Chapter 5 Sections 5 2 5 3 Units
















































- Slides: 71

Chapter 5 Sections 5. 2 -5. 3 Units of Measurement Mrs. Baldessari Chemistry Objectives: Use the metric system to measure length, volume, and mass

Measurement At the conclusion of our time together, you should be able to: 1. Explain the difference between the two types of observations 2. Give examples of 5 basic SI measuring units 3. Give examples of 3 different derived units from the basic SI units 4. Vary the amounts of the SI measuring units with 10 different prefixes and define each 5. Explain the difference between mass and weight 6. List and explain 5 different units for volume.

Measurement You are making a measurement when you ¨Check you weight ¨Read your watch ¨Take your temperature ¨Weigh a cantaloupe What kinds of measurements did you make today?

Some Tools for Measurement

Learning Check From the previous slide, state the tool (s) you would use to measure A. temperature __________ B. volume always use a __________ never use a __________ C. time __________ D. weight __________

Solution From the previous slide, state the tool (s) you would use to measure A. temperature thermometer B. volume always use a: graduated cylinder never use a: Beaker C. time stop watch D. weight scale

Measurement in Chemistry In chemistry we ¨do experiments ¨measure quantities ¨use numbers to report measurements

Learning Check What are some U. S. units that are used to measure each of the following? A. length B. volume C. weight D. temperature

Solution Some possible answers are A. length inch, foot, yard, mile B. volume cup, teaspoon, gallon, pint, quart C. weight ounce, pound (lb), ton D. temperature °F

Metric System (SI) n Is a decimal system based on 10 n Used in most of the world n Used by scientists and hospitals

Units in the Metric System l length meter m l volume liter L l mass gram g l temperature Celsius °C

Stating a Measurement In every measurement there is a ¨Number followed by a ¨ Unit from measuring device

Quantitative Measurements in Chemistry Must Include Units!

Learning Check What is the unit of measurement in each of the following examples? A. The patient’s temperature is 38. 8 °C. B. The sack holds 2. 3 kg of potatoes. C. It is 12. 8 km miles from your house to school. D. The bottle holds 2 L of orange soda.

Solution A. °C (degrees Celcius) B. Kg (Kilograms) C. Km (Kilometers) D. L (liters)

Learning Check Identify the measurement in metric units. A. John’s height is 1) 1. 5 yards 2) 6 feet 3) 2 meters B. The volume of saline in the IV bottle is 1) 1 liters 2) 1 quart 3) 2 pints C. The mass of a lemon is 1) 12 ounces 2) 145 grams 3) 0. 6 pounds

Solution A. John’s height is 3) 2 meters B. The volume of saline in the IV bottle is 1) 1 liter C. The mass of a lemon is 2) 145 grams

Types of Observations and Measurements n n We make QUALITATIVE observations of reactions — changes in color and physical state. We also make QUANTITATIVE observations that involve MEASUREMENTS with numbers and units.

Stating a Quantitative Measurement In every measurement there is a ¨Number (Quantity) followed by a ¨ Unit or label from a measuring device ¨Remember: No naked numbers

Quantitative Measurements in Chemistry Must Include Units!

Standards of Quantitative Measurement When we measure, we use a measuring tool to compare some dimension of an object to a standard. For example, at one time the standard for length was the king’s foot. What are some problems with this standard?

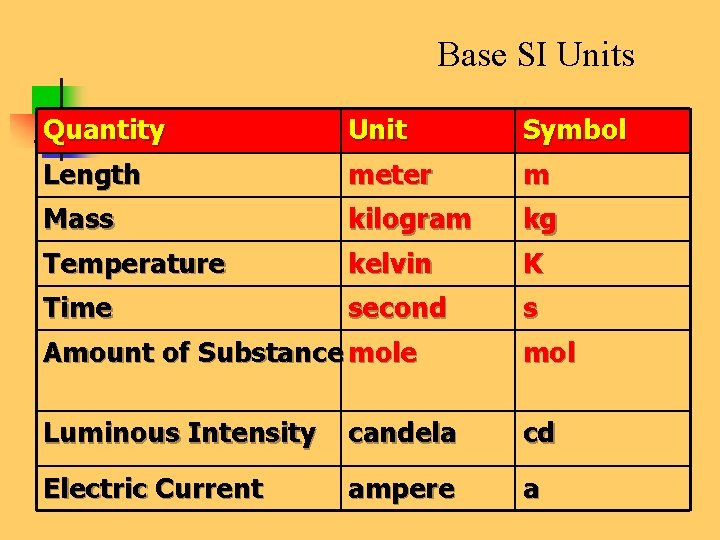
Base SI Units Quantity Unit Symbol Length meter m Mass kilogram kg Temperature kelvin K Time second s Amount of Substance mol Luminous Intensity candela cd Electric Current ampere a

Units Of Measurement You Must Know Use SI units — based on the metric system Length meter, m Mass kilogram, kg Volume Time Temperature liter, L seconds, s kelvin, K

Unit for Length 1 Meter or centimeter Note: Not Foot or inches !!

