The Quantum Model Part II Electron Configurations Quantum











- Slides: 27

The Quantum Model : Part II Electron Configurations

Quantum Numbers Describe the properties of atomic orbitals and the electrons that occupy them… Quantum Number Describes l Principle main E. level l Angular Momentum shape of Orbital l Magnetic orientation of orbital l Spin orientation of electron l

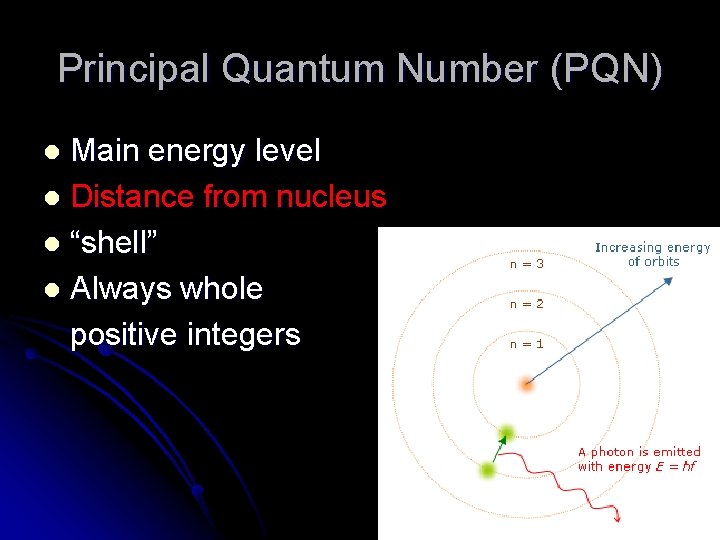
Principal Quantum Number (PQN) Main energy level l Distance from nucleus l “shell” l Always whole positive integers l

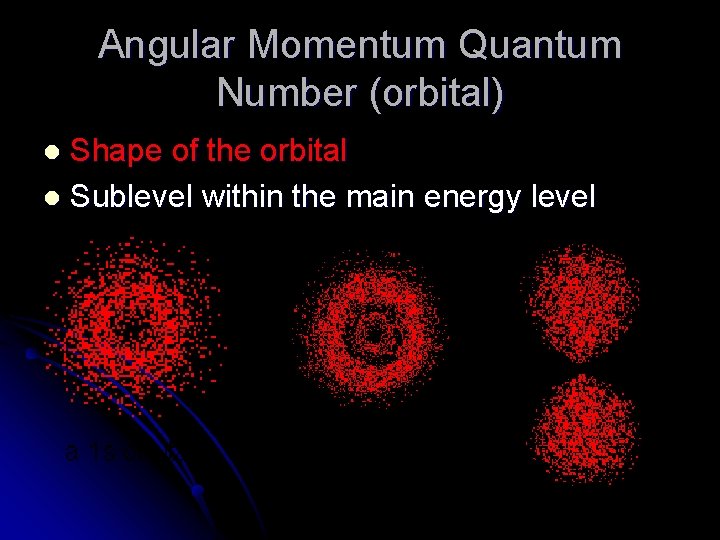
Angular Momentum Quantum Number (orbital) Shape of the orbital l Sublevel within the main energy level l

