Chapter 2 Part A Chemistry Comes Alive Matter








- Slides: 62

Chapter 2 Part A Chemistry Comes Alive

Matter Anything that has mass and occupies space States of matter: 1. Solid—definite shape and volume 2. Liquid—definite volume, changeable shape 3. Gas—changeable shape and volume

Energy Capacity to do work or put matter into motion Types of energy: ◦ Kinetic—energy in action ◦ Potential—stored (inactive) energy PLAY Animation: Energy Concepts

Forms of Energy Chemical energy—stored in bonds of chemical substances Electrical energy—results from movement of charged particles Mechanical energy—directly involved in moving matter Radiant or electromagnetic energy—exhibits wavelike properties (i. e. , visible light, ultraviolet light, and X-rays)

Energy Form Conversions Energy can neither be created nor destroyed (1 st law of thermodynamics) Energy may be converted from one form to another Conversion is inefficient because some energy is “lost” as heat

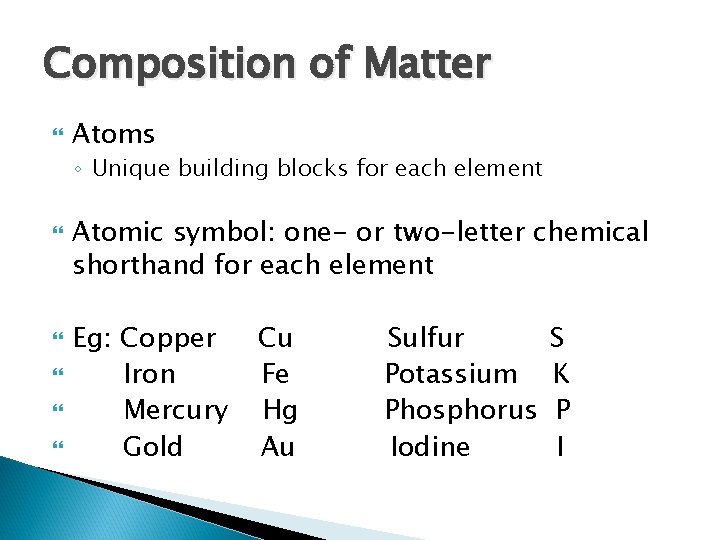
Composition of Matter Elements ◦ Cannot be broken down by ordinary chemical means ◦ Each has unique properties: Physical properties Are detectable with our senses, or are measurable Chemical properties How atoms interact (bond) with one another

Composition of Matter Atoms ◦ Unique building blocks for each element Atomic symbol: one- or two-letter chemical shorthand for each element Eg: Copper Iron Mercury Gold Cu Fe Hg Au Sulfur Potassium Phosphorus Iodine S K P I

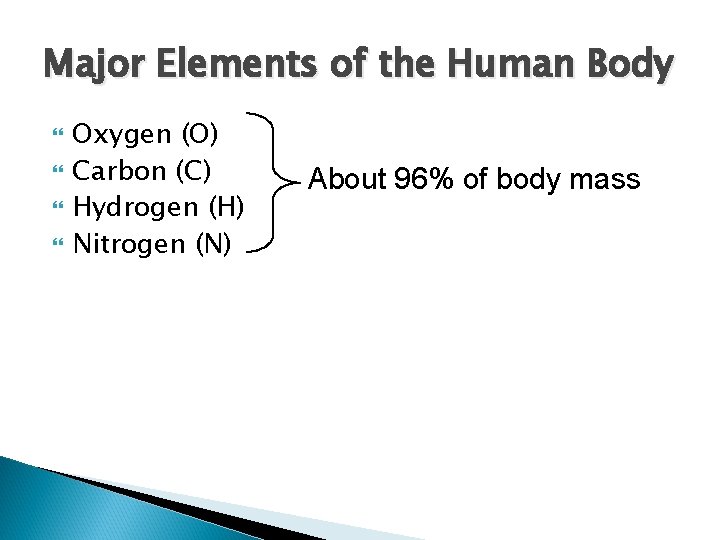
Major Elements of the Human Body Oxygen (O) Carbon (C) Hydrogen (H) Nitrogen (N) About 96% of body mass

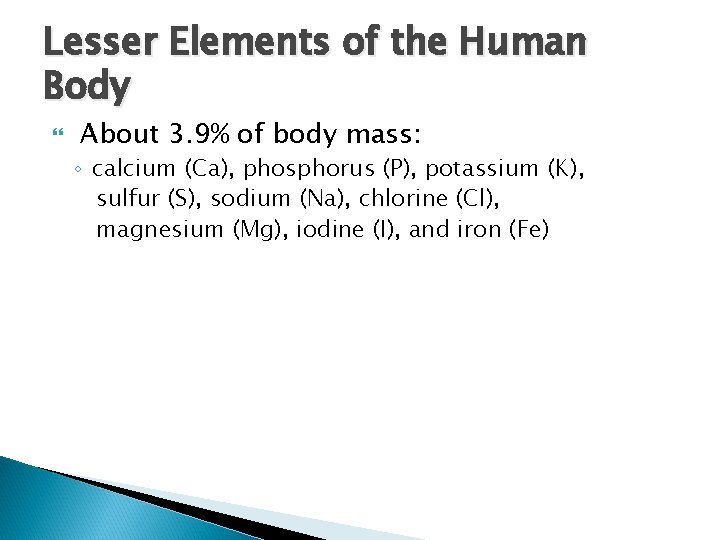
Lesser Elements of the Human Body About 3. 9% of body mass: ◦ calcium (Ca), phosphorus (P), potassium (K), sulfur (S), sodium (Na), chlorine (Cl), magnesium (Mg), iodine (I), and iron (Fe)