Metric Prefixes n Kilo- means 1000 of that unit n n n 1 kilometer (km) = 1000 meters (m) Centi- means 1/100 of that unit n 1 meter (m) = 100 centimeters (cm) n 1 dollar = 100 cents Milli- means 1/1000 of that unit n 1 meter (m) = 1000 millimeters (mm)

Metric Prefixes You Need to Know

The pneumonic: King Henry Died Unexpectedly Drinking Chocolate Milk Memorize this!

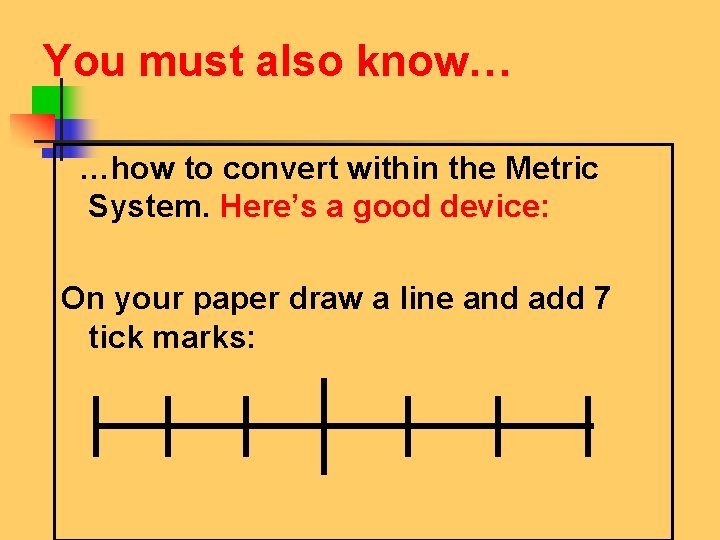
You must also know… …how to convert within the Metric System. Here’s a good device: On your paper draw a line and add 7 tick marks:

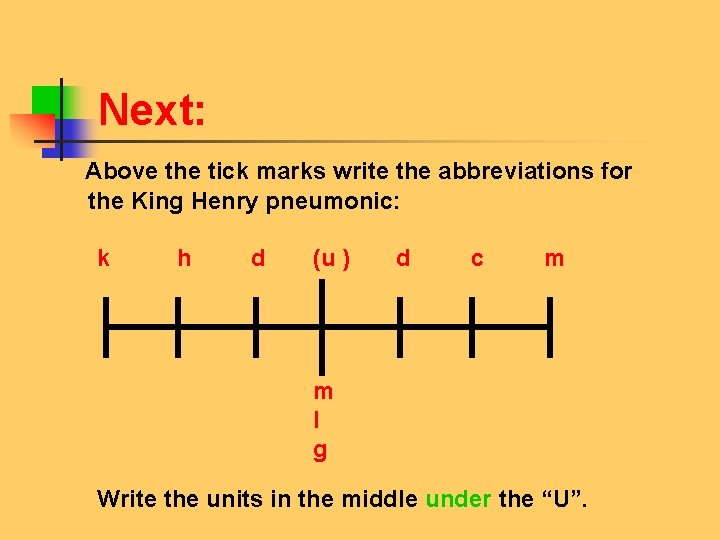
Next: Above the tick marks write the abbreviations for the King Henry pneumonic: k h d (u ) d c m m l g Write the units in the middle under the “U”.

Let’s add the meter line: k h d u d c m km hm dam m dm cm mm L g

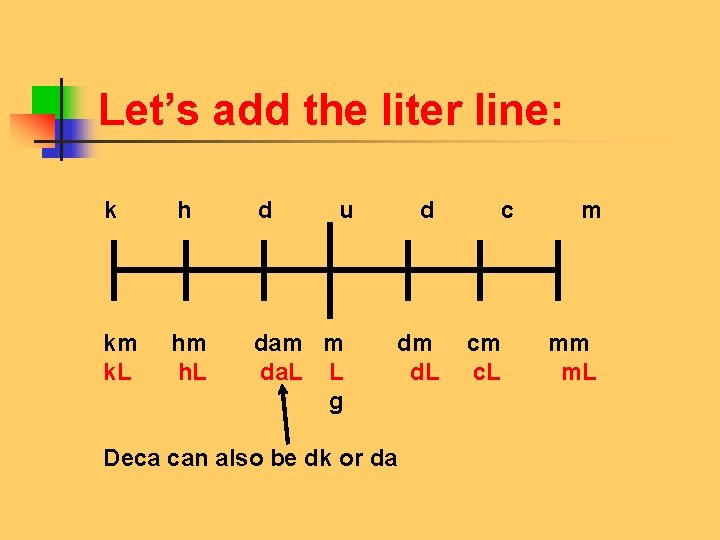
Let’s add the liter line: k h d u d c m km hm dam m dm cm mm k. L h. L da. L L d. L c. L m. L g Deca can also be dk or da

