CH 3 Many molecules are very small micromolecules





















- Slides: 73

CH 3

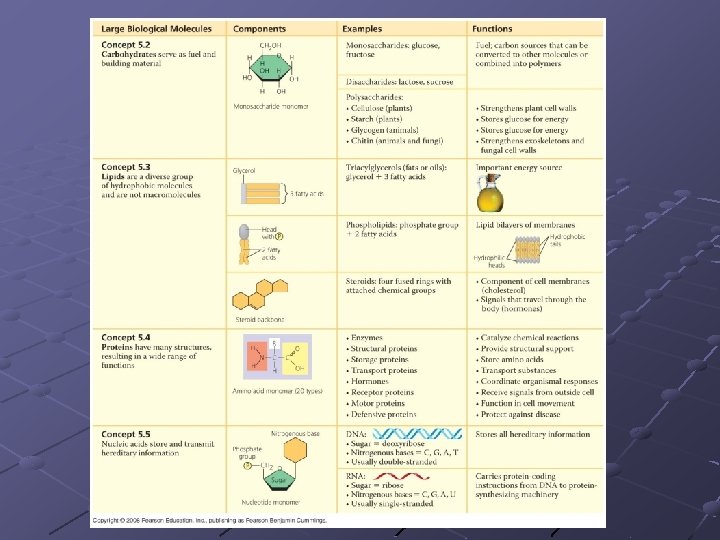
Many molecules are very small (micromolecules) Others are very large (macromolecules) 4 types---organic molecules n n Carbohydrates Lipids Proteins Nucleic acids

Organic compounds are structural (building) or informational (heredity) Many are polymers (repeating units) n n Monosaccharides complex carbs Amino acids protein/polypeptides Nucleotides nucleic acids (DNA, RNA) Fatty acids/glycerol lipids (not polymers)


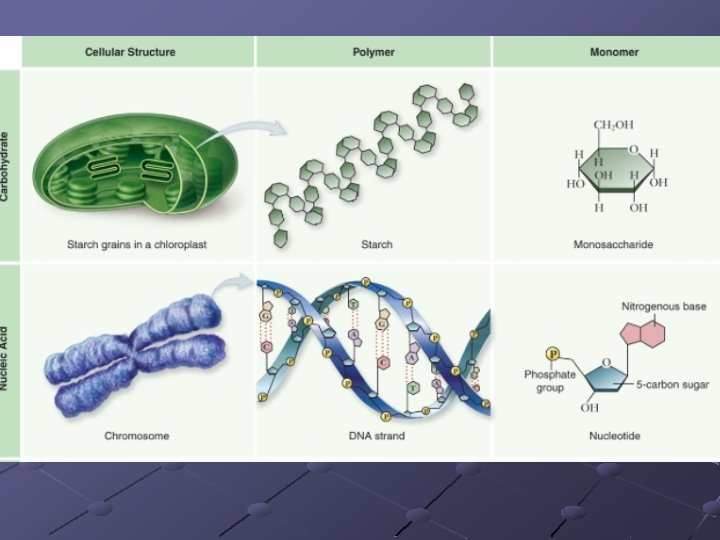
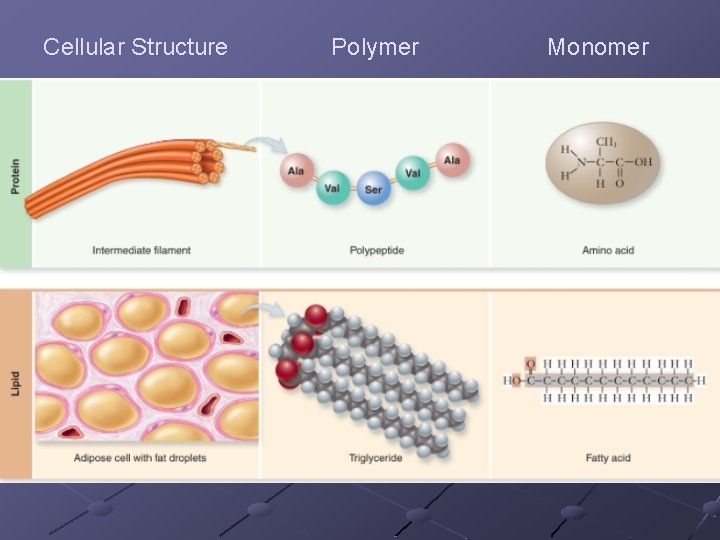
Cellular Structure Polymer Monomer


Subunits Function Examples

Subunits Function Examples

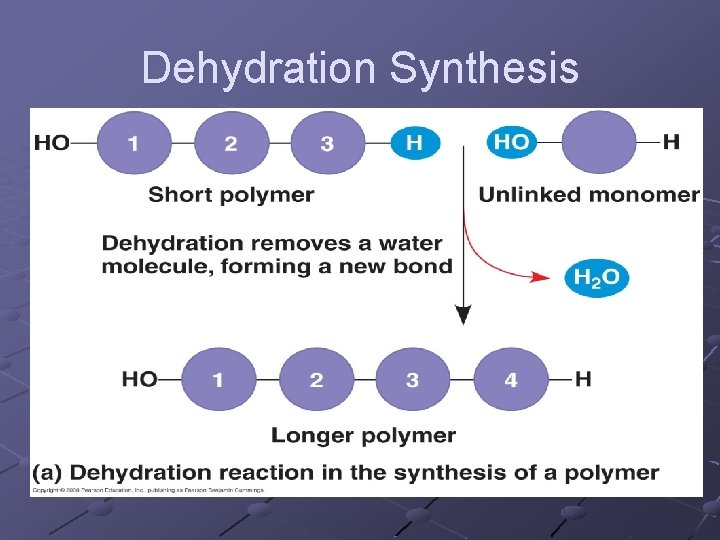
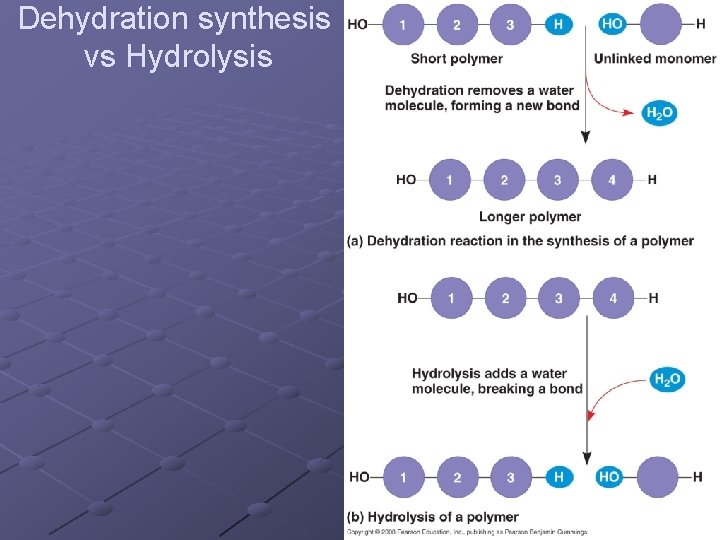
Building Macromolecules Subunits joined by covalent bonds n n -OH removed from one subunit -H removed from other subunit DEHYDRATION SYNTHESIS (aka Condensation)one molecule of water is removed as subunits are linked --anabolic (build up) --requires input of energy --stressing and breaking of bonds is carried out by enzymes---catalysis

Dehydration Synthesis

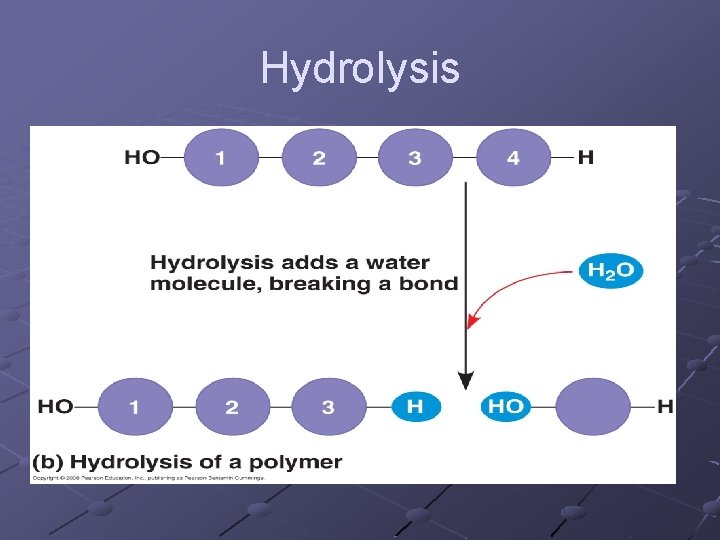
HYDROLYSIS- (hydro – add water, lysis – to split) - molecule can be broken down into subunits as water is added n n Catabolic Energy released

