Chapter 5 Quantum Theory and Electron Configurations QuantumMechanical












- Slides: 45

Chapter 5

Quantum Theory and Electron Configurations

Quantum-Mechanical Model of the Atom • Since the Bohr model had a very limited use, a new and very different model of the atom exists • The Quantum Mechanical Model (1926) contains: F Quantum energy levels F Dual wave/particle nature of electrons F Electron clouds • In the new model, don’t know exactly where electrons are - only know probabilities of where they could be

Heisenberg Uncertainty Principle • Heisenberg Uncertainty Principle = impossible to know both the velocity (or momentum) and position of an electron at the same time

The Quantum Mechanical Model Chapter 5

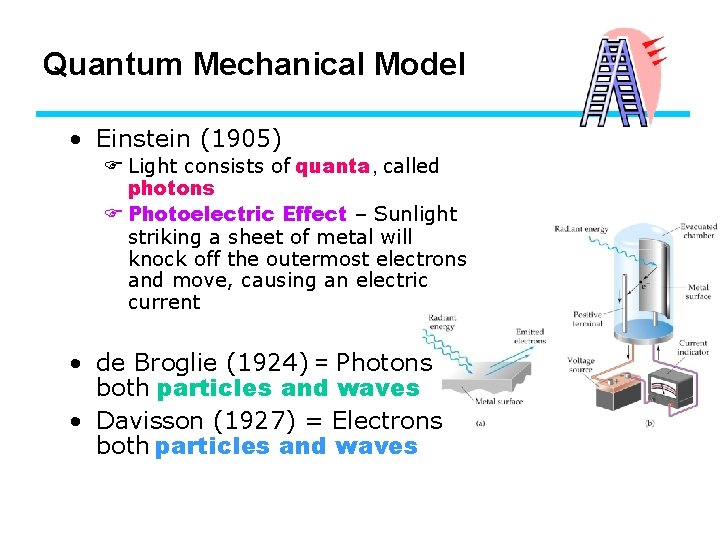
Quantum Mechanical Model • Einstein (1905) F Light consists of quanta, called photons F Photoelectric Effect – Sunlight striking a sheet of metal will knock off the outermost electrons and move, causing an electric current • de Broglie (1924) = Photons both particles and waves • Davisson (1927) = Electrons both particles and waves

Quantum-Mechanical Model of the Atom • Orbital = region around nucleus where an electron with a given energy level will probably (90%) be found • Four kinds of orbitals F s - spherical in shape, lowest orbital for every energy level F p - dumbbell shaped, second orbital F d - complex “flower” shape, third orbital F f - very complex shape, highest orbital

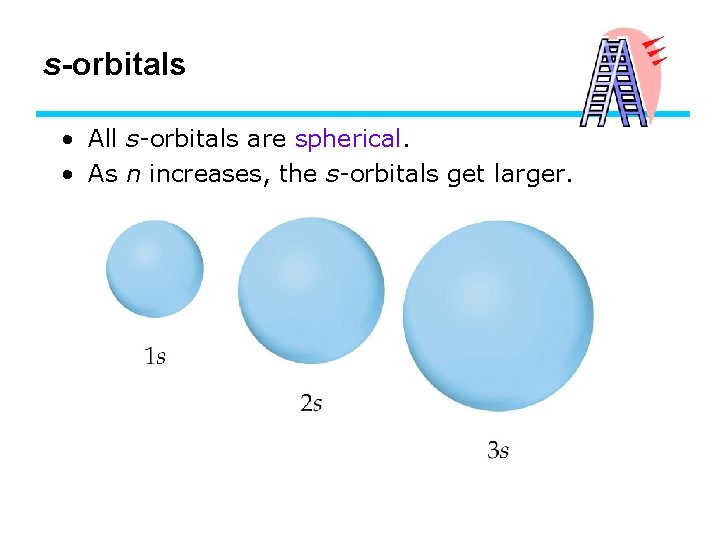
s-orbitals • All s-orbitals are spherical. • As n increases, the s-orbitals get larger.