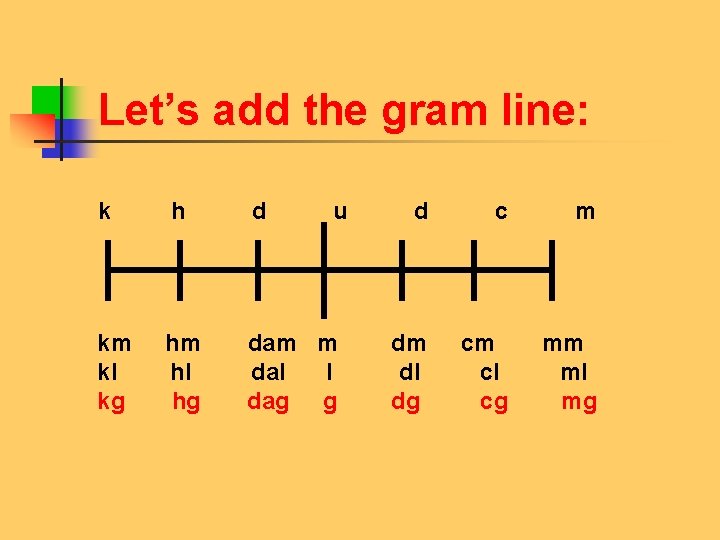
Let’s add the gram line: k h d u d c m km hm dam m dm cm mm kl hl dal l dl cl ml kg hg dag g dg cg mg

How to use this device: 1. Look at the problem. Look at the unit that has a number. On the device put your pencil on that unit. 2. Move to new unit, counting jumps and noticing the direction of the jump. 3. Move decimal in original number the same # of spaces and in the same direction.

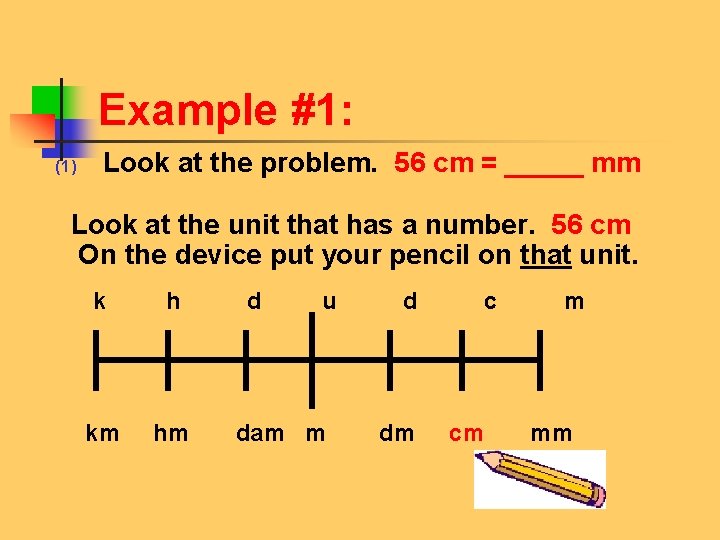
Example #1: (1) Look at the problem. 56 cm = _____ mm Look at the unit that has a number. 56 cm On the device put your pencil on that unit. k h d u d c m km hm dam m dm cm mm