Hydrolysis

Dehydration synthesis vs Hydrolysis

Crash Course Biological Molecules

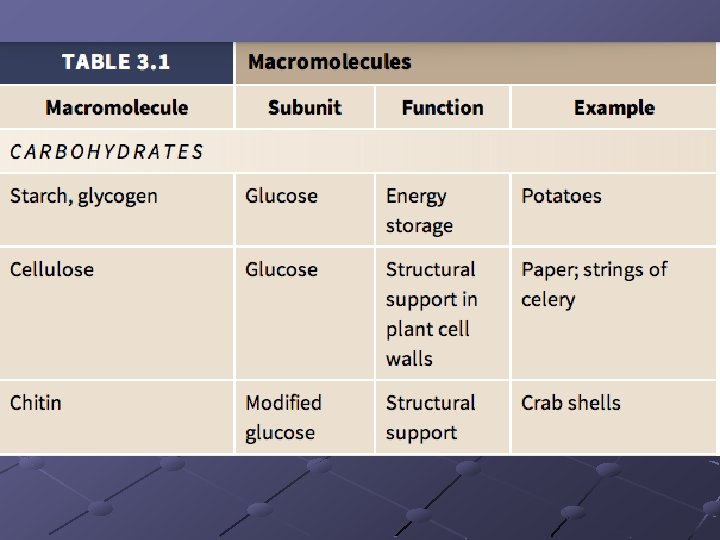
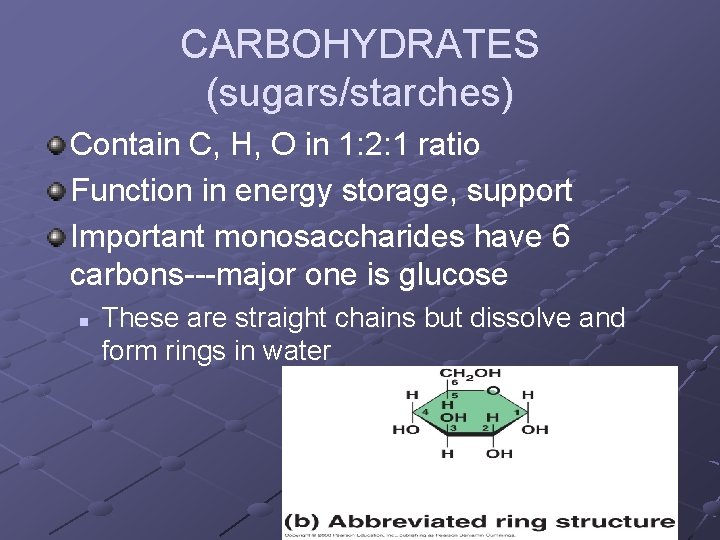
CARBOHYDRATES (sugars/starches) Contain C, H, O in 1: 2: 1 ratio Function in energy storage, support Important monosaccharides have 6 carbons---major one is glucose n These are straight chains but dissolve and form rings in water

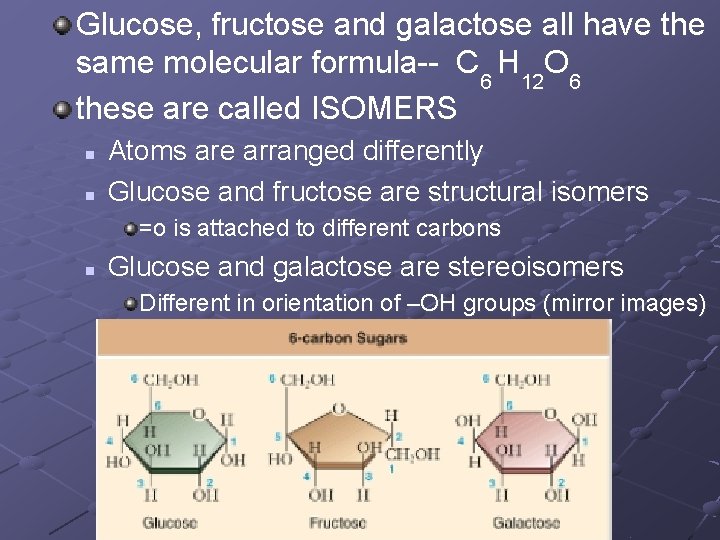
Glucose, fructose and galactose all have the same molecular formula-- C 6 H 12 O 6 these are called ISOMERS n n Atoms are arranged differently Glucose and fructose are structural isomers =o is attached to different carbons n Glucose and galactose are stereoisomers Different in orientation of –OH groups (mirror images) mirror images

Again…ISOMERS Molecules that have same molecular formula but different structure… n Ex. glucose, fructose, galactose

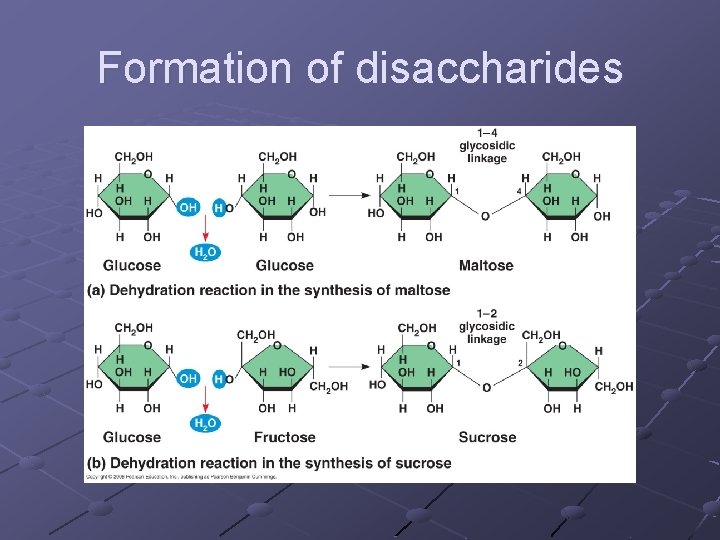
DISACCHARIDES n n n “double sugars” 2 monosaccharides joined by a covalent bond Can play a role in transport of sugars…transport disaccharides Transport disacch. Protects sugar from being metabolized during transport…. less readily used for energy when in this form Some organisms(specifically plants), glucose is converted to a transport form before being circulated glucose + glucose = maltose glucose + fructose = sucrose (table sugar) glucose + galacatose = lactose (milk sugar)

Formation of disaccharides

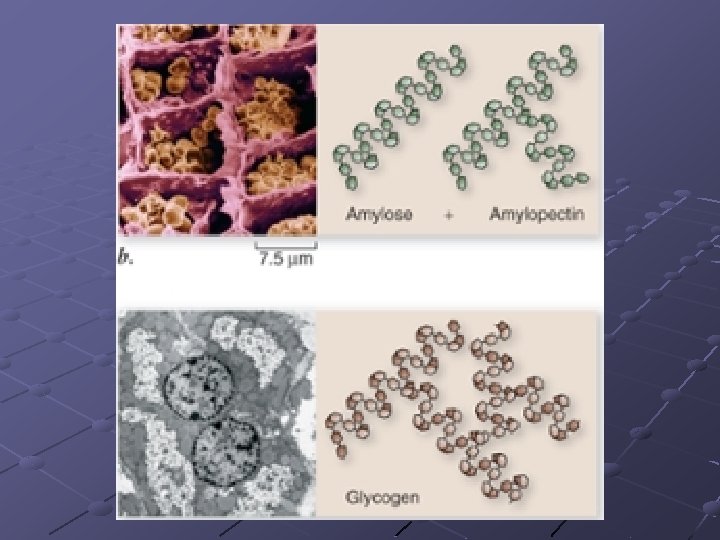
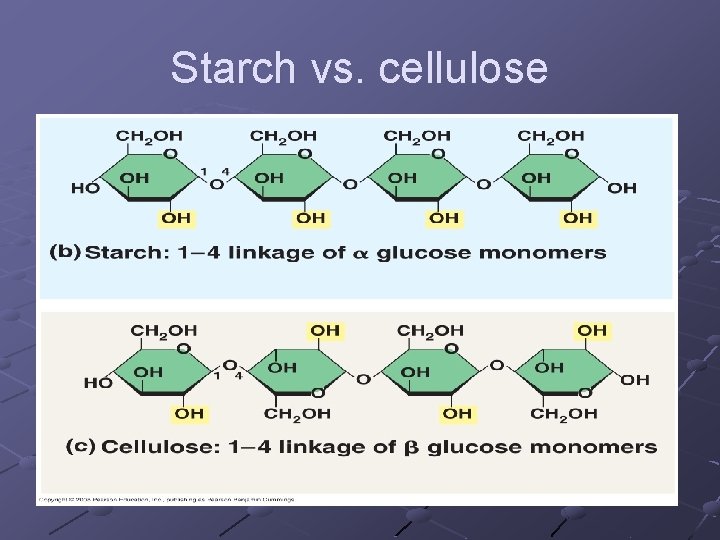
POLYSACCHARIDES n n Starches—insoluble in water Consists of glucose molecules linked in chains Energy storage, structural support Amylose, pectins, cellulose, glycogen,