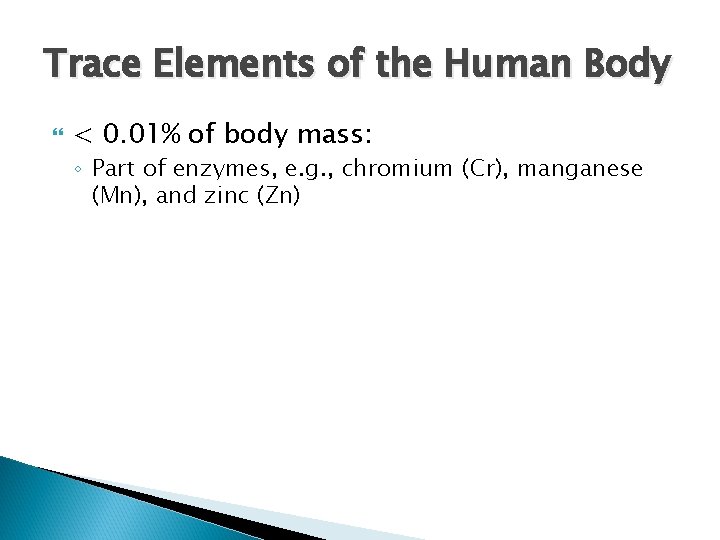
Trace Elements of the Human Body < 0. 01% of body mass: ◦ Part of enzymes, e. g. , chromium (Cr), manganese (Mn), and zinc (Zn)

Atomic Structure Determined by numbers of subatomic particles Nucleus consists of neutrons and protons

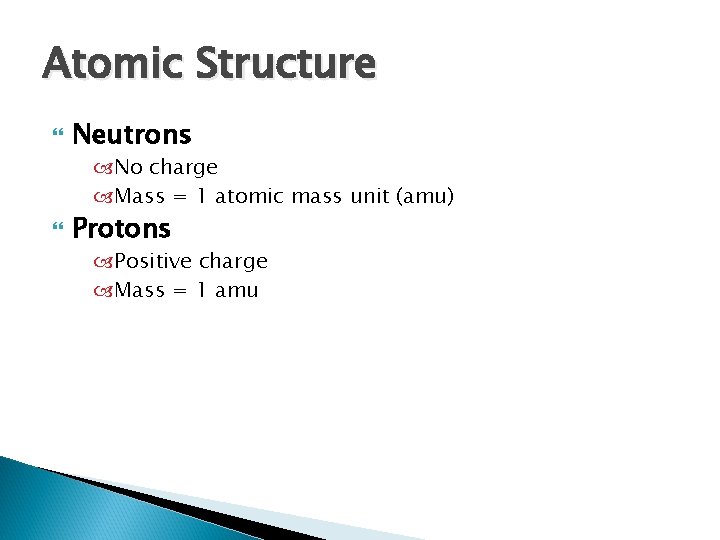
Atomic Structure Neutrons No charge Mass = 1 atomic mass unit (amu) Protons Positive charge Mass = 1 amu

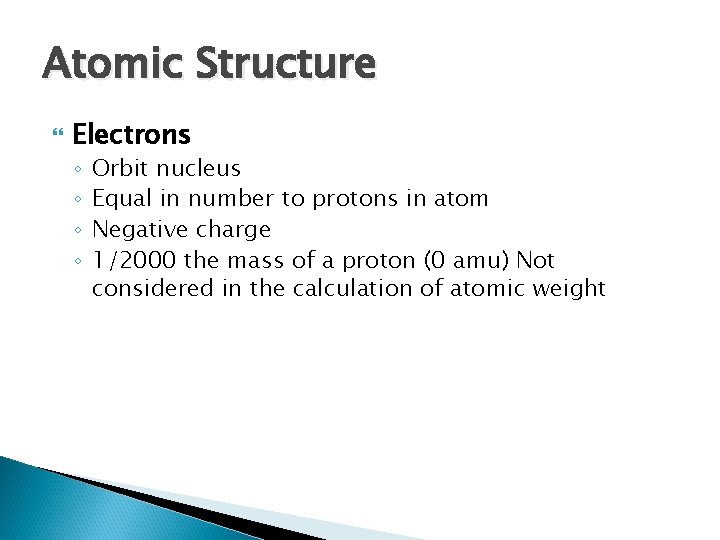
Atomic Structure Electrons ◦ ◦ Orbit nucleus Equal in number to protons in atom Negative charge 1/2000 the mass of a proton (0 amu) Not considered in the calculation of atomic weight

Model of the Atom Planetary model ◦ Depicts fixed circular electron paths ◦ Useful for illustrations (as in the text)

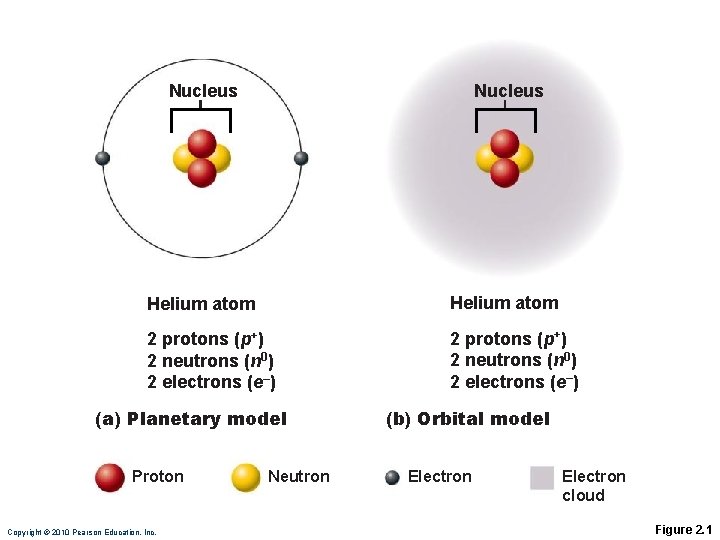
Nucleus Helium atom 2 protons (p+) 2 neutrons (n 0) 2 electrons (e–) (a) Planetary model Proton Copyright © 2010 Pearson Education, Inc. Neutron (b) Orbital model Electron cloud Figure 2. 1