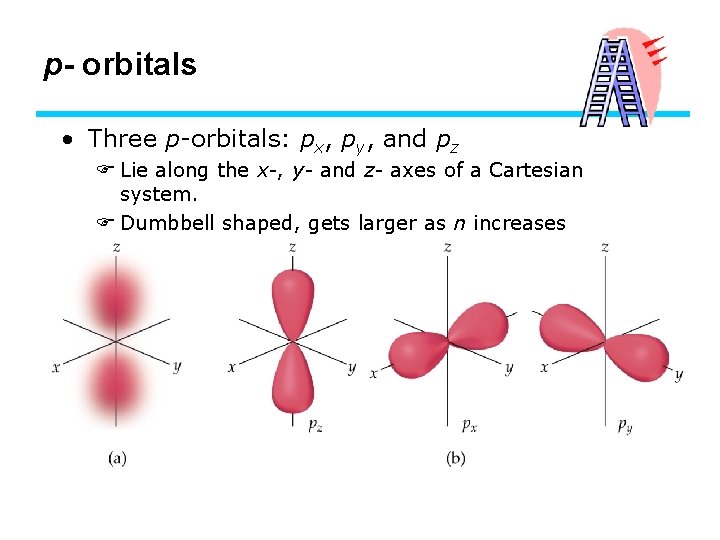
p- orbitals • Three p-orbitals: px, py, and pz F Lie along the x-, y- and z- axes of a Cartesian system. F Dumbbell shaped, gets larger as n increases

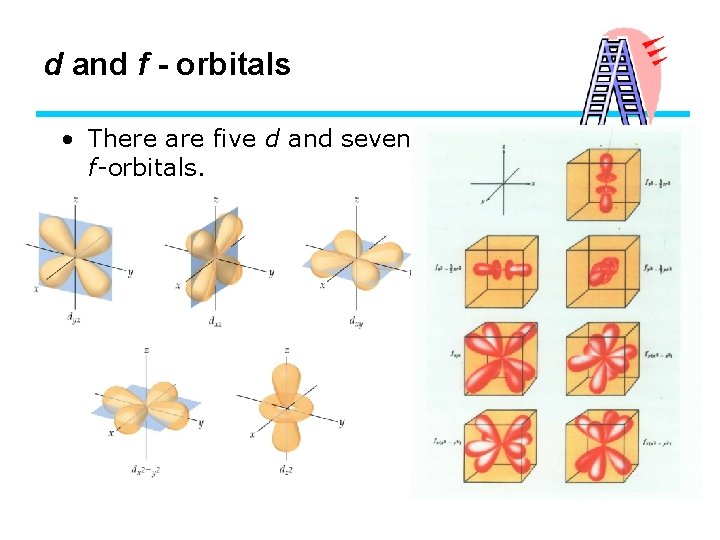
d and f - orbitals • There are five d and seven f-orbitals.


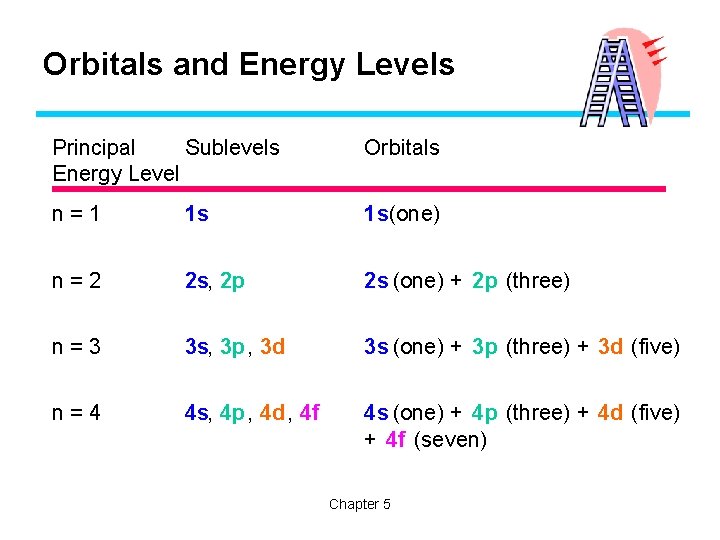
Quantum Mechanical Model • Principle Energy Levels (n) F Labeled from 1 -7 F First energy level is n=1 F Contains sublevels (s, p, d and f) • Each energy level contains the number of sublevels equal to it’s value for n – If n=3, there are three sublevels