Amylose - simplest starch, hundreds of glucose linked in unbranched chains. Potato Starch 20% amylose Pectins - branched chains with short linear amylose branches n Amylopectin is the part of a starch that prevents it from being solulable Glycogen - animal storage product, stored in liver


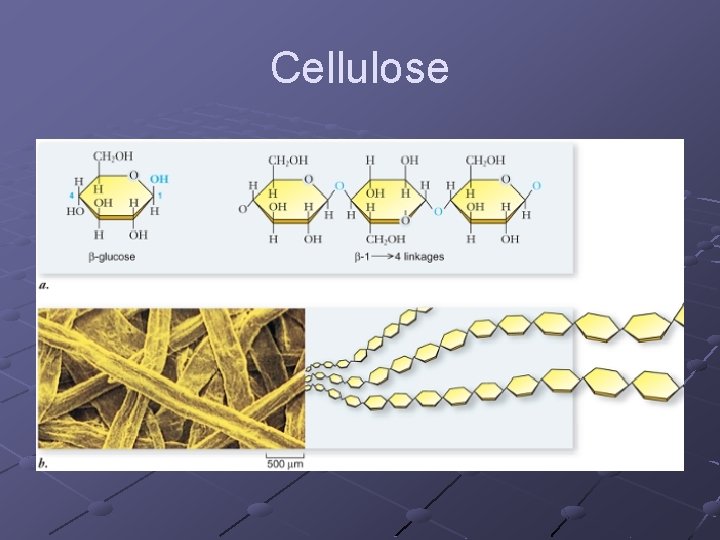
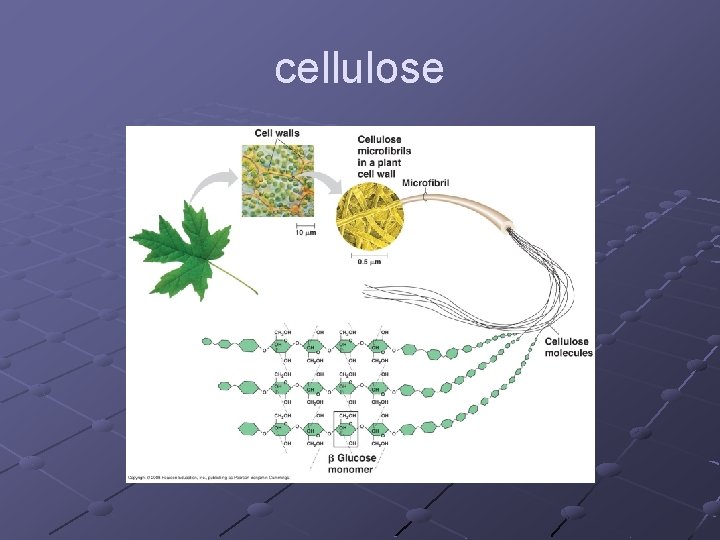
Cellulosen Orientation of glucose subunits(CH OH) 2 are on alternate sides so difficult for enzymes to break bonds dietary fiber, component of plant cell wall

Cellulose

cellulose

Starch vs. cellulose



The carbohydrates produced or consumed by an organism are used in 3 ways… n n n 1. maintained as glucose/immediate energy 2. converted to transport disaccharides 3. converted to starch, glycogen or fat(adipose) reserved for future use

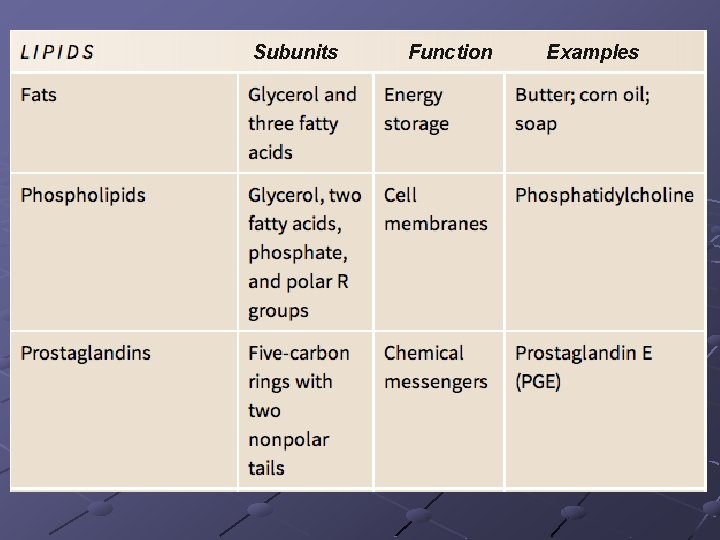
LIPIDS fats, oils, waxes Contain C, H, O >2: 1 ratio of H: O Insoluble in water— n Many lipid molecules cluster together when put in water and expose their hydrophilic (polar) groups while protecting their hydrophobic (non-polar) groups (important in cell membrane) Phospholipids, fats, oils, terpenes, steroids, prostaglandins are types of lipids