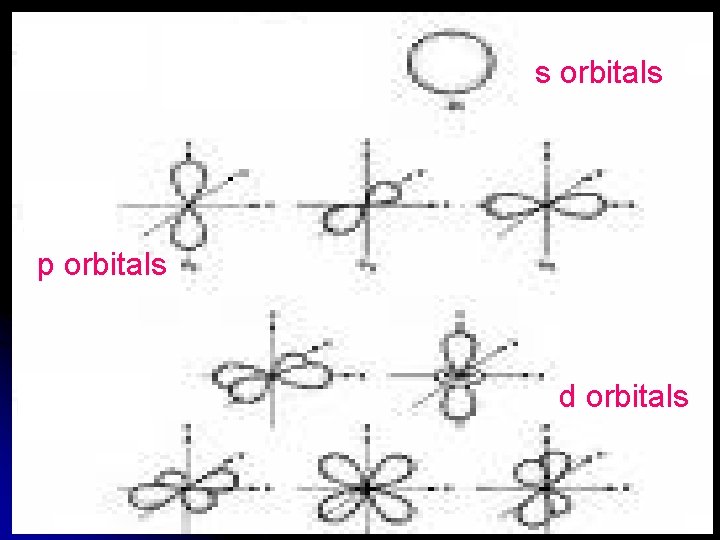
s orbitals p orbitals d orbitals

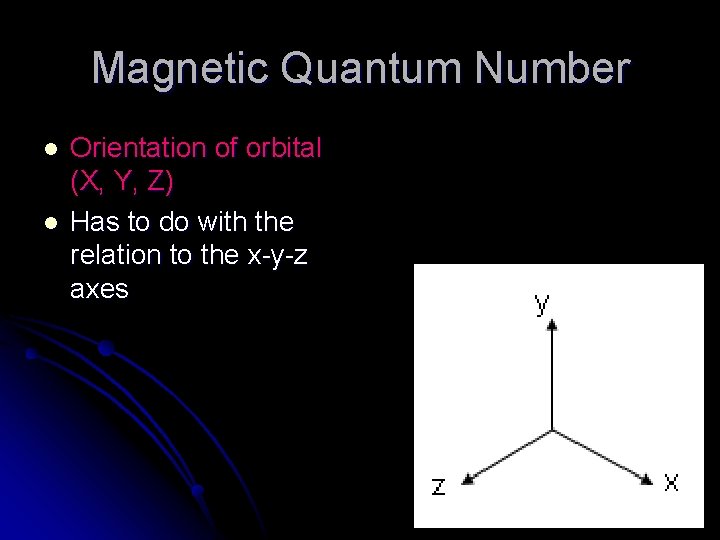
Magnetic Quantum Number l l Orientation of orbital (X, Y, Z) Has to do with the relation to the x-y-z axes

s Orbitals have only one possible orientation.

