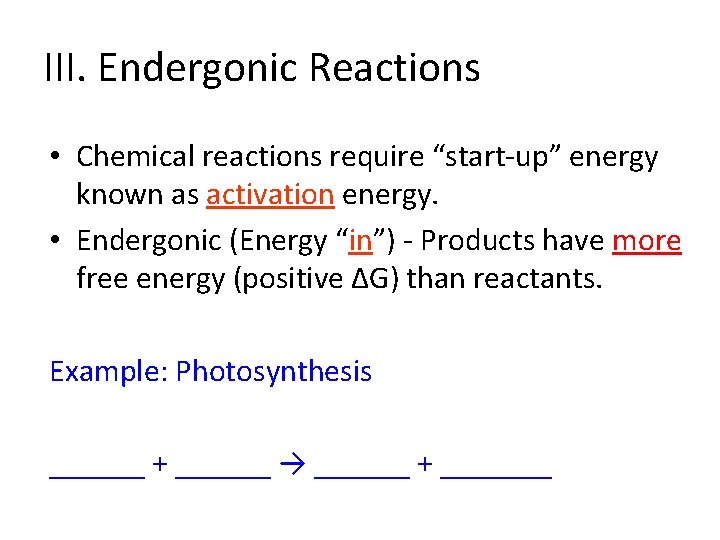
III. Endergonic Reactions • Chemical reactions require “start-up” energy known as _____energy.

III. Endergonic Reactions • Chemical reactions require “start-up” energy known as activation energy. • Endergonic (Energy “___”) - Products have _____ free energy (positive ∆G) than reactants.

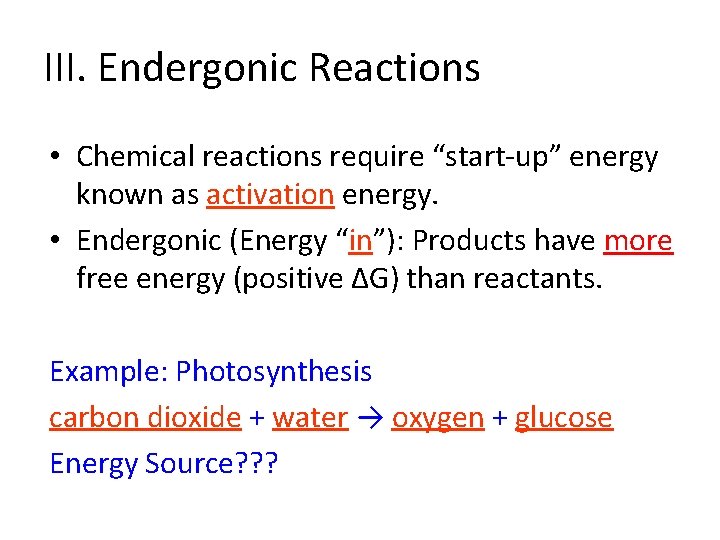
III. Endergonic Reactions • Chemical reactions require “start-up” energy known as activation energy. • Endergonic (Energy “in”) - Products have more free energy (positive ∆G) than reactants. Example: Photosynthesis ______ + ______ → ______ + _______

III. Endergonic Reactions • Chemical reactions require “start-up” energy known as activation energy. • Endergonic (Energy “in”): Products have more free energy (positive ∆G) than reactants. Example: Photosynthesis carbon dioxide + water → oxygen + glucose Energy Source? ? ?