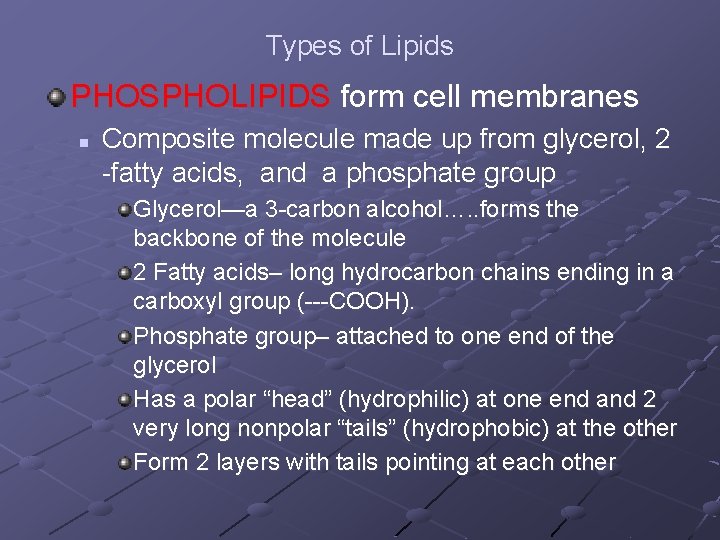
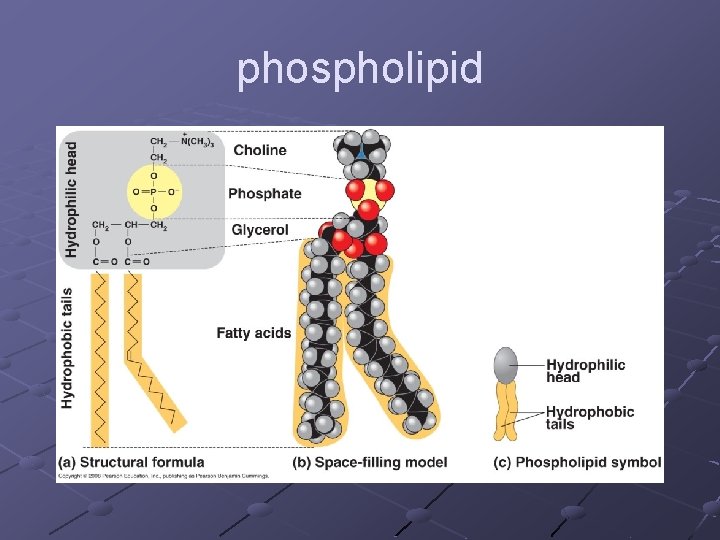
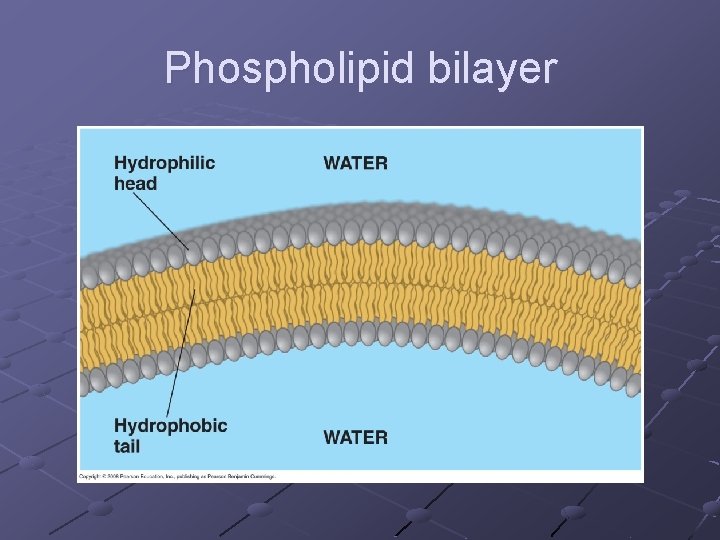
Types of Lipids PHOSPHOLIPIDS form cell membranes n Composite molecule made up from glycerol, 2 -fatty acids, and a phosphate group Glycerol—a 3 -carbon alcohol…. . forms the backbone of the molecule 2 Fatty acids– long hydrocarbon chains ending in a carboxyl group (---COOH). Phosphate group– attached to one end of the glycerol Has a polar “head” (hydrophilic) at one end and 2 very long nonpolar “tails” (hydrophobic) at the other Form 2 layers with tails pointing at each other

phospholipid

Phospholipid bilayer

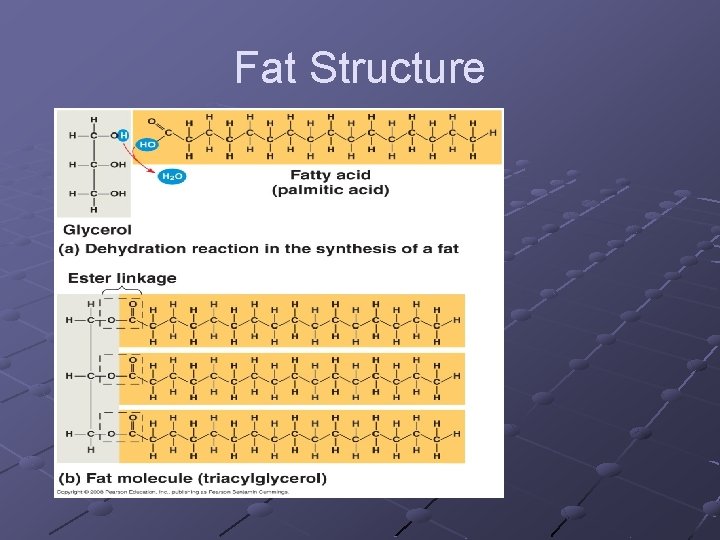
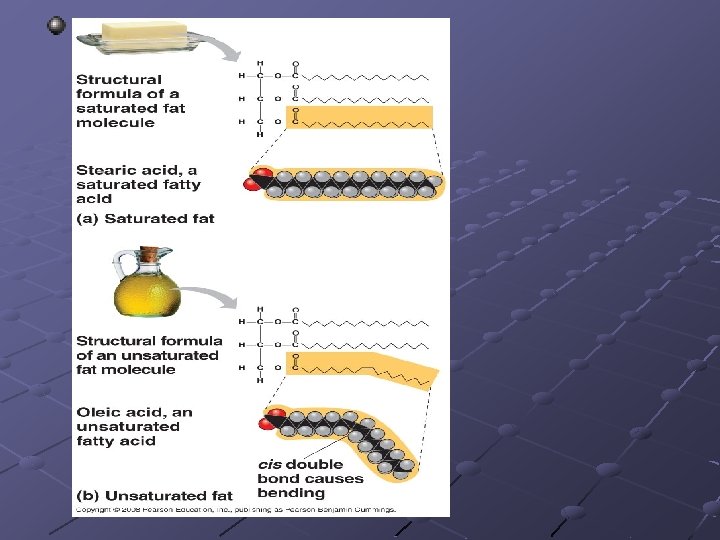
FATS n n n n n A glycerol attached to 3 fatty acids Called a triglyceride Store energy for long periods in the many C-H bonds Because they are insoluble in water, they can be deposited at specific locations within organisms Animal fats, oils, earwax, beeswax If all internal carbons have single bonds between them in the fatty acid chains—saturated If the fatty acids have double /triple bond between them—unsaturated More than 1 double/triple bond—polyunsaturated Form micelles (droplets) in water

Fat Structure

Fats made from polyunsaturated fatty acids have a low melting point ---fatty acid chains bend at the double bonds, preventing the fatty acids from aligning Poly. Fat(corn oil) is usually liquid at room temp and is called an oil. PLANT FATS Most saturated fats(butter) are solid at room temp ANIMAL FATS Possible to convert oil solid by adding hydrogen (hydrogenated) However, artificially hydrogenating unsaturated fats eliminates the health advantage

. nn

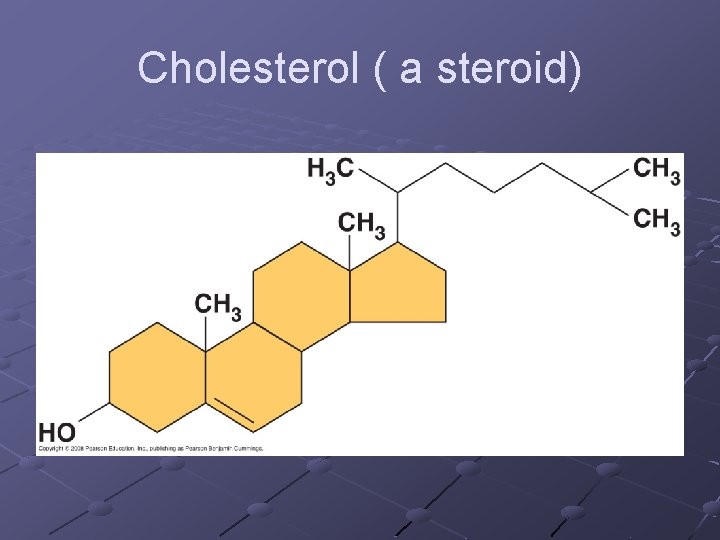
Types of lipids (cont) Terpenes-long chain lipids that are components of many biological pigments(chlorophyll, retinal). Rubber is also a terpene Steroids-composed of 4 carbon rings. Cholesterol (in cell membrane), testosterone and estrogen (hormones) Prostaglandins-a group of 20 lipids that are modified fatty acids…act as local chemical messengers (neural modulators) in vertebrates n Aspirin inhibits prostaglandin synthesis so reduces pain/inflammation…

Cholesterol ( a steroid)

Fats are a more efficient energy storage molecule than carbs n n n Most fats contain over 40 carbon atoms; ratio of C-H bonds to carbon atoms is twice that of carbs Fats yield 9 kcal of energy per gram Carbs yield 4 kcal of energy per gram