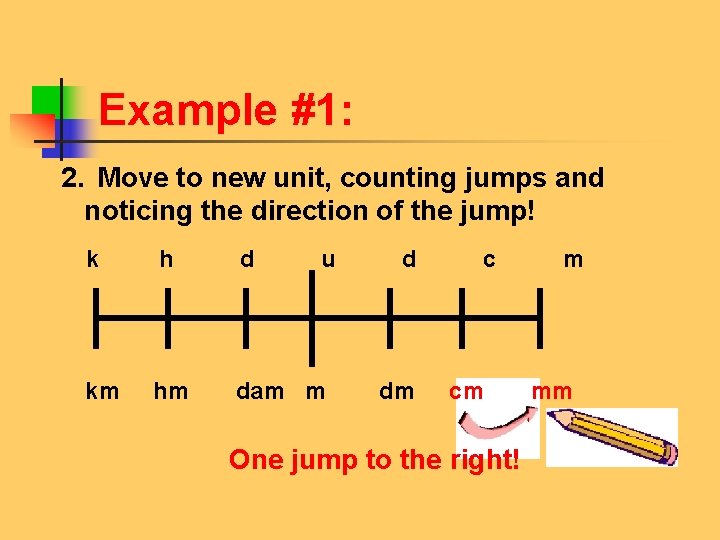
Example #1: 2. Move to new unit, counting jumps and noticing the direction of the jump! k h d u d c m km hm dam m dm cm mm One jump to the right!

Example #1: 3. Move decimal in original number the same # of spaces and in the same direction. 56 cm = _____ mm 56. 0. One jump to the right! Move decimal one jump to the right. Add a zero as a placeholder.

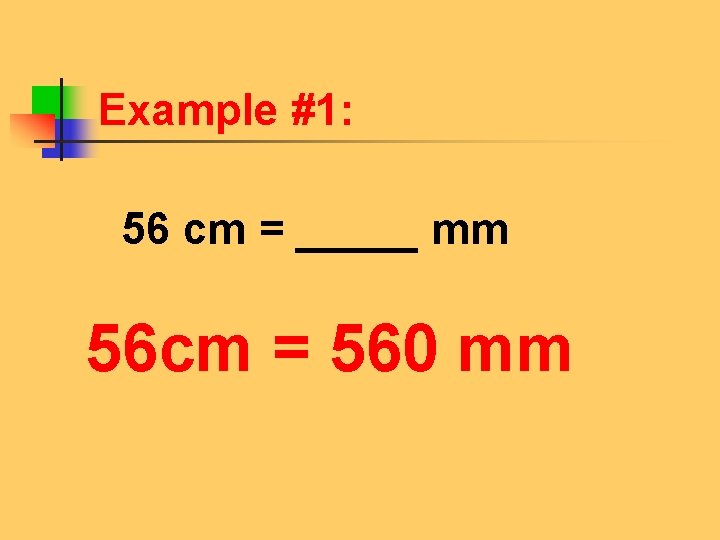
Example #1: 56 cm = _____ mm 56 cm = 560 mm

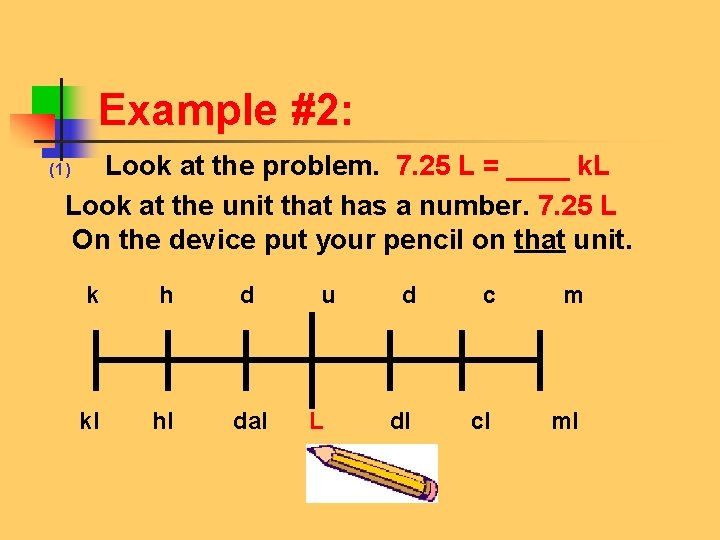
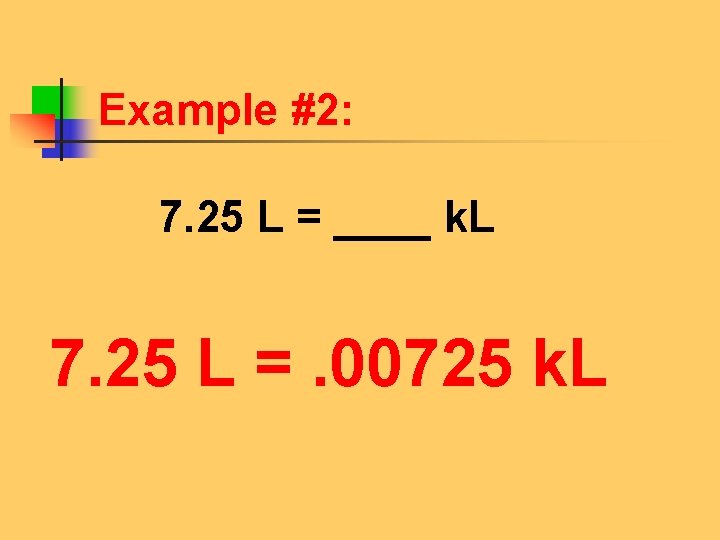
Example #2: Look at the problem. 7. 25 L = ____ k. L Look at the unit that has a number. 7. 25 L On the device put your pencil on that unit. (1) k h d u d c m kl hl dal L dl cl ml