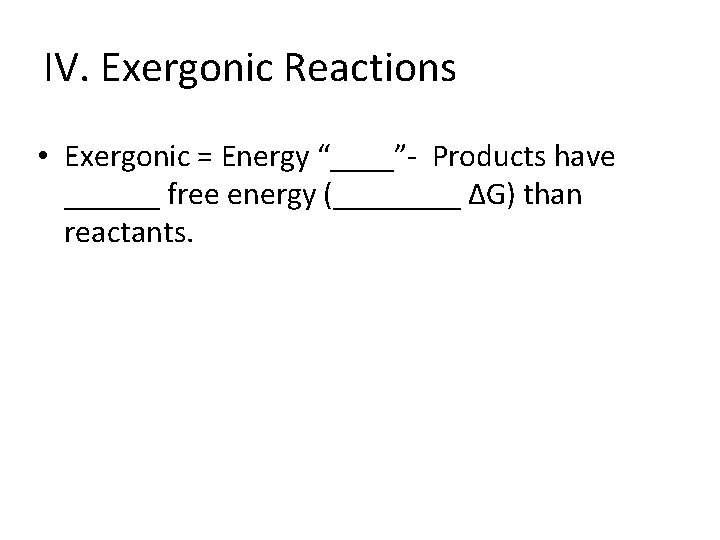
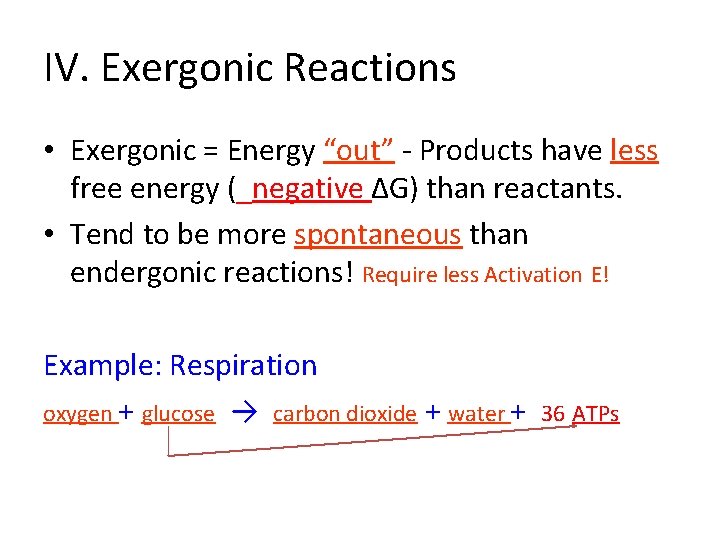
IV. Exergonic Reactions • Exergonic = Energy “____”- Products have ______ free energy (____ ∆G) than reactants.

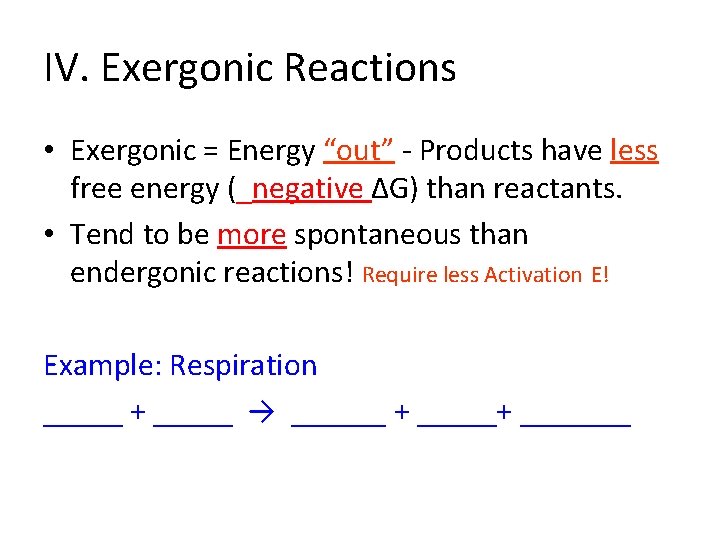
IV. Exergonic Reactions • Exergonic = Energy “out” - Products have less free energy (_____∆G) than reactants.

IV. Exergonic Reactions • Exergonic = Energy “out” - Products have less free energy (_negative ∆G) than reactants.

IV. Exergonic Reactions • Exergonic = Energy “out” - Products have less free energy (_negative ∆G) than reactants. • Tend to be _____ spontaneous than endergonic reactions!