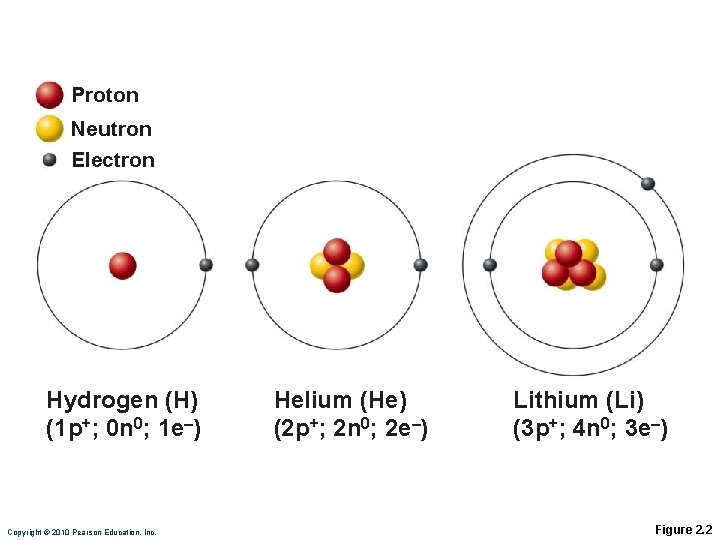
Identifying Elements Atoms of different elements contain different numbers of subatomic particles ◦ Compare hydrogen, helium and lithium (next slide)

Proton Neutron Electron Hydrogen (H) (1 p+; 0 n 0; 1 e–) Copyright © 2010 Pearson Education, Inc. Helium (He) (2 p+; 2 n 0; 2 e–) Lithium (Li) (3 p+; 4 n 0; 3 e–) Figure 2. 2

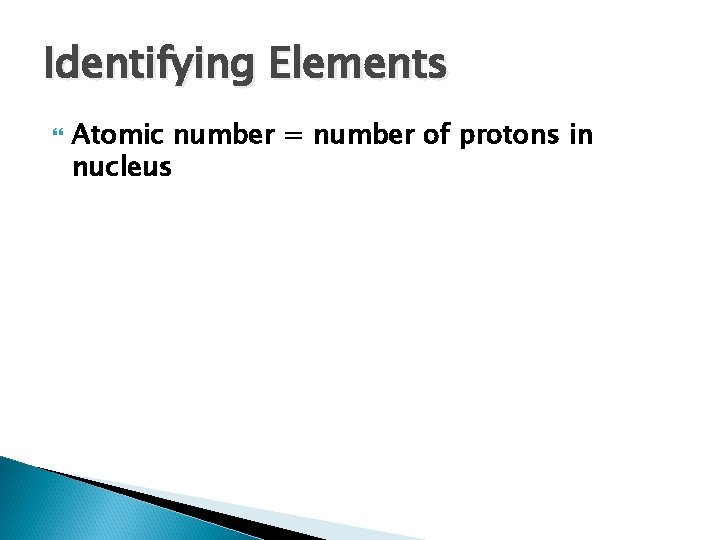
Identifying Elements Atomic number = number of protons in nucleus

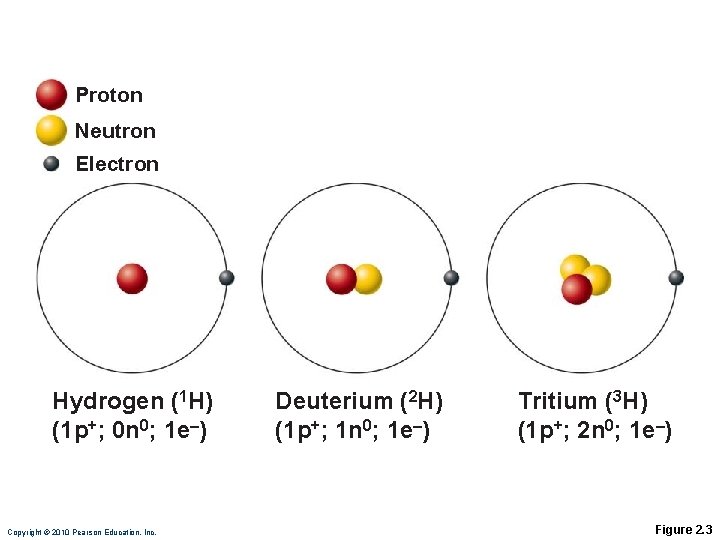
Identifying Elements Atomic weight = mass of the protons and neutrons

Proton Neutron Electron Hydrogen (1 H) (1 p+; 0 n 0; 1 e–) Copyright © 2010 Pearson Education, Inc. Deuterium (2 H) (1 p+; 1 n 0; 1 e–) Tritium (3 H) (1 p+; 2 n 0; 1 e–) Figure 2. 3

Radioisotopes Spontaneous decay (radioactivity) Similar chemistry to stable isotopes Can be detected with scanners

Radioisotopes Valuable tools for biological research and medicine Cause damage to living tissue: ◦ Useful against localized cancers ◦ Radon from uranium decay causes lung cancer

Molecules and Compounds Most atoms combine chemically with other atoms to form molecules and compounds ◦ Molecule—two or more atoms of same element bonded together (e. g. , H + H = H 2 ) ◦ Compound—two or more atoms of different elements bonded together (e. g. , C 6 H 12 O 6)

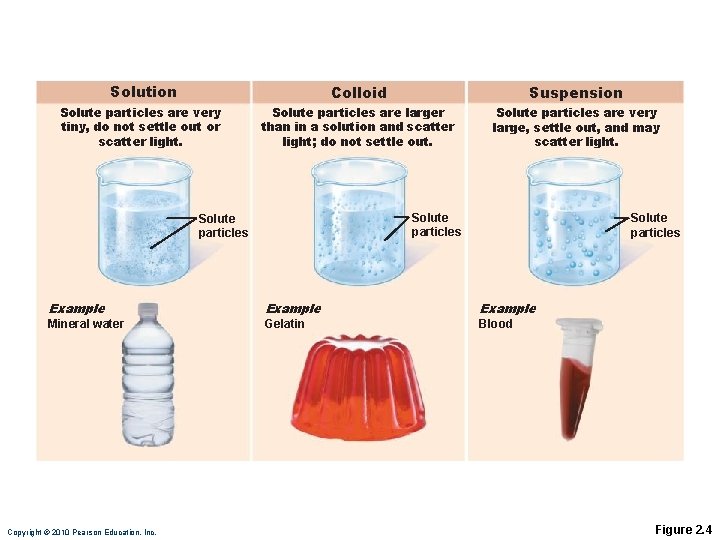
Mixtures Most matter exists as mixtures ◦ Two or more components physically intermixed Three types of mixtures ◦ Solutions ◦ Colloids ◦ Suspensions