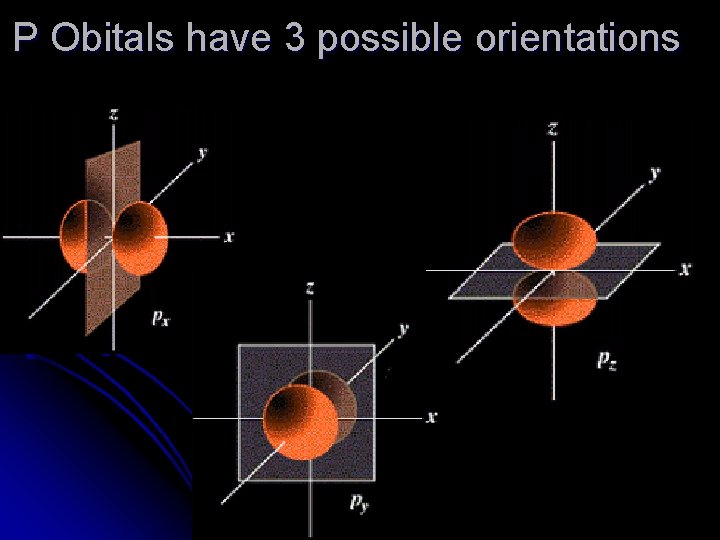
P Obitals have 3 possible orientations

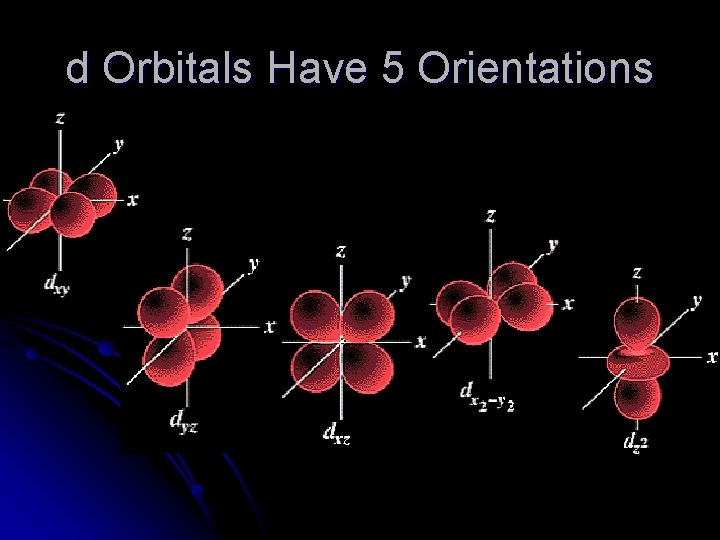
d Orbitals Have 5 Orientations

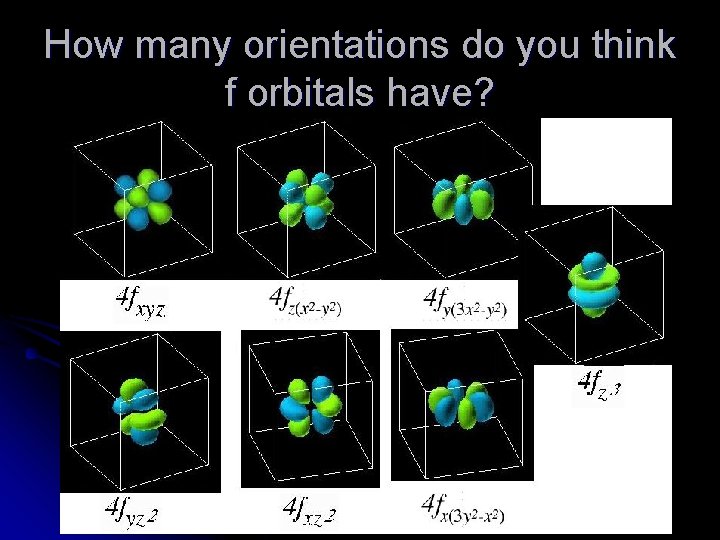
How many orientations do you think f orbitals have?

SPIN QUANTUM NUMBER The orientation of the electron in the orbital. l “Direction of its spin”. l +1/2 or -1/2 l

Atomic Orbitals and Quantum Numbers Use your notes to make a chart showing important information about each of the four quantum numbers. Make chart for only n=1, 2, 3, 4 l Principal Quantum Number l Angular Momentum Quantum Number l Magnetic Quantum Number l Spin Quantum Number

HOMEWORK l Read pages 143 – 146 l Answer questions #2 -4 on page 146 l Study for Quiz #2 next class

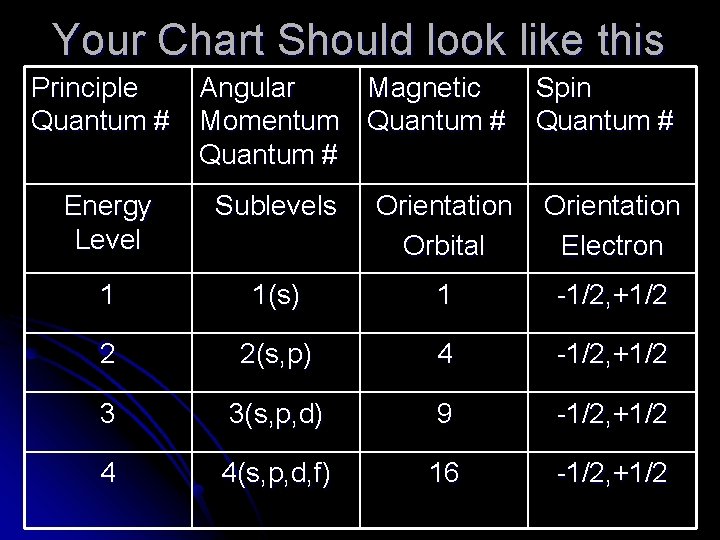
Your Chart Should look like this Principle Quantum # Angular Magnetic Momentum Quantum # Spin Quantum # Energy Level Sublevels Orientation Orbital Orientation Electron 1 1(s) 1 -1/2, +1/2 2 2(s, p) 4 -1/2, +1/2 3 3(s, p, d) 9 -1/2, +1/2 4 4(s, p, d, f) 16 -1/2, +1/2