IV. Exergonic Reactions • Exergonic = Energy “out” - Products have less free energy (_negative ∆G) than reactants. • Tend to be more spontaneous than endergonic reactions! Require less Activation E! Example: Respiration _____ + _____ → ______ + _______

IV. Exergonic Reactions • Exergonic = Energy “out” - Products have less free energy (_negative ∆G) than reactants. • Tend to be more spontaneous than endergonic reactions! Require less Activation E! Example: Respiration oxygen + glucose → carbon dioxide + water + 36 ATPs

V. Free Energy Changes in a Reaction Lead to Changes in Entropy, Stability, and Capacity to do Work

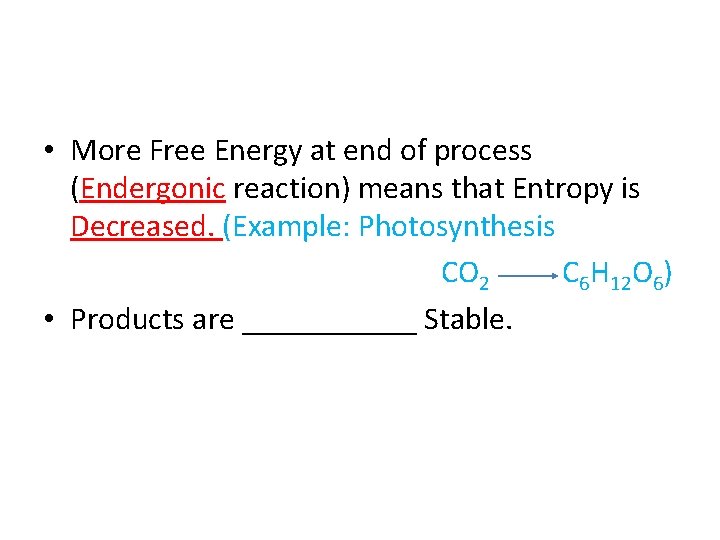
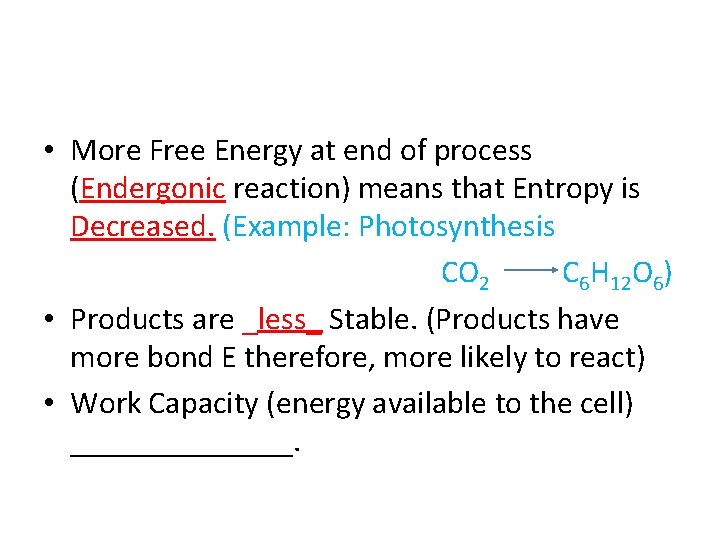
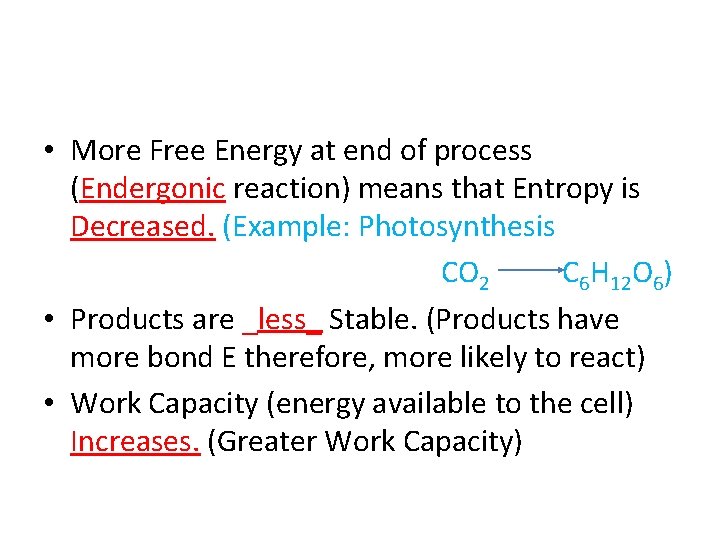
• More Free Energy at end of process (______ reaction) means that Entropy is _______