Solutions are homogeneous mixtures Usually transparent, e. g. , atmospheric air or seawater ◦ Solvent Present in greatest amount, usually a liquid ◦ Solute(s) Present in smaller amounts

Colloids and Suspensions Colloids (emulsions) ◦ Heterogeneous translucent mixtures, e. g. , cytosol ◦ Large solute particles that do not settle out ◦ Undergo sol-gel transformations Suspensions: ◦ Heterogeneous mixtures, e. g. , blood ◦ Large visible solutes tend to settle out

Solution Colloid Suspension Solute particles are very tiny, do not settle out or scatter light. Solute particles are larger than in a solution and scatter light; do not settle out. Solute particles are very large, settle out, and may scatter light. Solute particles Example Mineral water Gelatin Blood Copyright © 2010 Pearson Education, Inc. Figure 2. 4

Mixtures vs. Compounds Mixtures ◦ No chemical bonding between components ◦ Can be separated physically, such as by straining or filtering ◦ Heterogeneous or homogeneous Compounds ◦ Can be separated only by breaking bonds ◦ All are homogeneous

Chemical Bonds Electrons occupy up to seven electron shells (energy levels) around nucleus Octet rule: Except for the first shell which is full with two electrons, atoms interact in a manner to have eight electrons in their outermost energy level (valence shell)

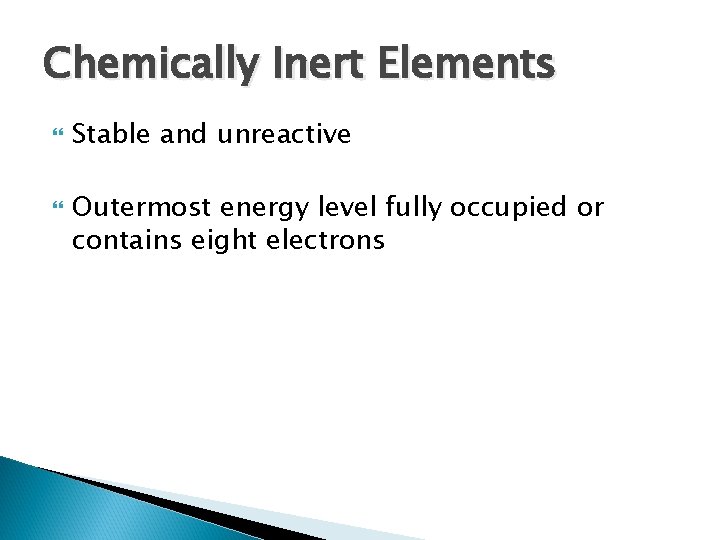
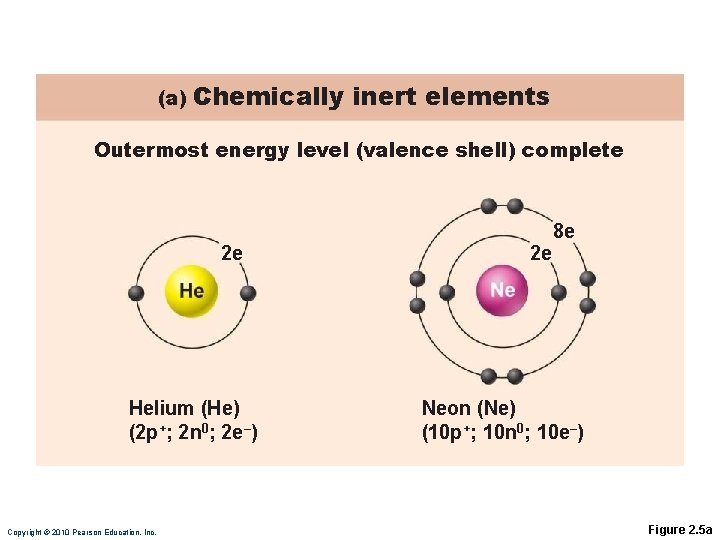
Chemically Inert Elements Stable and unreactive Outermost energy level fully occupied or contains eight electrons

(a) Chemically inert elements Outermost energy level (valence shell) complete 2 e Helium (He) (2 p+; 2 n 0; 2 e–) Copyright © 2010 Pearson Education, Inc. 2 e 8 e Neon (Ne) (10 p+; 10 n 0; 10 e–) Figure 2. 5 a

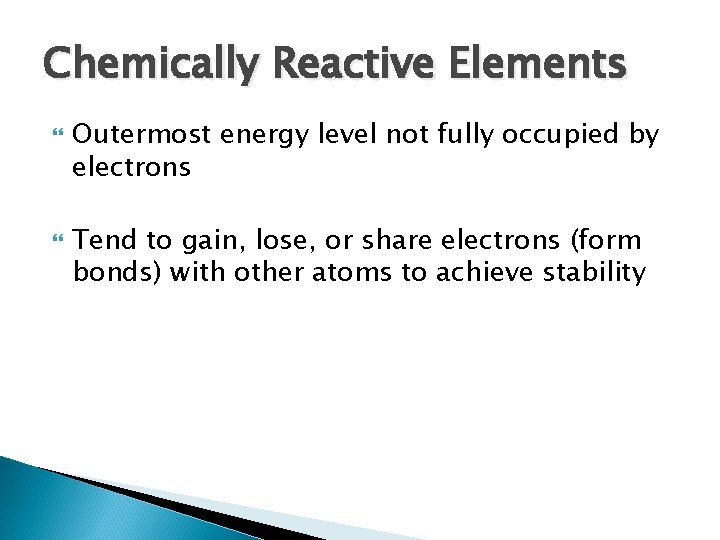
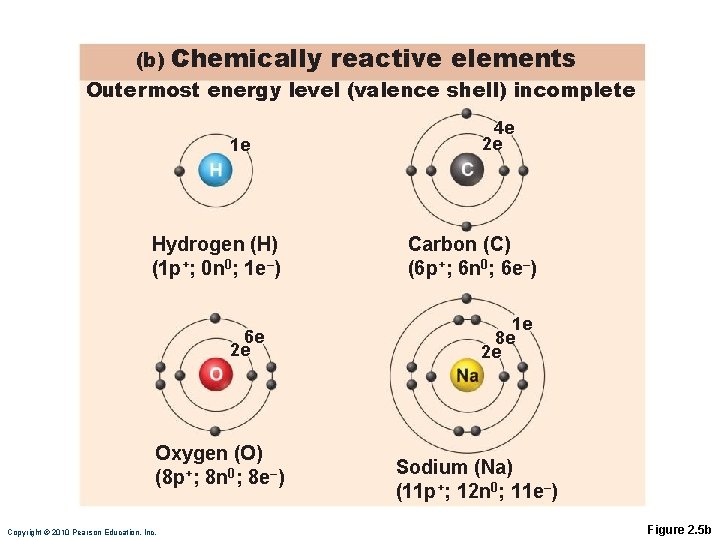
Chemically Reactive Elements Outermost energy level not fully occupied by electrons Tend to gain, lose, or share electrons (form bonds) with other atoms to achieve stability