Electron Configuration Show the placement of electrons in specific levels, sublevels and orbitals. l Make use of the four quantum numbers and a few simple rules. l Each orbital can only hold a maximum of 2 electrons l Before we learn the rules lets look at a few examples! l

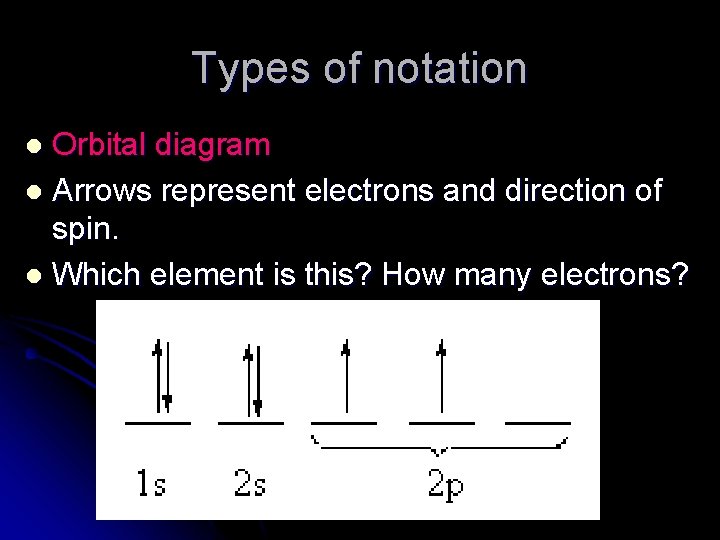
Types of notation Orbital diagram l Arrows represent electrons and direction of spin. l Which element is this? How many electrons? l

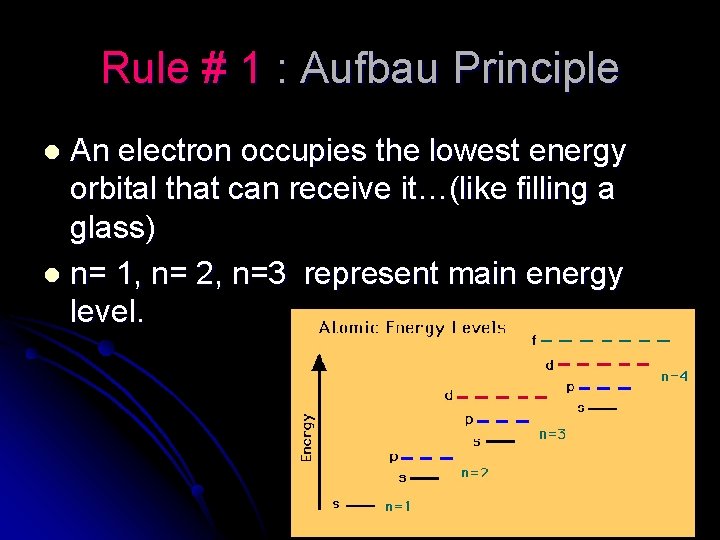
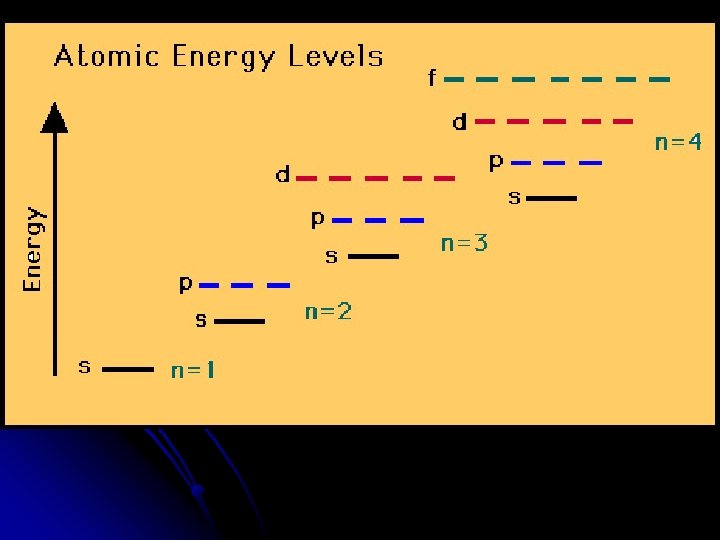
Rule # 1 : Aufbau Principle An electron occupies the lowest energy orbital that can receive it…(like filling a glass) l n= 1, n= 2, n=3 represent main energy level. l