• More Free Energy at end of process (Endergonic reaction) means that Entropy is _______

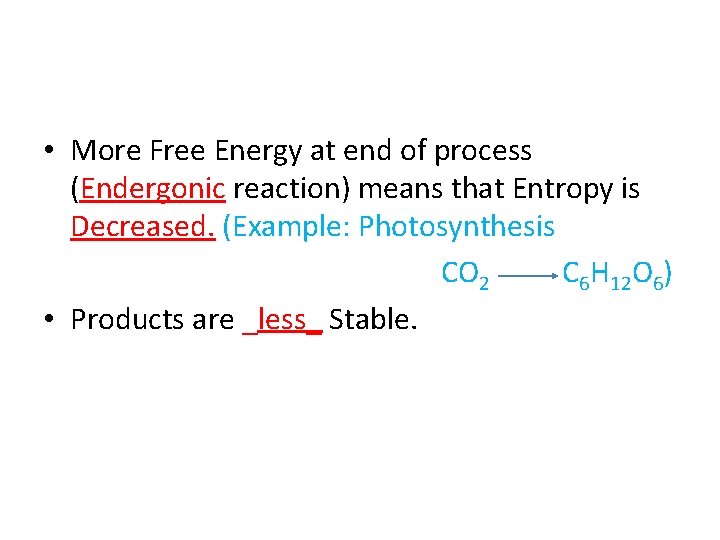
• More Free Energy at end of process (Endergonic reaction) means that Entropy is Decreased. (Example: Photosynthesis CO 2 C 6 H 12 O 6)

• More Free Energy at end of process (Endergonic reaction) means that Entropy is Decreased. (Example: Photosynthesis CO 2 C 6 H 12 O 6) • Products are ______ Stable.

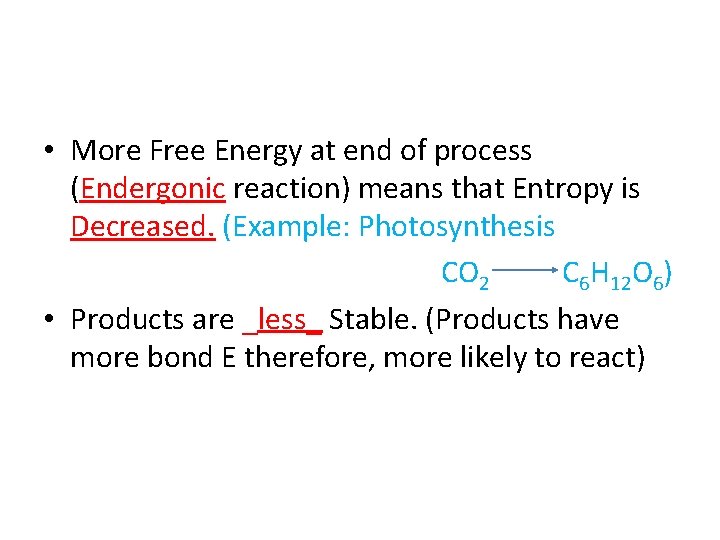
• More Free Energy at end of process (Endergonic reaction) means that Entropy is Decreased. (Example: Photosynthesis CO 2 C 6 H 12 O 6) • Products are _less_ Stable.

• More Free Energy at end of process (Endergonic reaction) means that Entropy is Decreased. (Example: Photosynthesis CO 2 C 6 H 12 O 6) • Products are _less_ Stable. (Products have more bond E therefore, more likely to react)

• More Free Energy at end of process (Endergonic reaction) means that Entropy is Decreased. (Example: Photosynthesis CO 2 C 6 H 12 O 6) • Products are _less_ Stable. (Products have more bond E therefore, more likely to react) • Work Capacity (energy available to the cell) _______.