(b) Chemically reactive elements Outermost energy level (valence shell) incomplete 1 e Hydrogen (H) (1 p+; 0 n 0; 1 e–) 6 e 2 e Oxygen (O) (8 p+; 8 n 0; 8 e–) Copyright © 2010 Pearson Education, Inc. 4 e 2 e Carbon (C) (6 p+; 6 n 0; 6 e–) 1 e 8 e 2 e Sodium (Na) (11 p+; 12 n 0; 11 e–) Figure 2. 5 b

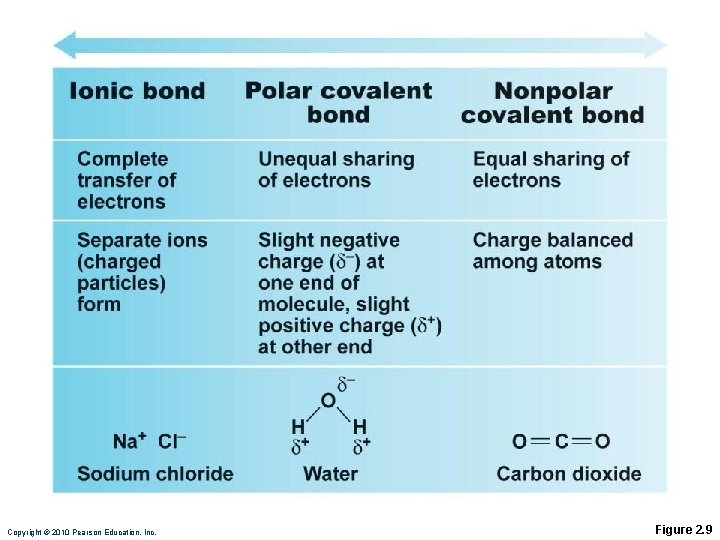
Types of Chemical Bonds Ionic Covalent Hydrogen

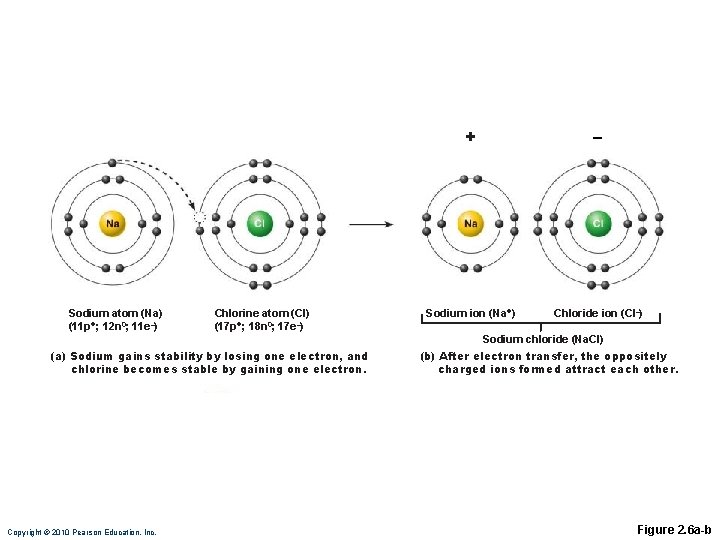
Ionic Bonds Ions are formed by transfer of valence shell electrons between atoms ◦ Anions (– charge) have gained one or more electrons ◦ Cations (+ charge) have lost one or more electrons Attraction of opposite charges results in an ionic bond

Sodium atom (Na) (11 p+; 12 n 0; 11 e–) Chlorine atom (Cl) (17 p+; 18 n 0; 17 e–) + – Sodium ion (Na+) Chloride ion (Cl–) Sodium chloride (Na. Cl) (a) Sodium gains stability by losing one electron, and chlorine becomes stable by gaining one electron. Copyright © 2010 Pearson Education, Inc. (b) After electron transfer, the oppositely charged ions formed attract each other. Figure 2. 6 a-b

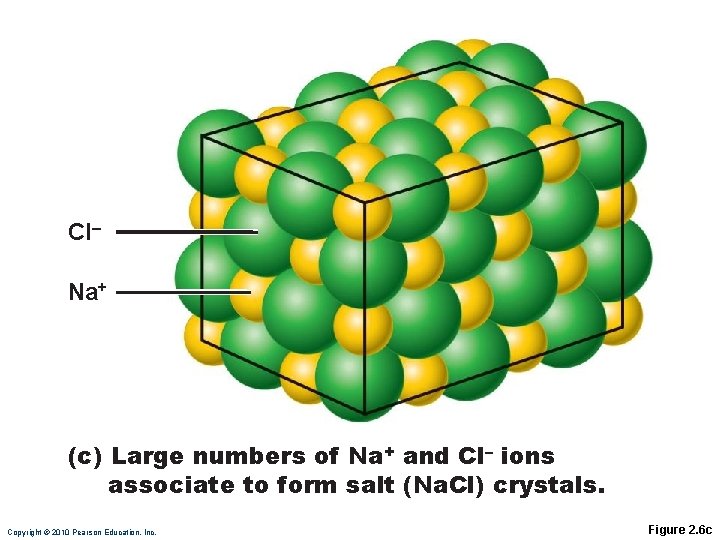
Formation of an Ionic Bond Ionic compounds form crystals instead of individual molecules ◦ Na. Cl (sodium chloride)