Organize the levels and sublevels Main energy level, 1, 2, 3 etc. ) l How many sublevels exist at each main energy level? l What are the sublevels at each main energy level? l 1__ 2__ 3__ 4__ 5__ 5__

Organize the levels and sublevels l Principle Quantum Number (main energy level) ↓ 1 s 2 s 3 s 4 s 5 s 2 p 3 p 4 p 5 p 3 d 4 d 5 d 4 f 5 f 5 g

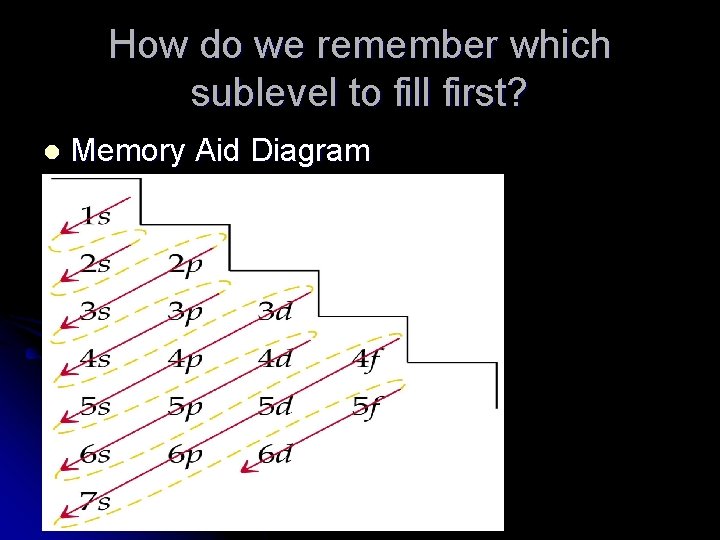
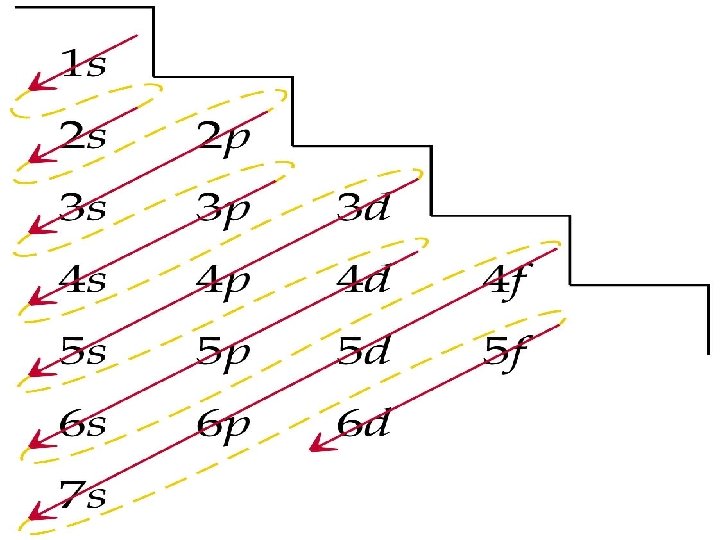
How do we remember which sublevel to fill first? l Memory Aid Diagram