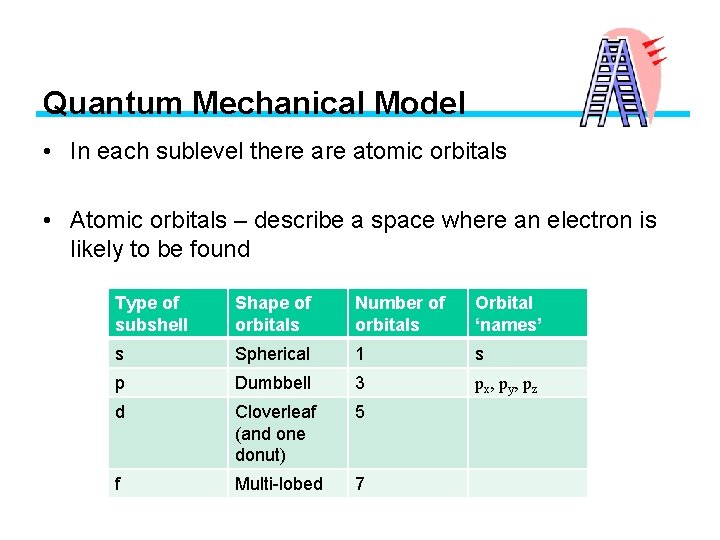
Quantum Mechanical Model • In each sublevel there atomic orbitals • Atomic orbitals – describe a space where an electron is likely to be found Type of subshell Shape of orbitals Number of orbitals Orbital ‘names’ s Spherical 1 s p Dumbbell 3 px, py, pz d Cloverleaf (and one donut) 5 f Multi-lobed 7

Quantum Mechanical Model • Each orbital can contain two electrons. • Since negative-negative repel, these electrons occupy the orbital with opposite spins.

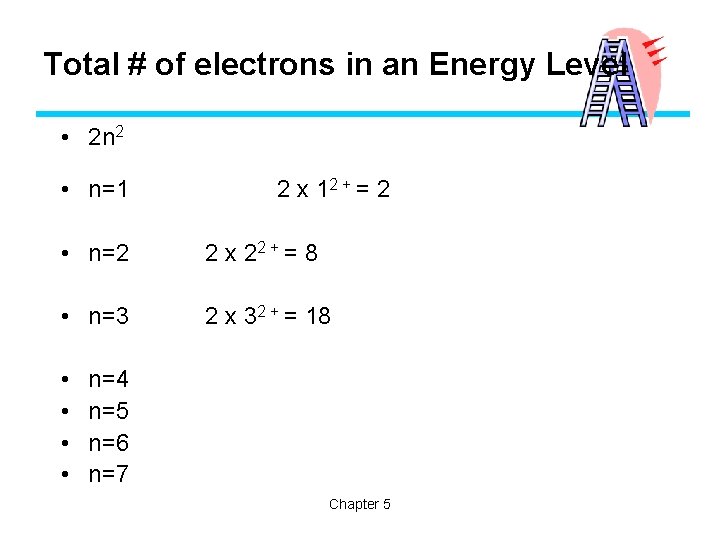
Quantum Mechanical Model • The total number of orbitals of an energy level is n 2. F For the third principle energy level, n=3, which means there are 9 orbitals • These orbitals are 3 s, 3 px, 3 py, 3 pz and the 5 d orbitals • Remember, we no longer think of orbitals as concentric circles, but we can say that n=4 extends farther from the nucleus than n=1.