CI– Na+ (c) Large numbers of Na+ and Cl– ions associate to form salt (Na. Cl) crystals. Copyright © 2010 Pearson Education, Inc. Figure 2. 6 c

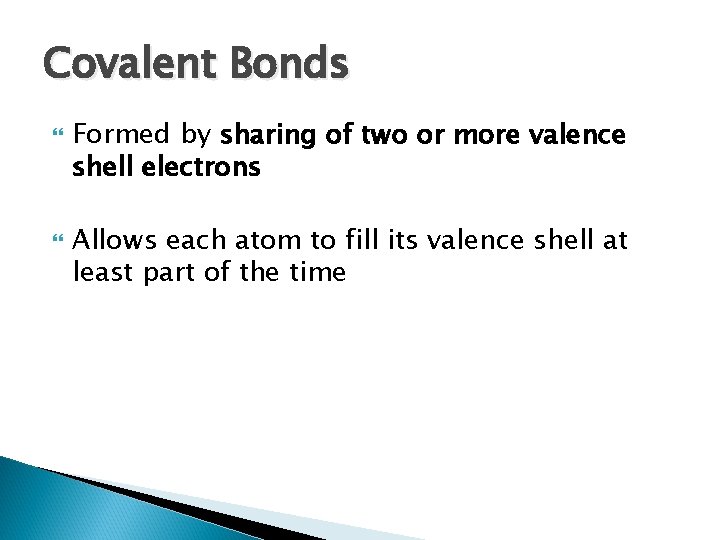
Covalent Bonds Formed by sharing of two or more valence shell electrons Allows each atom to fill its valence shell at least part of the time

Reacting atoms Resulting molecules + Molecule of Hydrogen Carbon methane gas (CH 4) atoms atom (a) Formation of four single covalent bonds: carbon shares four electron pairs with four hydrogen atoms. Copyright © 2010 Pearson Education, Inc. or Structural formula shows single bonds. Figure 2. 7 a

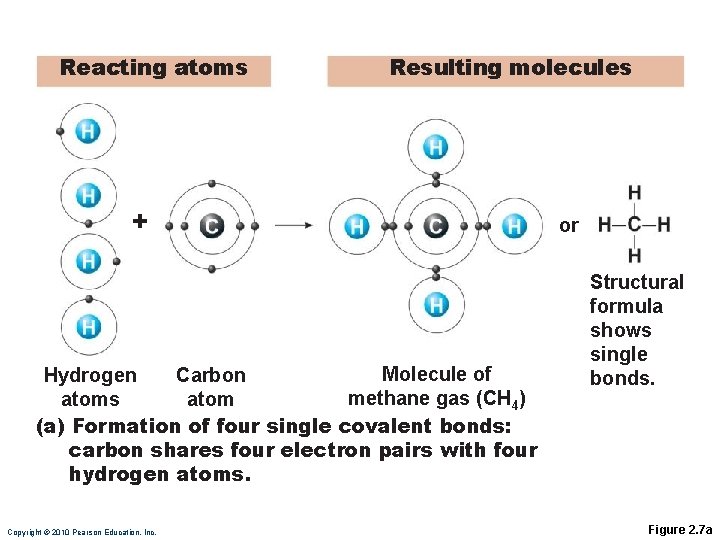
Reacting atoms Resulting molecules + Oxygen atom or Oxygen atom Molecule of oxygen gas (O 2) (b) Formation of a double covalent bond: Two oxygen atoms share two electron pairs. Copyright © 2010 Pearson Education, Inc. Structural formula shows double bond. Figure 2. 7 b

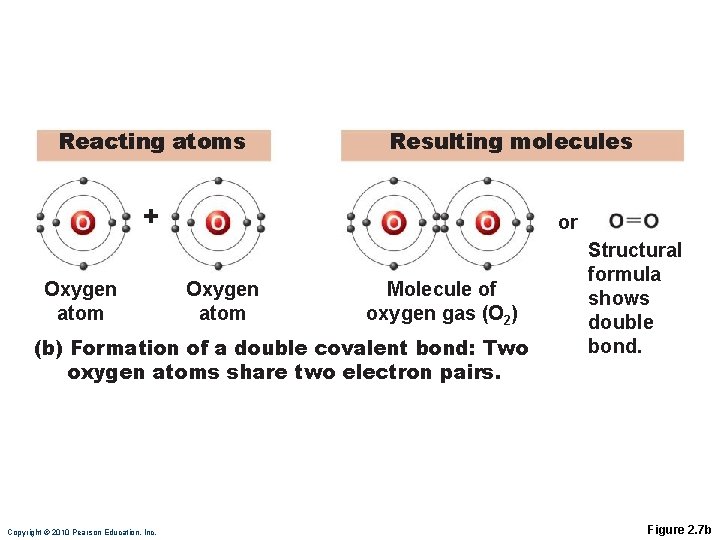
Reacting atoms Resulting molecules + Nitrogen atom or Nitrogen atom Molecule of nitrogen gas (N 2) (c) Formation of a triple covalent bond: Two nitrogen atoms share three electron pairs. Copyright © 2010 Pearson Education, Inc. Structural formula shows triple bond. Figure 2. 7 c