Example #2: Move to new unit, counting jumps and noticing the direction of the jump! 2. k h d u d c m kl hl dal L dl cl ml Three jumps to the left!

Example #2: (3) Move decimal in original number the same # of spaces and in the same direction. 7. 25 L = ____ k. L . 007. 25 Three jumps to the left! Move decimal to the left three jumps. Add two zeros as placeholders.

Example #2: 7. 25 L = ____ k. L 7. 25 L =. 00725 k. L

Example #3: Try this problem on your own: 45, 000 g = ____mg k h d u d c m kg hg dag g dg cg mg

Example #3: k h d u d c m kg hg dag g dg cg mg Three jumps to the right! 45, 000.

Example #3: 45, 000 g = 45, 000 mg Three jumps to the right!

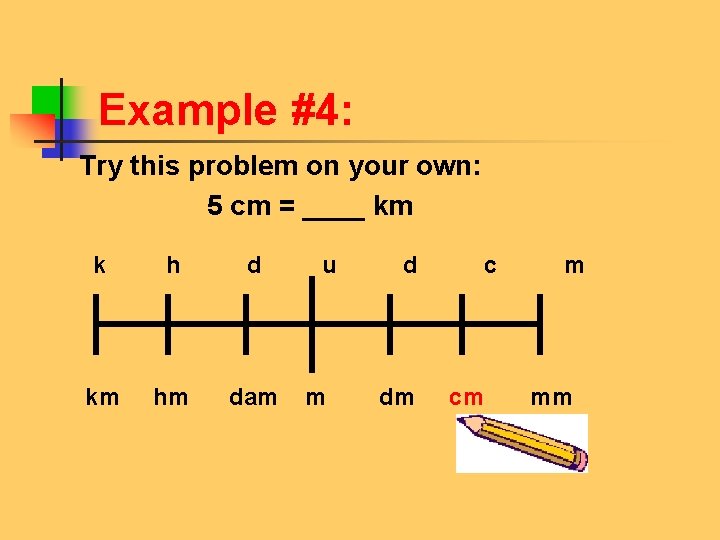
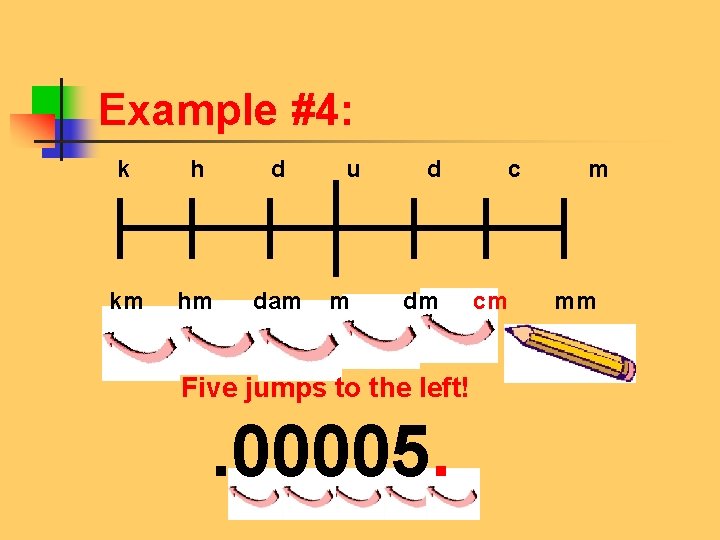
Example #4: Try this problem on your own: 5 cm = ____ km k h d u d c m km hm dam m dm cm mm

Example #4: k h d u d c m km hm dam m dm cm mm Five jumps to the left! . 00005.

Example #4: 5 cm =. 00005 km Five jumps to the left!

Examples #5 -9: Solve these five problems on your own. Show your answers to your teacher. You may choose to use the King Henry Notes. You may choose the King Henry Staircase. You may also choose to make a King Henry Slider.

Examples #5 -9: (5) 35 mm = ____ cm (6) 14, 443 L = ____ k. L (7) 0. 00056 kg = ____ g (8)35. 4 L = ____ m. L (9)16 mm = ____ km

One last caution: Be careful NOT to count the spot you start from, where you put your pencil point. Only count the jumps!