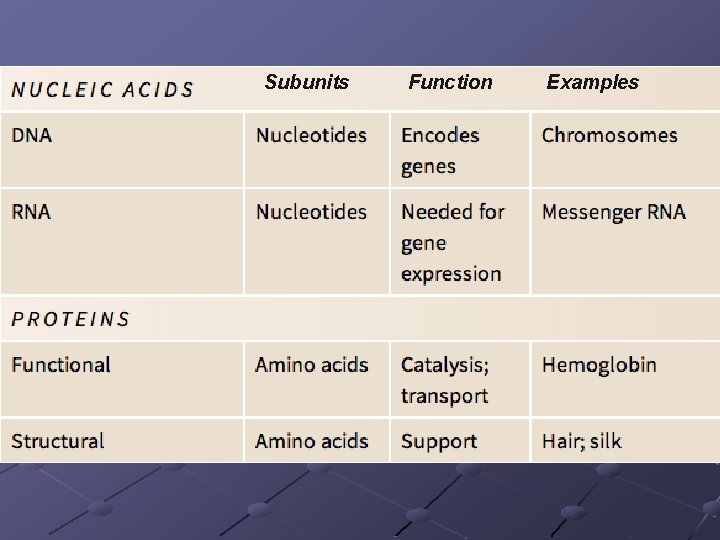
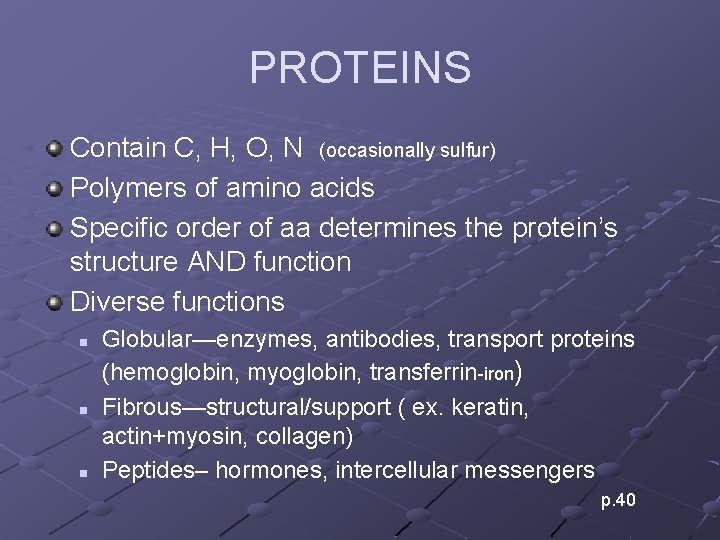
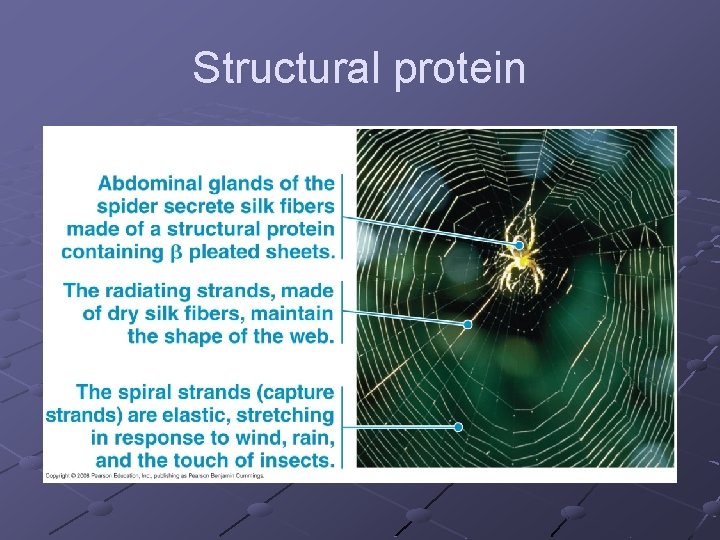
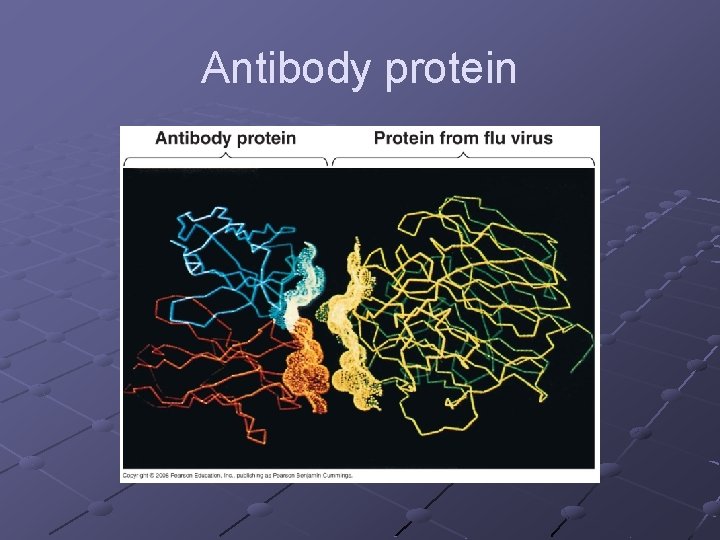
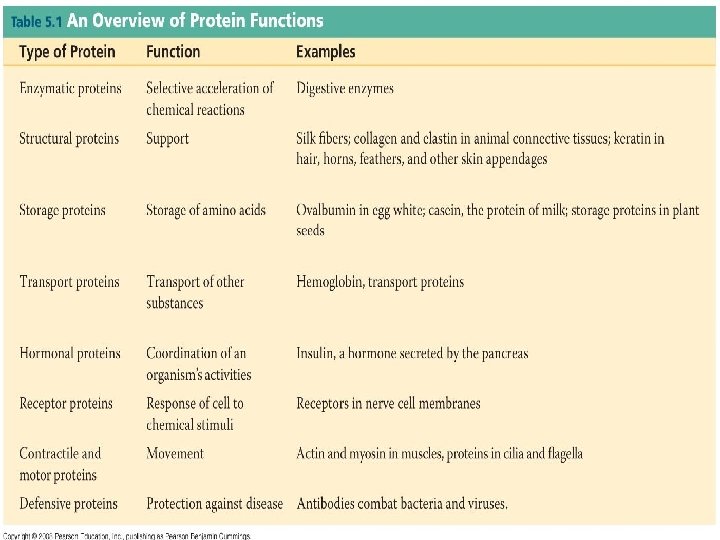
PROTEINS Contain C, H, O, N (occasionally sulfur) Polymers of amino acids Specific order of aa determines the protein’s structure AND function Diverse functions n n n Globular—enzymes, antibodies, transport proteins (hemoglobin, myoglobin, transferrin-iron) Fibrous—structural/support ( ex. keratin, actin+myosin, collagen) Peptides– hormones, intercellular messengers p. 40

Structural protein

Antibody protein

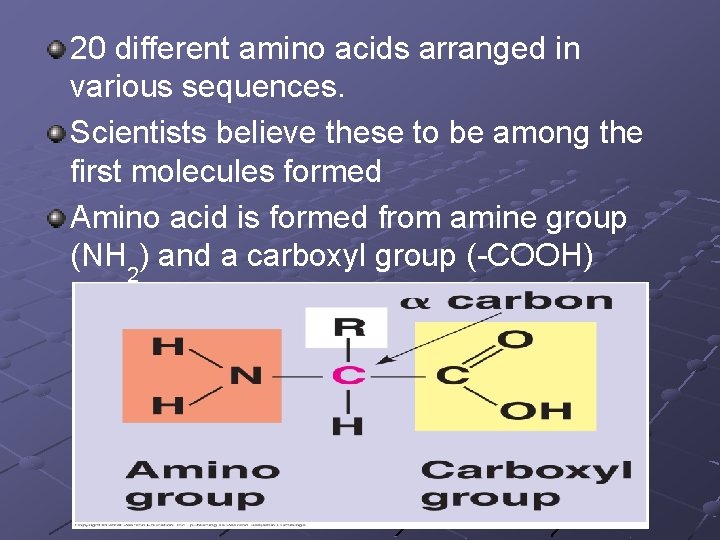
20 different amino acids arranged in various sequences. Scientists believe these to be among the first molecules formed Amino acid is formed from amine group (NH 2) and a carboxyl group (-COOH)