• More Free Energy at end of process (Endergonic reaction) means that Entropy is Decreased. (Example: Photosynthesis CO 2 C 6 H 12 O 6) • Products are _less_ Stable. (Products have more bond E therefore, more likely to react) • Work Capacity (energy available to the cell) Increases. (Greater Work Capacity)

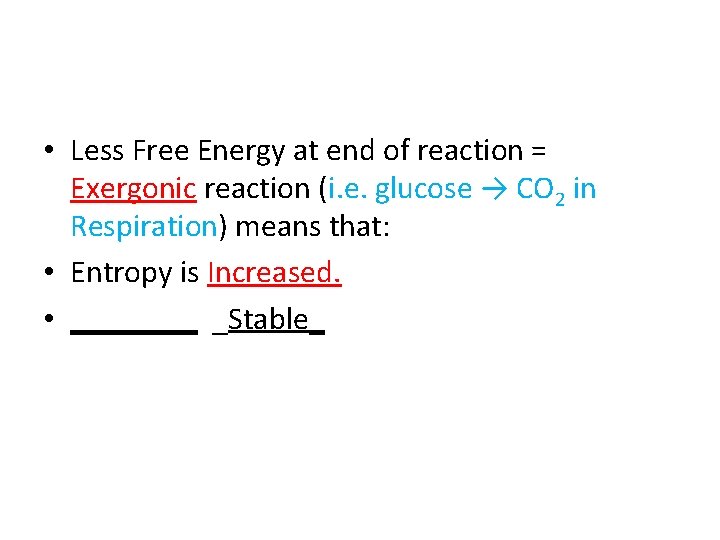
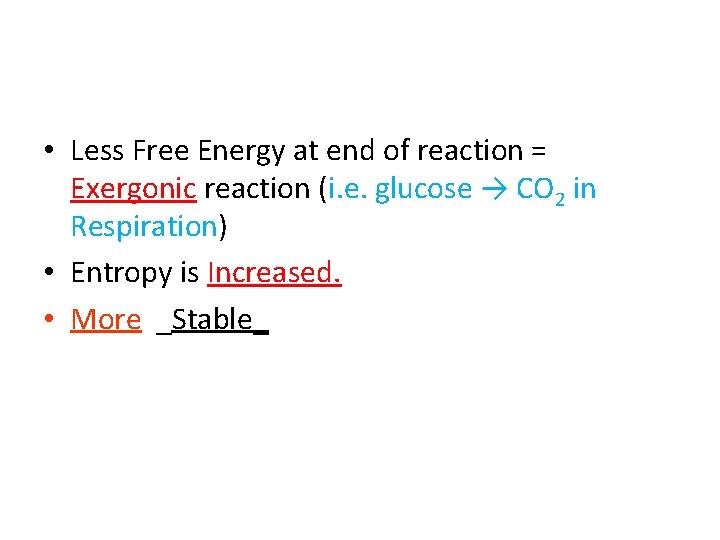
• Less Free Energy at end of reaction = ______ reaction (i. e. glucose → CO 2 in Respiration) means that:

• Less Free Energy at end of reaction = Exergonic reaction (i. e. glucose → CO 2 in Respiration) means that:

• Less Free Energy at end of reaction = Exergonic reaction (i. e. glucose → CO 2 in Respiration) means that: • Entropy is ______.

• Less Free Energy at end of reaction = Exergonic reaction (i. e. glucose → CO 2 in Respiration) means that: • Entropy is Increased.

• Less Free Energy at end of reaction = Exergonic reaction (i. e. glucose → CO 2 in Respiration) means that: • Entropy is Increased. • ____ _Stable_

• Less Free Energy at end of reaction = Exergonic reaction (i. e. glucose → CO 2 in Respiration) • Entropy is Increased. • More _Stable_

• Less Free Energy at end of reaction = Exergonic reaction (i. e. glucose → CO 2 in Respiration) means that: • Entropy is Increased. • More _Stable_ (Products have less bond E, therefore, are less likely to react)

• Less Free Energy at end of reaction = Exergonic reaction (i. e. glucose → CO 2 in Respiration) means that: • Entropy is Increased. • More _Stable_(Products have less bond E, therefore, are less likely to react) • ______ Work Capacity

• Less Free Energy at end of reaction = Exergonic reaction (i. e. glucose → CO 2 in Respiration) means that: • Entropy is Increased. • More _Stable_(Products have less bond E, therefore, are less likely to react) • Decreased Work Capacity