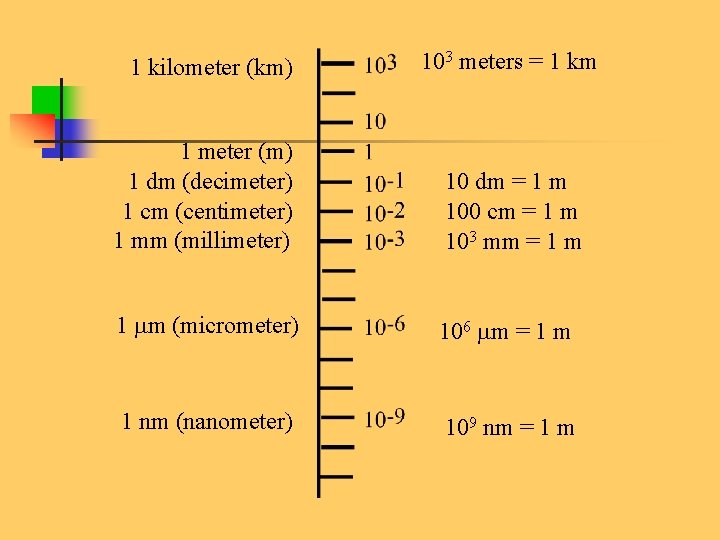
1 kilometer (km) 103 meters = 1 km 1 meter (m) 1 dm (decimeter) 1 cm (centimeter) 1 mm (millimeter) 10 dm = 1 m 100 cm = 1 m 103 mm = 1 m (micrometer) 106 m = 1 m 1 nm (nanometer) 109 nm = 1 m

Mass and Weight • Weight is force of the gravitational pull on an object. It would be different on the moon than it is on earth. • Mass is a measure of the amount of matter in an object.

Mass and Weight • Mass and weight are directly related as long as we remain on earth at the same elevation. That is, if one object has twice the mass of another, then its weight on earth would also be twice as large. • However, if we take the same object to Denver, Colorado, the mass stays the same but the weight would be different. Why? ?

Mass vs. Weight Mass: Amount of Matter n (grams, measured with a BALANCE) n Weight: Force exerted by the mass, only present with gravity n (pounds, measured with a scale) n Can you hear me now?

Unit for Volume 1 Meter Cubed Note: A Derived Unit!! Too Big, So Generally Use the Liter

What is a Liter? ?

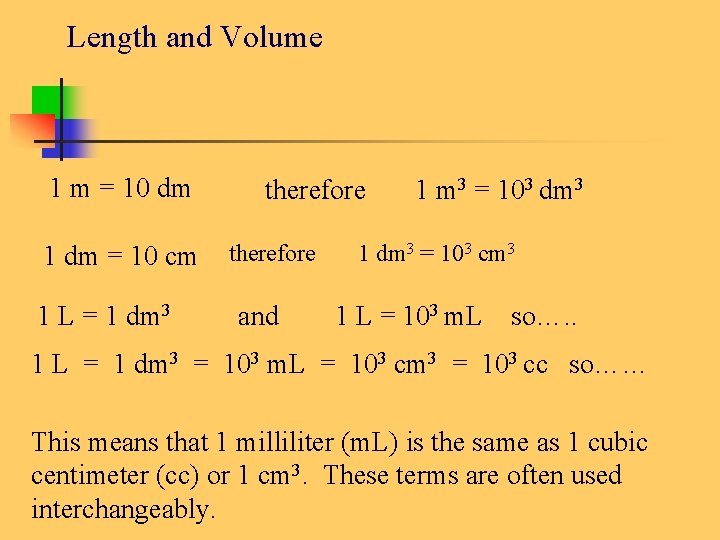
Length and Volume 1 m = 10 dm 1 dm = 10 cm 1 L = 1 dm 3 therefore and 1 m 3 = 103 dm 3 1 dm 3 = 103 cm 3 1 L = 103 m. L so…. . 1 L = 1 dm 3 = 103 m. L = 103 cm 3 = 103 cc so…… This means that 1 milliliter (m. L) is the same as 1 cubic centimeter (cc) or 1 cm 3. These terms are often used interchangeably.

Units for Volume m 3 cm 3 1 dm 3 = 1 L dm 3 L m. L Liter 1 cm 3 = 1 m. L

Measuring Volume: n The volume of a liquid is measured with a graduated cylinder. When liquid is poured into the cylinder, a curved surface called the meniscus is formed.