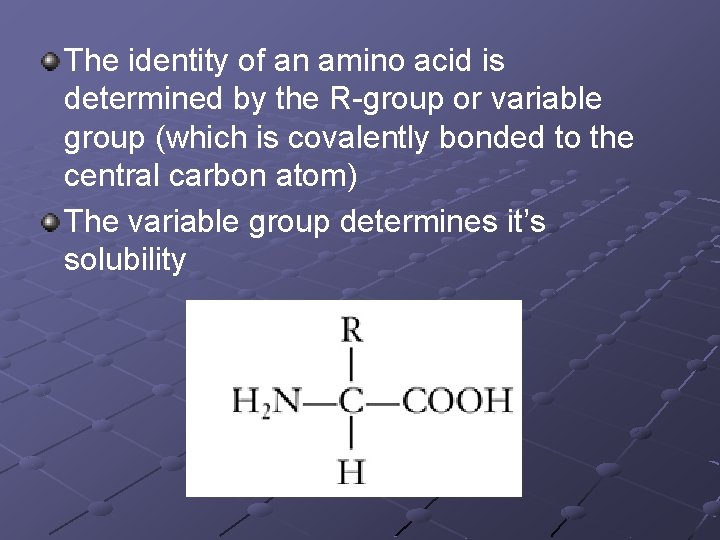
The identity of an amino acid is determined by the R-group or variable group (which is covalently bonded to the central carbon atom) The variable group determines it’s solubility

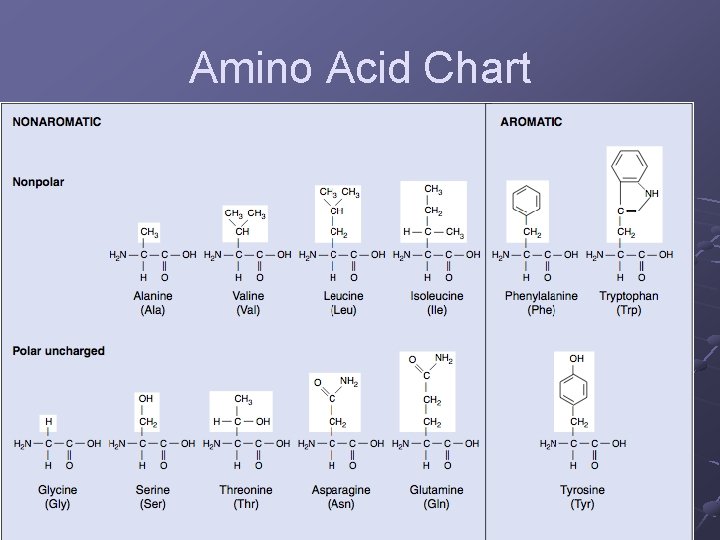
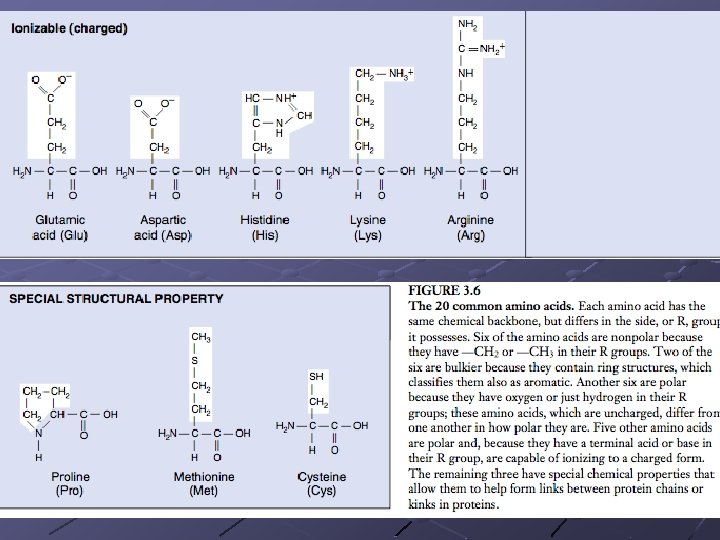
5 Classes of Amino Acids (based on the R group ) Nonpolar - R groups contain CH 2 or CH 3, Polar (uncharged)-R groups contain only H or OH, ex. Threonine Ionizable - R groups contain acids/bases, ex. glutamic acid Aromatic - R groups contain an organic ring with alternating single + double bonds. Nonpolar, ex. phenylalanine Special function - have specific properties n n n Methionine - initiates protein synthesis Proline - caused kinks in chain Cysteine - links chains together pg. 45

Amino Acid Chart

Amino Acid Chart continued

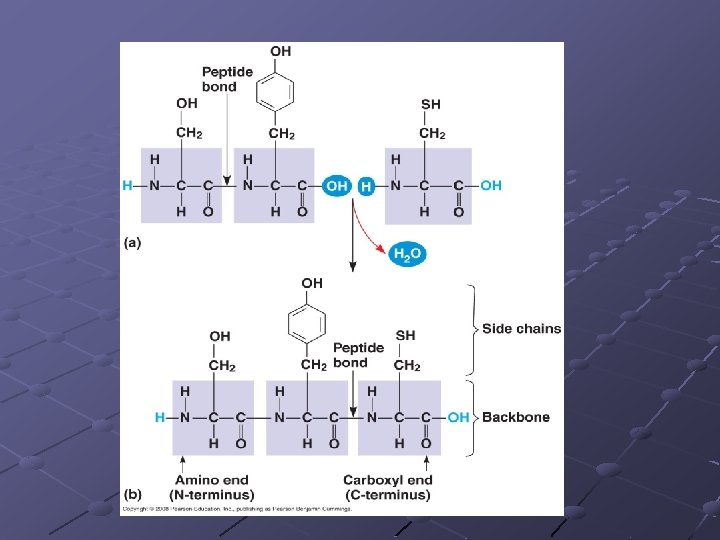
Amino acids are joined by dehydration synthesis and linked by peptide bonds 2 amino acids joined creates a molecule called a dipeptide


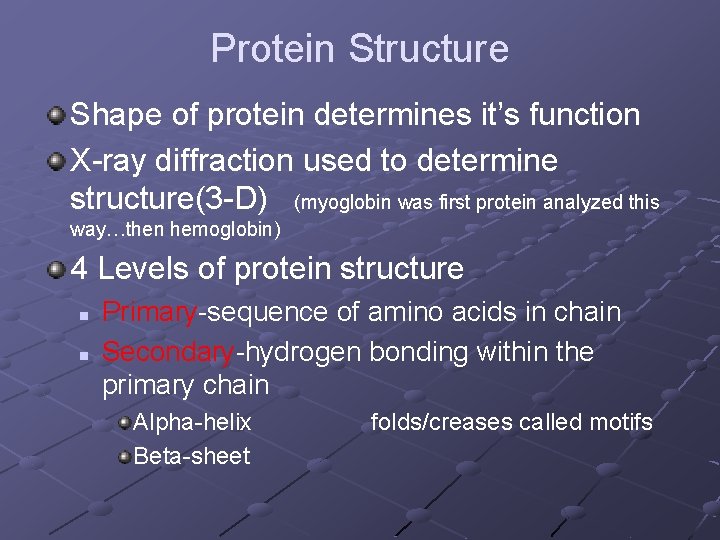
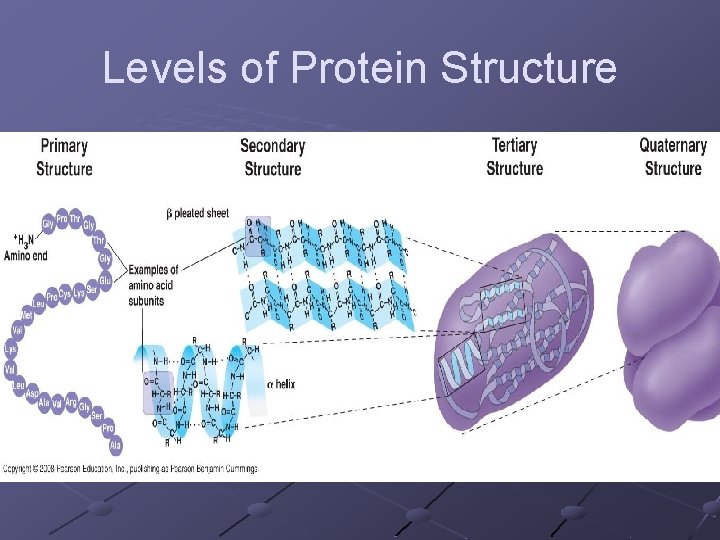
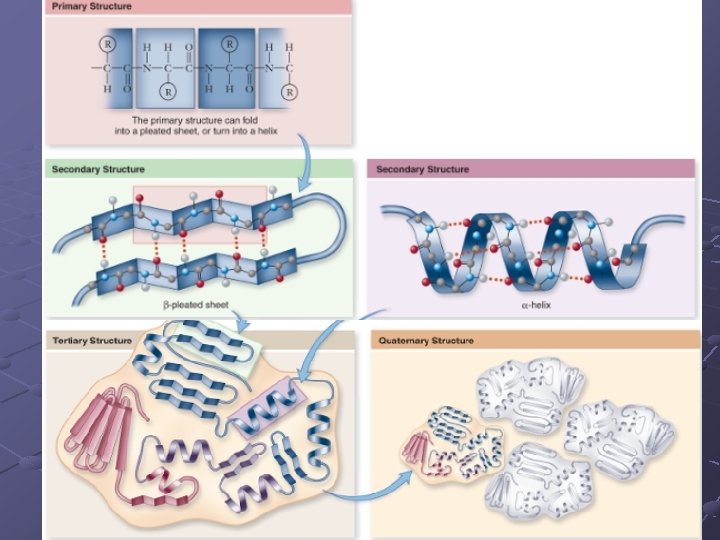
Protein Structure Shape of protein determines it’s function X-ray diffraction used to determine structure(3 -D) (myoglobin was first protein analyzed this way…then hemoglobin) 4 Levels of protein structure n n Primary-sequence of amino acids in chain Secondary-hydrogen bonding within the primary chain Alpha-helix Beta-sheet folds/creases called motifs