Valence Electrons • Only those electrons in the highest principle energy level

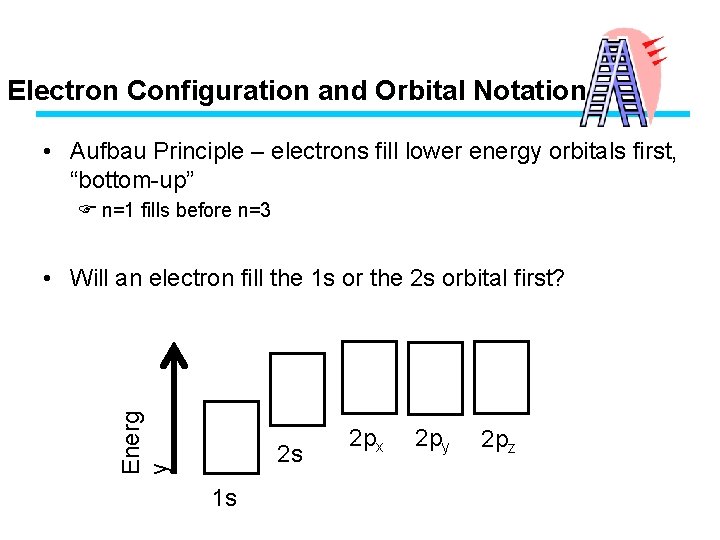
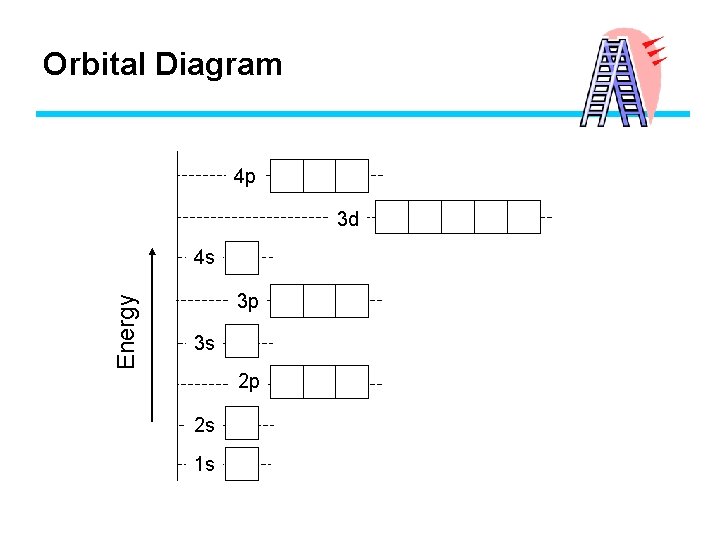
Electron Configuration and Orbital Notation • Aufbau Principle – electrons fill lower energy orbitals first, “bottom-up” F n=1 fills before n=3 Energ y • Will an electron fill the 1 s or the 2 s orbital first? 2 s 1 s 2 px 2 py 2 pz

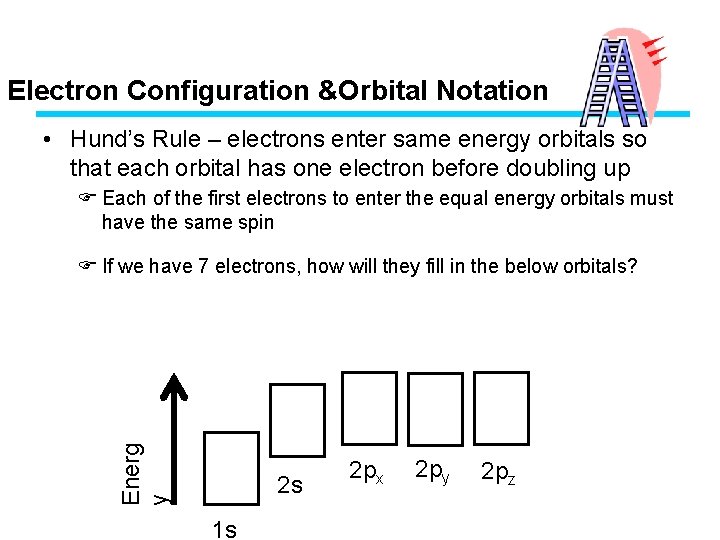
Electron Configuration &Orbital Notation • Hund’s Rule – electrons enter same energy orbitals so that each orbital has one electron before doubling up F Each of the first electrons to enter the equal energy orbitals must have the same spin Energ y F If we have 7 electrons, how will they fill in the below orbitals? 2 s 1 s 2 px 2 py 2 pz