Other Derived SI Units Quantity Unit Symbol Volume cubic meter m 3 Density kilograms per cubic meter kg/m 3 Speed meter per second m/s Newton kg m/ s 2 N Energy 2/s 2) Joule (kg m J Pressure 2) Pascal (kg/ms Pa

Units for Energy n Joule n calorie J 1 cal = 4. 184 J 1 cal = quantity of heat needed to raise the temperature of 1 g of water by 1 o. C. 1 kcal = 1000 cal

Measurement Let’s see if you can: 1. Explain the difference between the two types of observations 2. Give examples of 5 basic SI measuring units 3. Give examples of 3 different derived units from the basic SI units 4. Vary the amounts of the SI measuring units with 10 different prefixes and define each 5. Explain the difference between mass and weight 6. List and explain 5 different units for volume.

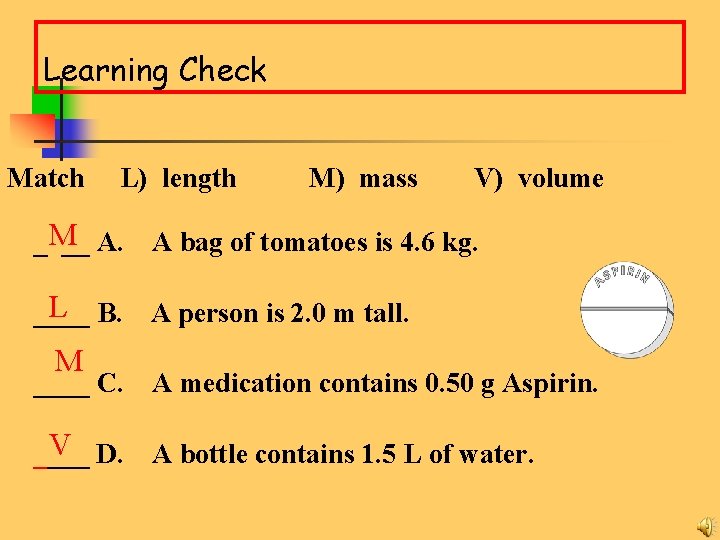
Learning Check Match L) length M) mass V) volume ____ A. A bag of tomatoes is 4. 6 kg. ____ B. A person is 2. 0 m tall. ____ C. A medication contains 0. 50 g Aspirin. ____ D. A bottle contains 1. 5 L of water.

Learning Check Match L) length M) mass V) volume _M __ A. A bag of tomatoes is 4. 6 kg. L B. ____ A person is 2. 0 m tall. M ____ C. A medication contains 0. 50 g Aspirin. V D. ____ A bottle contains 1. 5 L of water.

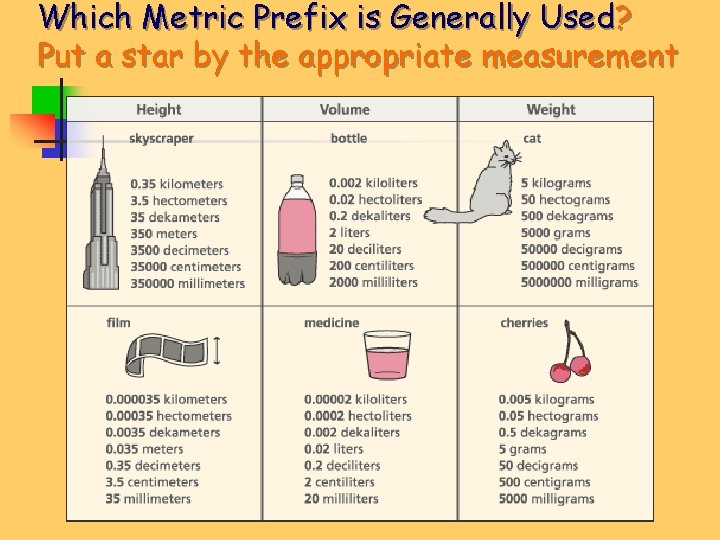
Which Metric Prefix is Generally Used? Put a star by the appropriate measurement

Which Metric Prefix is Generally Used?