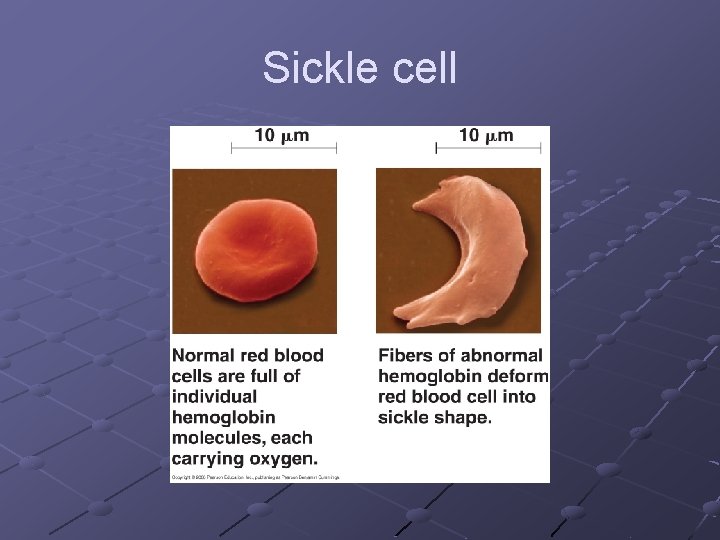
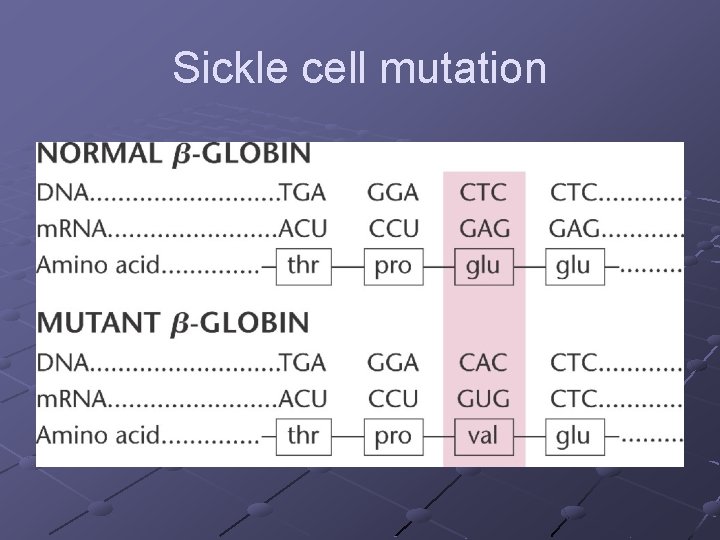
n Tertiary-final folded shape of a protein Spontaneous. . driven by hydrophobic interactions w/water A mutation that changes a single amino acid within protein’s interior can disrupt the protein’s stability and have major structural consequences n Replacing a polar a. a with a nonpolar one can cause a “sticky patch” on surface of protein…hence sickle-cell anemia (nonpolar valine replaces polar glutamate) Domains- sections of this structure with diff. functions (cofactor, active site…within 1 protein)

Quaternary-clustering of more than 1 polypeptide chain (each has it’s own tertiary structure). Each is called a subunit

Sickle cell

Sickle cell mutation

Levels of Protein Structure


If the nonpolar sticky interior of protein chains are exposed during their arranging, they can stick to other unwanted protein partners Chaperone proteins—help new proteins fold correctly. (scientists unsure how they work) ********************

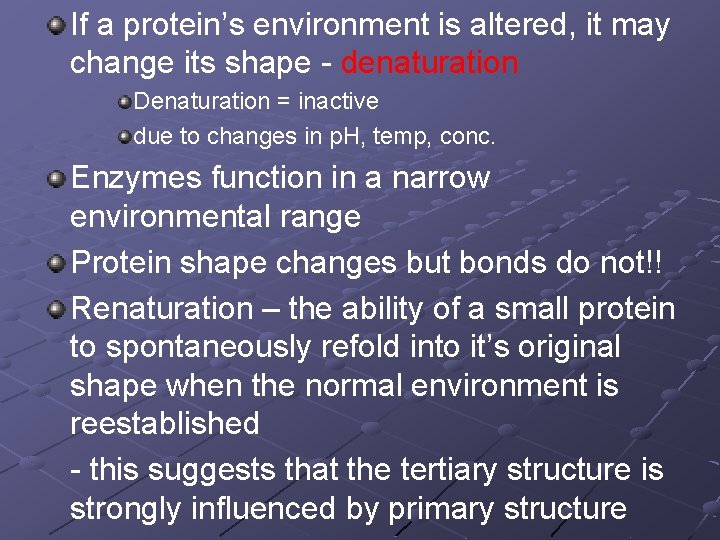
If a protein’s environment is altered, it may change its shape - denaturation Denaturation = inactive due to changes in p. H, temp, conc. Enzymes function in a narrow environmental range Protein shape changes but bonds do not!! Renaturation – the ability of a small protein to spontaneously refold into it’s original shape when the normal environment is reestablished - this suggests that the tertiary structure is strongly influenced by primary structure