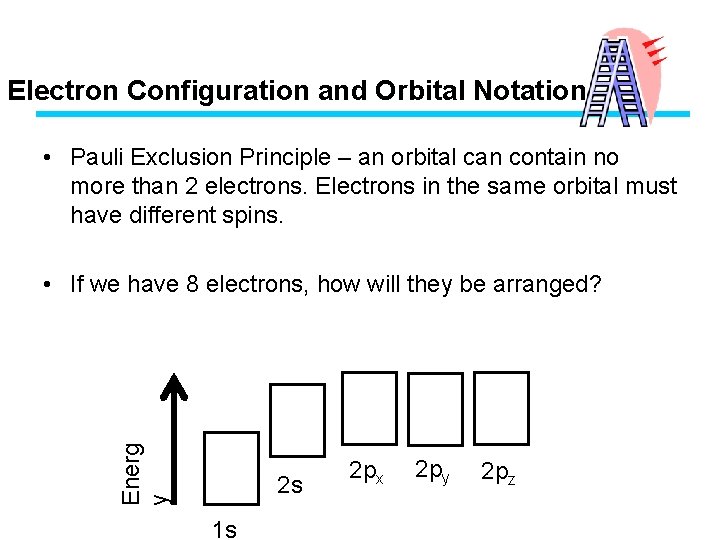
Electron Configuration and Orbital Notation • Pauli Exclusion Principle – an orbital can contain no more than 2 electrons. Electrons in the same orbital must have different spins. Energ y • If we have 8 electrons, how will they be arranged? 2 s 1 s 2 px 2 py 2 pz




Apartment Analogy • • Atom is the building Floors are energy levels Rooms are orbitals Only two people per room

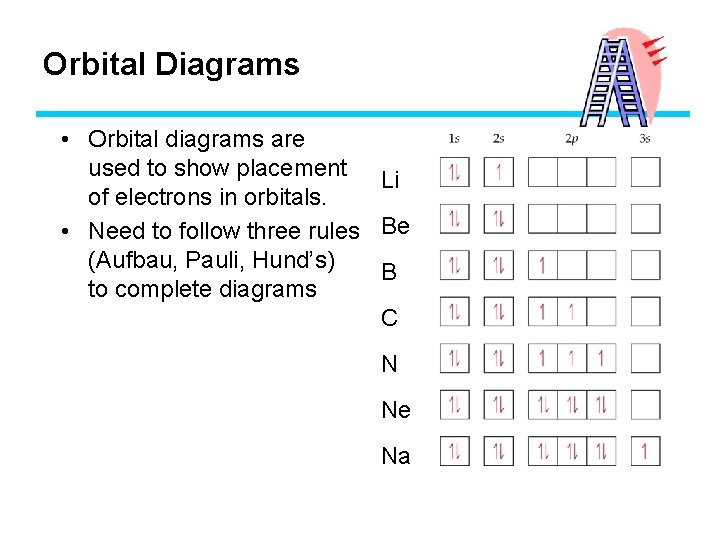
Orbital Diagrams • Draw each orbital as a box. • Each electron is represented using an arrow. F Up arrows – clockwise spin F Down arrows – counter-clockwise spin • Determine the total number of electrons involved. • Start with the lowest energy level (1 s) and start filling in the boxes according the rules we just learned.

Transition Metal Exceptions • Can move from the highest filled s orbital to create a fully filled, or half filled d or f • TRANSITION METAL EXCEPTIONS

Total # of electrons in an Energy Level • 2 n 2 • n=1 2 x 12 + = 2 • n=2 2 x 22 + = 8 • n=3 2 x 32 + = 18 • • n=4 n=5 n=6 n=7 Chapter 5