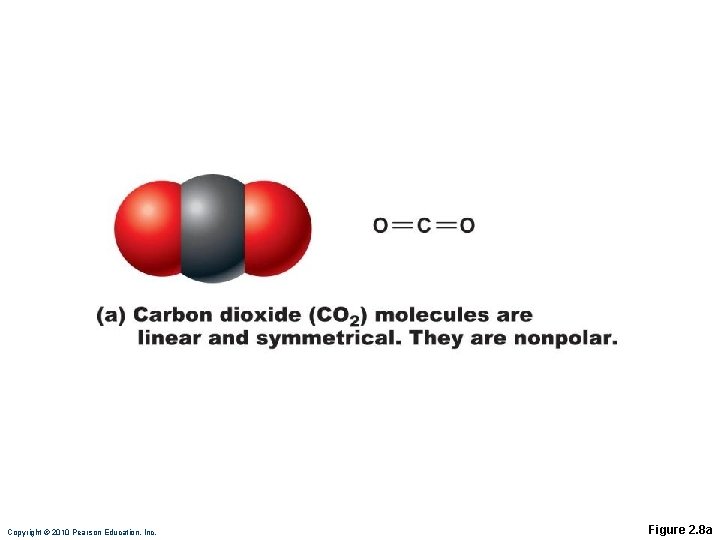
Covalent Bonds Sharing of electrons may be equal or unequal ◦ Equal sharing produces electrically balanced nonpolar molecules CO 2

Copyright © 2010 Pearson Education, Inc. Figure 2. 8 a

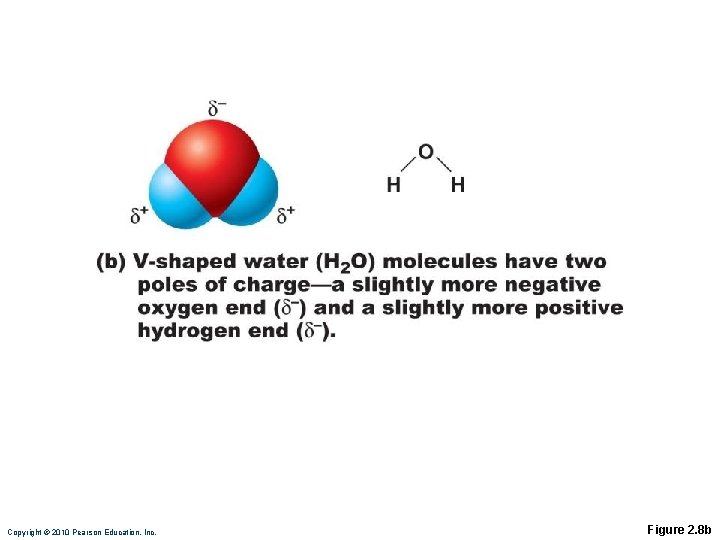
Covalent Bonds Unequal sharing by atoms with different electron-attracting abilities produces polar molecules ◦ H 2 O Atoms with six or seven valence shell electrons are electronegative, e. g. , oxygen Atoms with one or two valence shell electrons are electropositive, e. g. , sodium

Copyright © 2010 Pearson Education, Inc. Figure 2. 8 b

Copyright © 2010 Pearson Education, Inc. Figure 2. 9

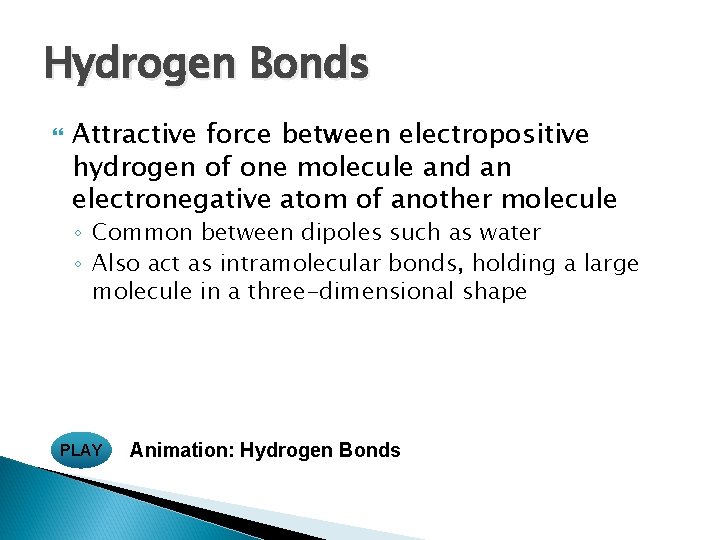
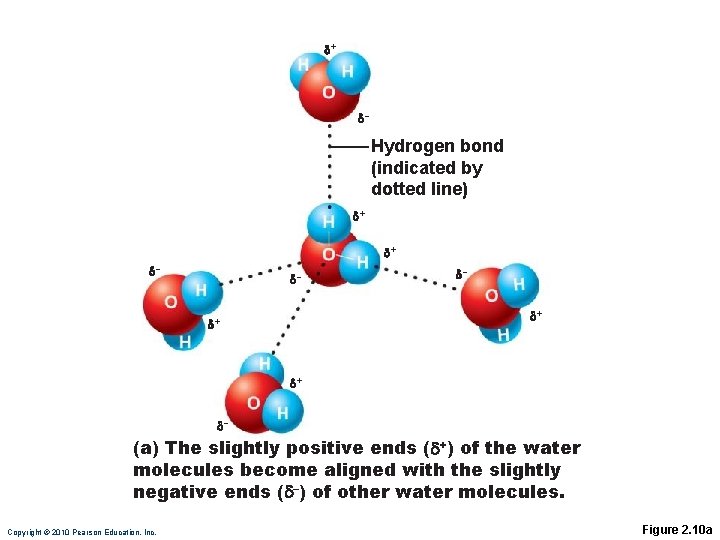
Hydrogen Bonds Attractive force between electropositive hydrogen of one molecule and an electronegative atom of another molecule ◦ Common between dipoles such as water ◦ Also act as intramolecular bonds, holding a large molecule in a three-dimensional shape PLAY Animation: Hydrogen Bonds

+ – Hydrogen bond (indicated by dotted line) + + – – – + + + – (a) The slightly positive ends ( +) of the water molecules become aligned with the slightly negative ends ( –) of other water molecules. Copyright © 2010 Pearson Education, Inc. Figure 2. 10 a

Chemical Reactions Occur when chemical bonds are formed, rearranged, or broken Represented as chemical equations Chemical equations contain: ◦ Molecular formula for each reactant and product ◦ Relative amounts of reactants and products, which should balance