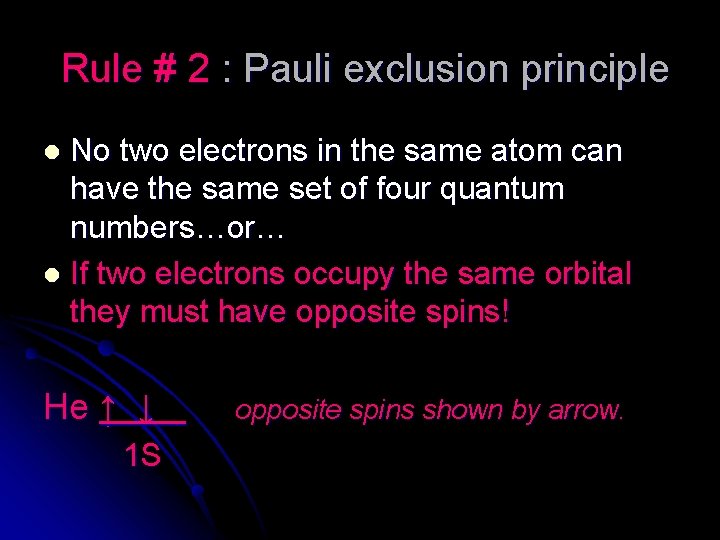
Rule # 2 : Pauli exclusion principle No two electrons in the same atom can have the same set of four quantum numbers…or… l If two electrons occupy the same orbital they must have opposite spins! l He ↑ ↓ 1 S opposite spins shown by arrow.

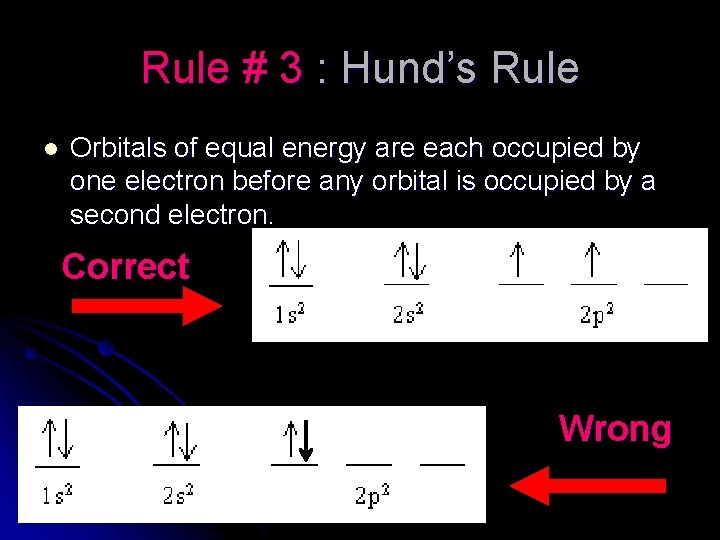
Rule # 3 : Hund’s Rule l Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron. Correct Wrong

Electron configuration notation 1 S 2 2 P 2 What does each character represent? Which element is this?

The Three Little Rules! l l l An electron occupies the lowest energy orbital that can receive it…(like filling a glass) If two electrons occupy the same orbital they must have opposite spins! Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron.

Homework l Read pg 147 – 153 Questions: pg 156 #6 l pg 157 #20, 22, 23, 27, 28 l l Quiz Wed 12/3 l Spectroscope and Flame Lab due 12/3