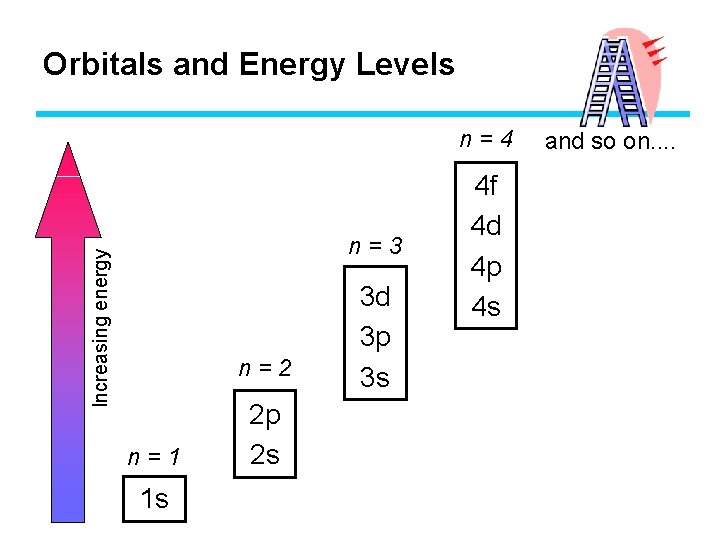
Orbitals and Energy Levels Principal Sublevels Energy Level Orbitals n=1 1 s 1 s(one) n=2 2 s, 2 p 2 s (one) + 2 p (three) n=3 3 s, 3 p , 3 d 3 s (one) + 3 p (three) + 3 d (five) n=4 4 s, 4 p, 4 d, 4 f 4 s (one) + 4 p (three) + 4 d (five) + 4 f (seven) Chapter 5

Summary # of Max shapes electrons s p d f Chapter 5 Starts at energy level

Orbitals and Energy Levels n=4 Increasing energy n=3 n=2 n=1 1 s 2 p 2 s 3 d 3 p 3 s 4 f 4 d 4 p 4 s and so on. .

Orbital Diagrams • Orbital diagrams are used to show placement Li of electrons in orbitals. • Need to follow three rules Be (Aufbau, Pauli, Hund’s) B to complete diagrams C N Ne Na

Orbital Diagram 4 p 3 d Energy 4 s 3 p 3 s 2 p 2 s 1 s

Electron Configuration • Let’s determine the electron configuration for Phosphorus • Need to account for 15 electrons Chapter 5

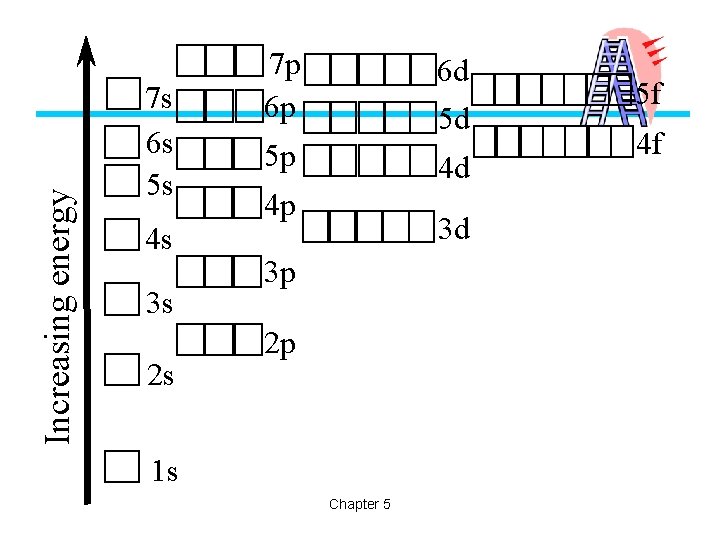
Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 7 p 6 p 6 d 5 d 5 p 4 d 4 p 3 d 3 p 2 p 1 s Chapter 5 5 f 4 f

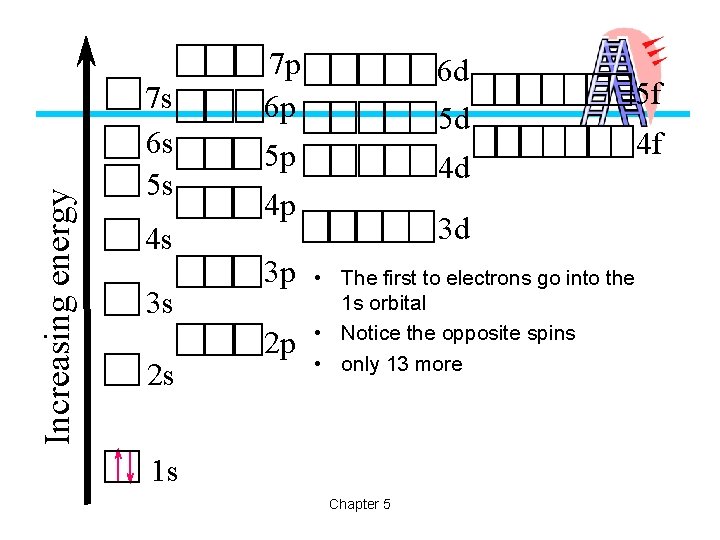
Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 7 p 6 p 6 d 5 d 5 p 4 d 4 p 3 p 2 p 3 d • The first to electrons go into the 1 s orbital • Notice the opposite spins • only 13 more 1 s Chapter 5 5 f 4 f