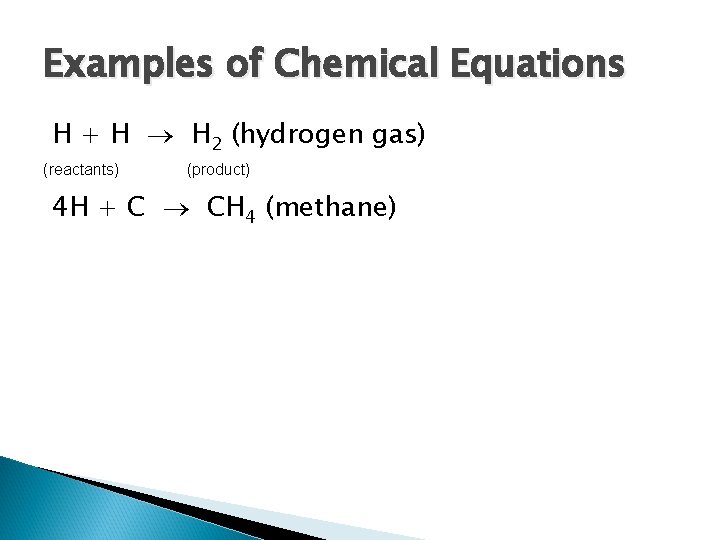
Examples of Chemical Equations H + H H 2 (hydrogen gas) (reactants) (product) 4 H + C CH 4 (methane)

Patterns of Chemical Reactions Synthesis (combination) reactions Decomposition reactions Exchange reactions

Synthesis Reactions A + B AB ◦ Always involve bond formation ◦ Anabolic

(a) Synthesis reactions Smaller particles are bonded together to form larger, more complex molecules. Example Amino acids are joined together to form a protein molecule. Amino acid molecules Protein molecule Copyright © 2010 Pearson Education, Inc. Figure 2. 11 a

Decomposition Reactions AB A + B ◦ Reverse synthesis reactions ◦ Involve breaking of bonds ◦ Catabolic

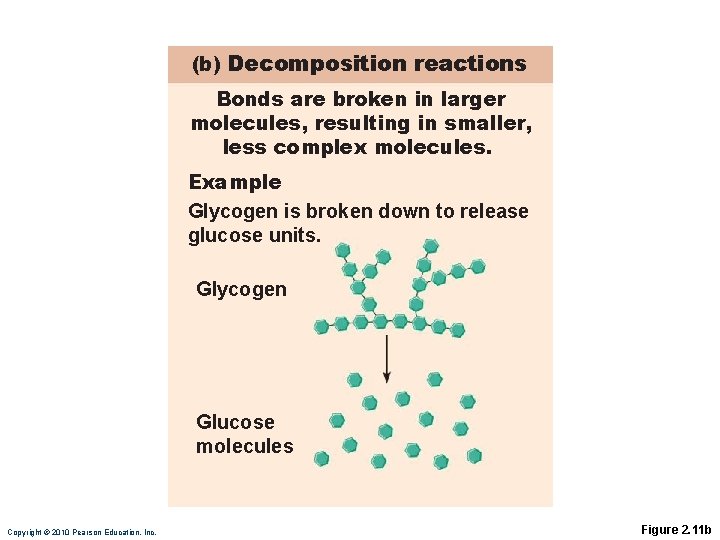
(b) Decomposition reactions Bonds are broken in larger molecules, resulting in smaller, less complex molecules. Example Glycogen is broken down to release glucose units. Glycogen Glucose molecules Copyright © 2010 Pearson Education, Inc. Figure 2. 11 b

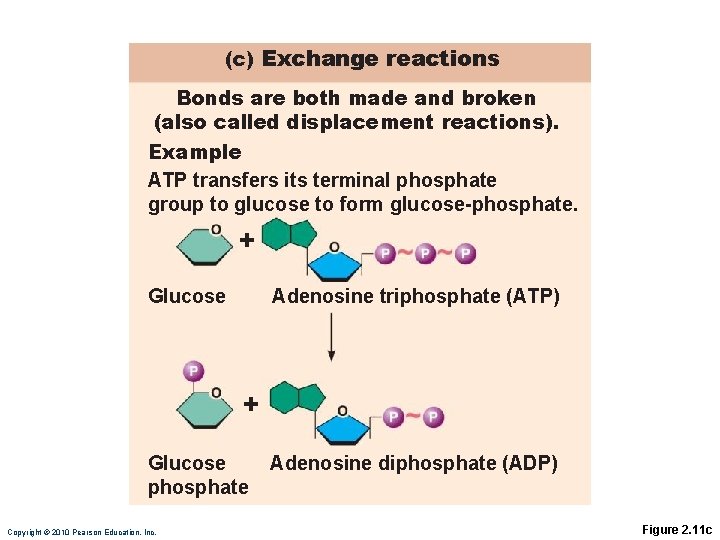
Exchange Reactions AB + C = AC + B AB + CD = AD + CB ◦ Also called displacement reactions ◦ Bonds are both made and broken

(c) Exchange reactions Bonds are both made and broken (also called displacement reactions). Example ATP transfers its terminal phosphate group to glucose to form glucose-phosphate. + Glucose Adenosine triphosphate (ATP) + Glucose phosphate Copyright © 2010 Pearson Education, Inc. Adenosine diphosphate (ADP) Figure 2. 11 c

Oxidation-Reduction (Redox) Reactions Decomposition reactions: Reactions in which fuel is broken down for energy Also called exchange reactions because electrons are exchanged or shared differently ◦ Electron donors lose electrons and are oxidized ◦ Electron acceptors receive electrons and become reduced

Chemical Reactions All chemical reactions are either exergonic or endergonic ◦ Exergonic reactions—release energy Catabolic reactions ◦ Endergonic reactions—form energy bonds: products contain more potential energy than did reactants Anabolic reactions

Chemical Reactions All chemical reactions are theoretically reversible ◦ A + B AB ◦ AB A + B Chemical equilibrium occurs if neither a forward nor reverse reaction is dominant Many biological reactions are essentially irreversible due to ◦ Energy requirements ◦ Removal of products

Rate of Chemical Reactions Rate of reaction is influenced by: ◦ temperature rate ◦ particle size rate ◦ concentration of reactant rate Catalysts: rate without being chemically changed: they reduce the activation energy needed for the reaction ◦ Enzymes are biological catalysts