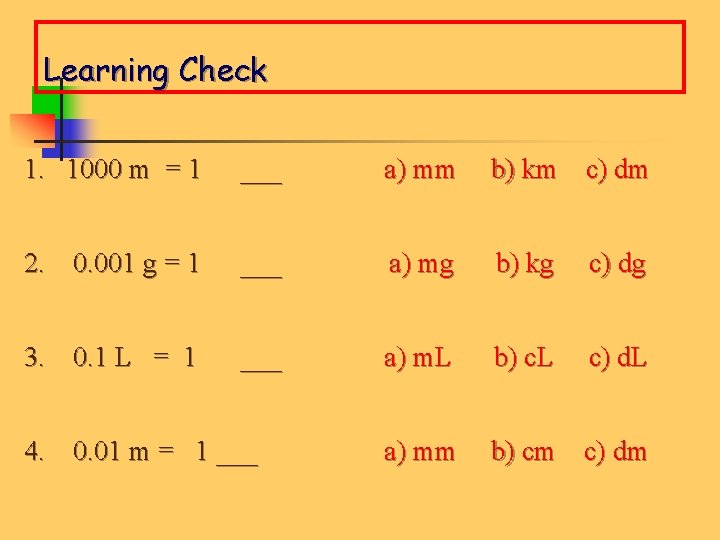
Learning Check 1. 1000 m = 1 ___ a) mm b) km c) dm 2. 0. 001 g = 1 ___ a) mg b) kg c) dg 3. 0. 1 L = 1 ___ a) m. L b) c. L c) d. L a) mm b) cm c) dm 4. 0. 01 m = 1 ___

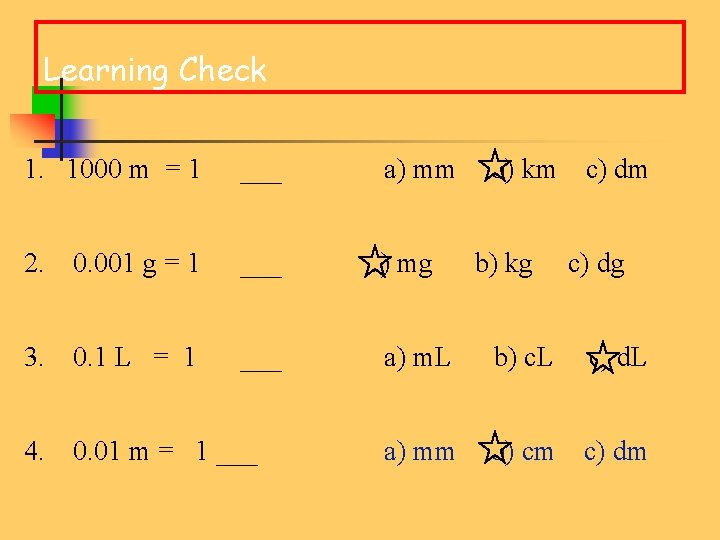
Learning Check 1. 1000 m = 1 ___ 2. 0. 001 g = 1 ___ 3. 0. 1 L = 1 ___ 4. 0. 01 m = 1 ___ a) mm a) mg b) km b) kg c) dm c) dg a) m. L b) c. L c) d. L a) mm b) cm c) dm

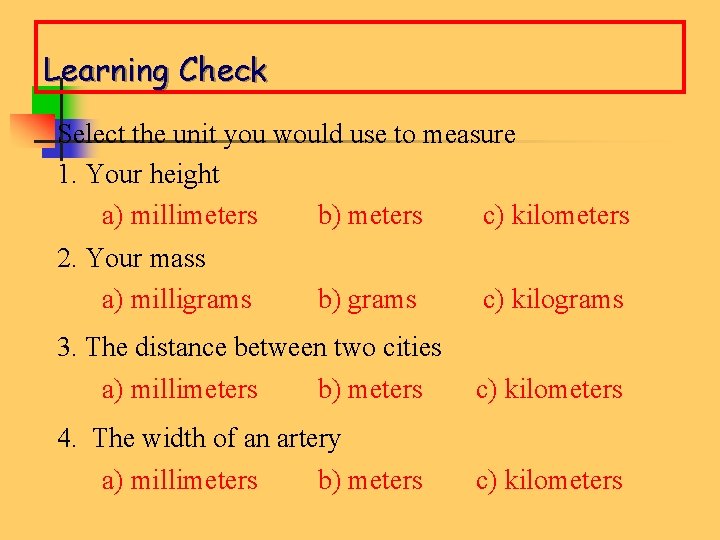
Learning Check Select the unit you would use to measure 1. Your height a) millimeters b) meters c) kilometers 2. Your mass a) milligrams b) grams c) kilograms 3. The distance between two cities a) millimeters b) meters c) kilometers 4. The width of an artery a) millimeters b) meters c) kilometers

Learning Check Select the unit you would use to measure 1. Your height a) millimeters b) meters c) kilometers 2. Your mass a) milligrams b) grams c) kilograms 3. The distance between two cities a) millimeters b) meters c) kilometers 4. The width of an artery a) millimeters b) meters c) kilometers

Complete 1 -10 metric measurement worksheet