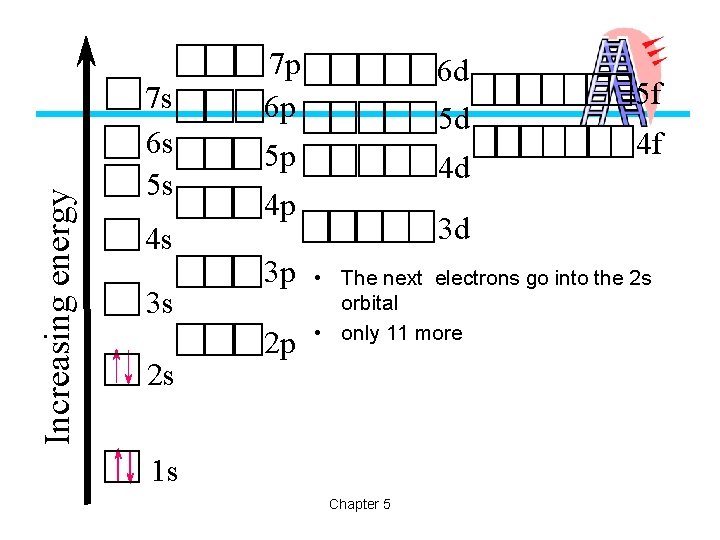
Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 7 p 6 p 6 d 5 d 5 p 4 d 4 p 3 p 2 p 5 f 4 f 3 d • The next electrons go into the 2 s orbital • only 11 more 1 s Chapter 5

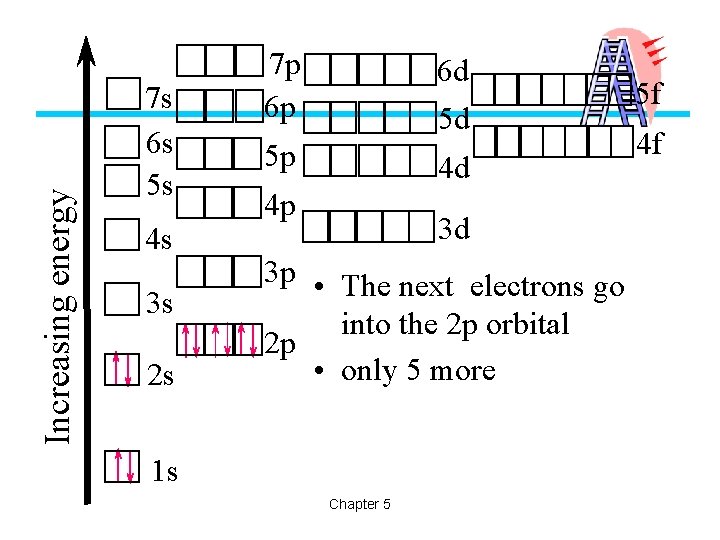
Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 7 p 6 p 6 d 5 d 5 p 4 d 4 p 3 d 3 p • The next electrons go into the 2 p orbital 2 p • only 5 more 1 s Chapter 5 5 f 4 f

Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 7 p 6 p 6 d 5 d 5 p 4 d 4 p 3 d 3 p • The next electrons go into the 3 s orbital 2 p • only 3 more 1 s Chapter 5 5 f 4 f