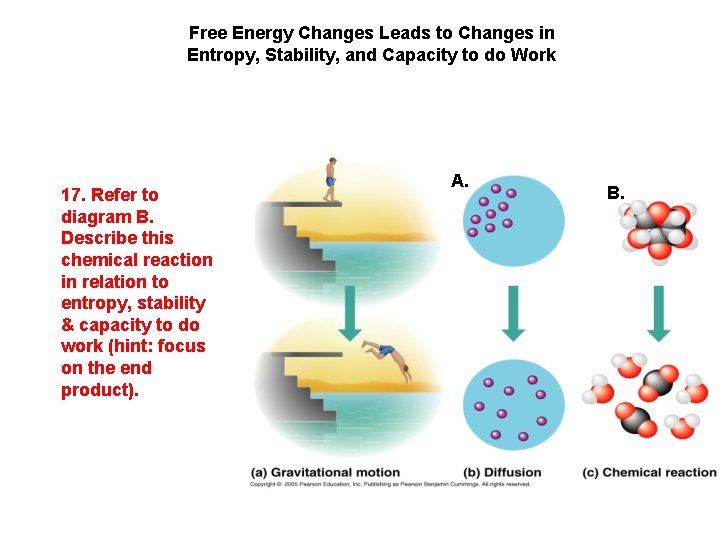
Free Energy Changes Leads to Changes in Entropy, Stability, and Capacity to do Work 17. Refer to diagram B. Describe this chemical reaction in relation to entropy, stability & capacity to do work (hint: focus on the end product). A. B.
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Stored procedure and stored function
Stored procedure and stored function Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations An example of redox reaction
An example of redox reaction Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chemical reactions that release energy are called
Chemical reactions that release energy are called Strain energy of hollow shaft
Strain energy of hollow shaft Energy stored in a spring
Energy stored in a spring Energy due to position
Energy due to position Elastic energy stored in a wire
Elastic energy stored in a wire Difference between capacitor and capacitance
Difference between capacitor and capacitance Is atp chemical energy
Is atp chemical energy The sudden release of energy stored in rocks causes a(n)
The sudden release of energy stored in rocks causes a(n) Stored energy solutions
Stored energy solutions Natural science grade 7 term 4
Natural science grade 7 term 4 Energy stored in parallel plate capacitor
Energy stored in parallel plate capacitor C=q/v
C=q/v Energy stored in capacitors
Energy stored in capacitors Energy stored in inductor
Energy stored in inductor Energy stored in a candy bar is an example of
Energy stored in a candy bar is an example of Energy stored in capacitor
Energy stored in capacitor Capacitor energy
Capacitor energy Electric potential energy
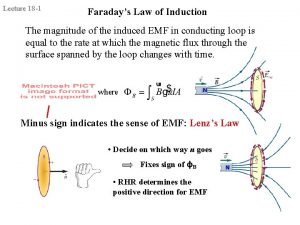
Electric potential energy Faraday law of electromagnetic induction
Faraday law of electromagnetic induction Inductor energy formula
Inductor energy formula Hand safety tool
Hand safety tool How is energy stored in a mousetrap
How is energy stored in a mousetrap Proportional relationships in chemical reactions
Proportional relationships in chemical reactions I intro
I intro Types of chemical reactions redox
Types of chemical reactions redox Types of reaction
Types of reaction Types of reactions chemistry
Types of reactions chemistry What are the 5 types of chemical reactions
What are the 5 types of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chapter assessment chemical reactions answers
Chapter 10 chapter assessment chemical reactions answers The calculation of quantities in chemical equations
The calculation of quantities in chemical equations Ouchterlony
Ouchterlony Predicting products of chemical reactions
Predicting products of chemical reactions Predicting synthesis reactions
Predicting synthesis reactions Combination reaction example
Combination reaction example Unit 11 chemical reactions
Unit 11 chemical reactions Toxic reactions chemical equations
Toxic reactions chemical equations Four types of chemical reactions
Four types of chemical reactions Biology-roots.com
Biology-roots.com Describing chemical reactions
Describing chemical reactions