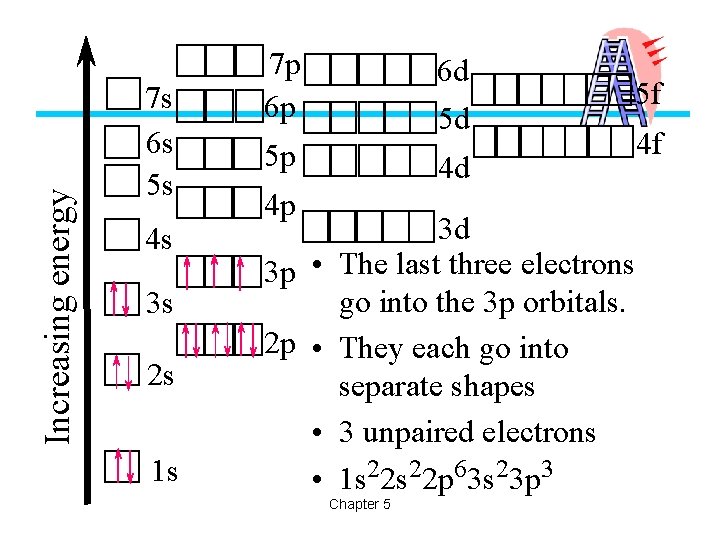
Increasing energy 7 s 6 s 5 s 4 s 3 s 2 s 1 s 7 p 6 p 6 d 5 d 5 p 5 f 4 d 4 p 3 p • 2 p • • • 3 d The last three electrons go into the 3 p orbitals. They each go into separate shapes 3 unpaired electrons 1 s 22 p 63 s 23 p 3 Chapter 5 4 f

Writing Electron Configuration • Determine the total number of electrons. • Write the principle energy level number as a coefficient, the letter for the subshell, and an exponent to represent the number of electrons in the subshell. • He: 1 s 2

The Kernel (Noble Gas) Notation • Determine the total number of electrons • Find the previous noble gas and put its symbol in brackets • Write the configuration from that noble gas forward as usual

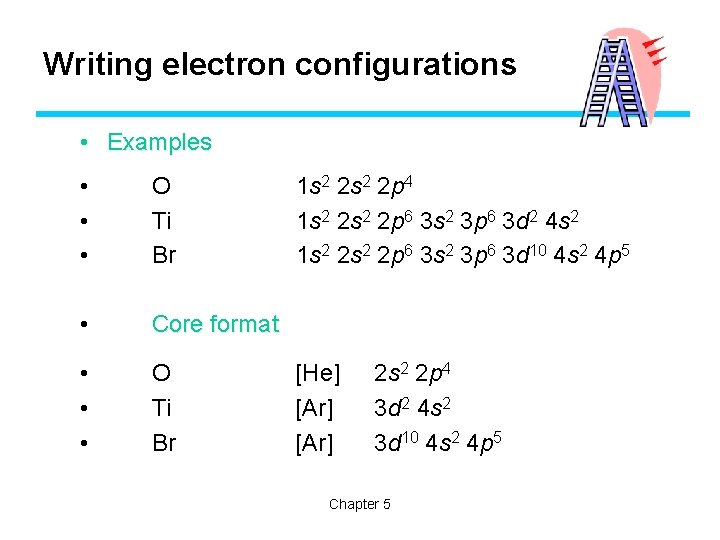
Writing electron configurations • Examples • • • O Ti Br • Core format • • • O Ti Br 1 s 2 2 p 4 1 s 2 2 p 6 3 s 2 3 p 6 3 d 2 4 s 2 1 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 5 [He] [Ar] 2 s 2 2 p 4 3 d 2 4 s 2 3 d 10 4 s 2 4 p 5 Chapter 5


Quantum Numbers • Each electron can be described by four numbers unique to that electron (like a fingerprint) • “n” – the principal quantum # describes the principal energy level, n=1, 2, 3…, 7 • “l” – describes the shape of subshell F s subshell = 0 F p subshell = 1 F d subshell = 2 F f subshell = 3 • “m” –describes the orientation , m = -l…. 0…. +l • “s” – describes the spin, s=1/2 or -1/2


Quantum Numbers • Example: Look at carbon’s orbital diagram which contains 6 electrons. What are the quantum #s for the last electron to be filled? • Example: Look at Vanadium’s Kernel notation. Do the orbital diagram for only the valence electrons. What are the quantum #’s for the second to last electron to be filled?