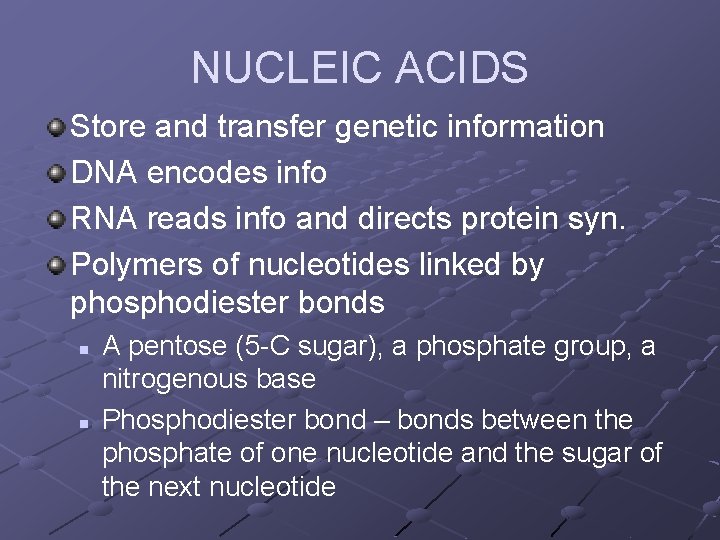
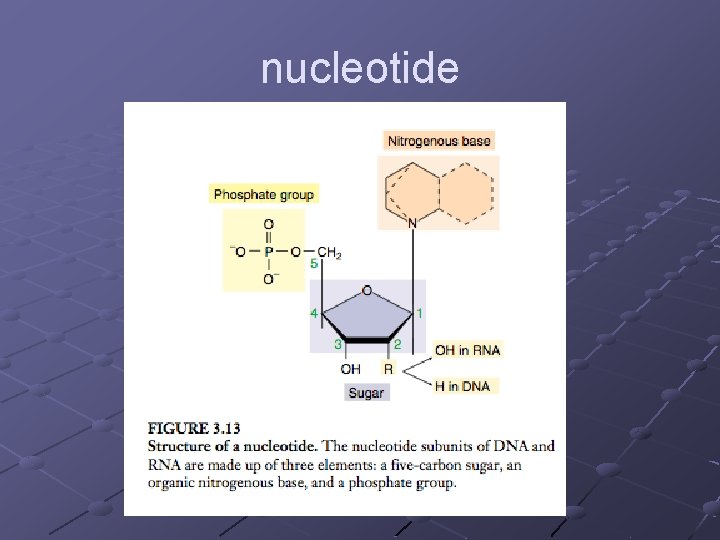
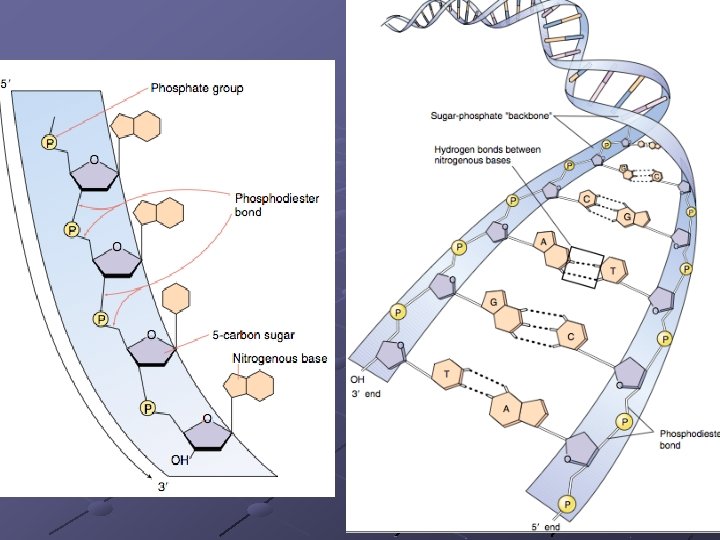
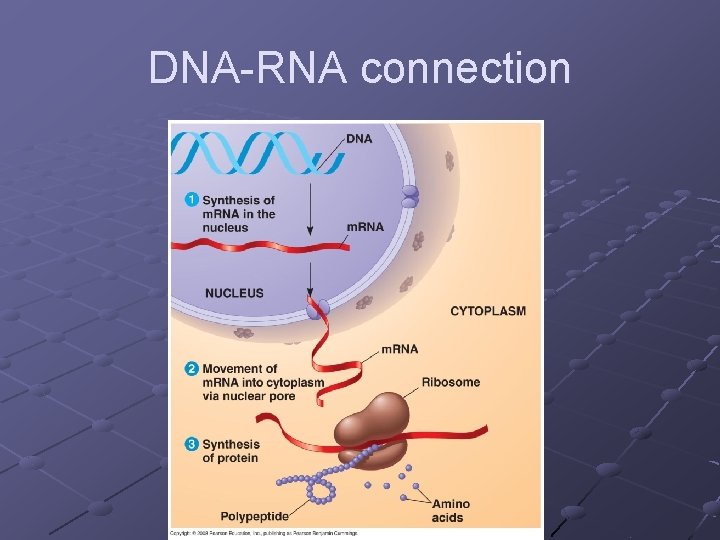
NUCLEIC ACIDS Store and transfer genetic information DNA encodes info RNA reads info and directs protein syn. Polymers of nucleotides linked by phosphodiester bonds n n A pentose (5 -C sugar), a phosphate group, a nitrogenous base Phosphodiester bond – bonds between the phosphate of one nucleotide and the sugar of the next nucleotide

nucleotide

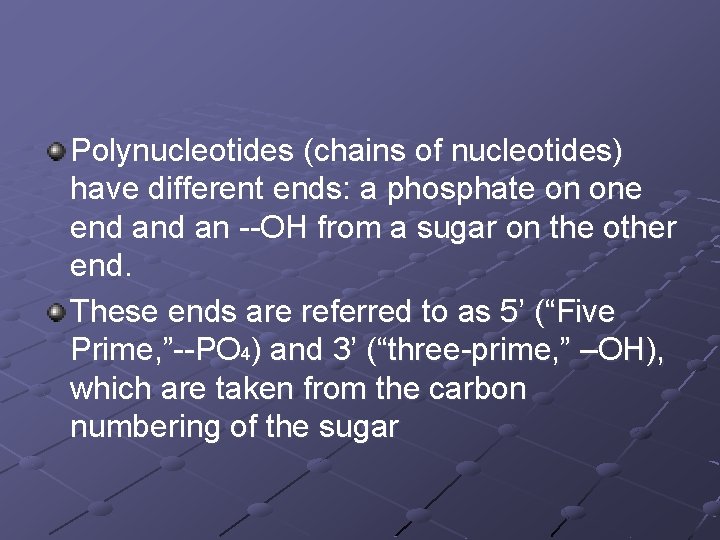
Polynucleotides (chains of nucleotides) have different ends: a phosphate on one end an --OH from a sugar on the other end. These ends are referred to as 5’ (“Five Prime, ”--PO 4) and 3’ (“three-prime, ” –OH), which are taken from the carbon numbering of the sugar


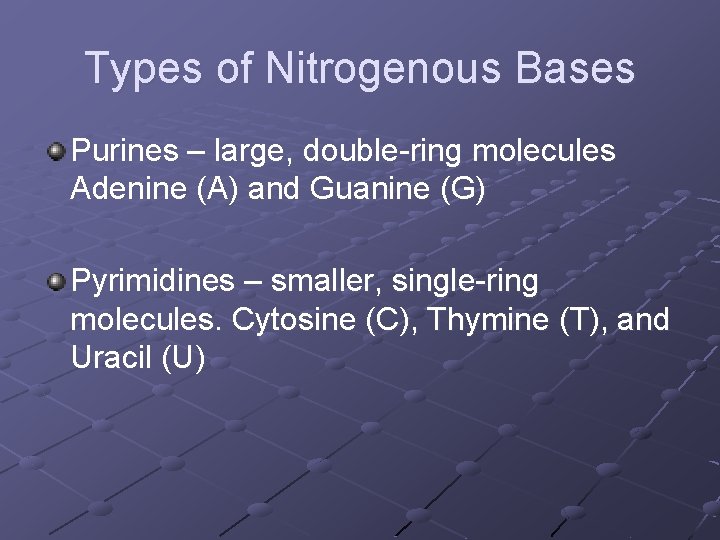
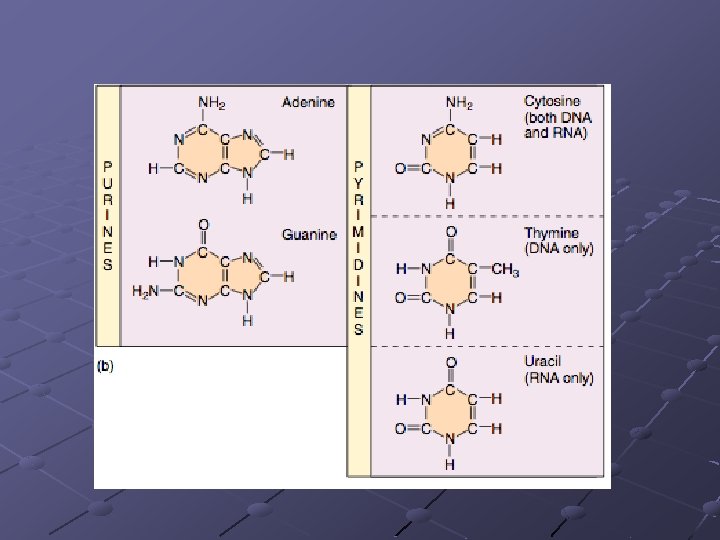
Types of Nitrogenous Bases Purines – large, double-ring molecules Adenine (A) and Guanine (G) Pyrimidines – smaller, single-ring molecules. Cytosine (C), Thymine (T), and Uracil (U)


DNA-RNA connection

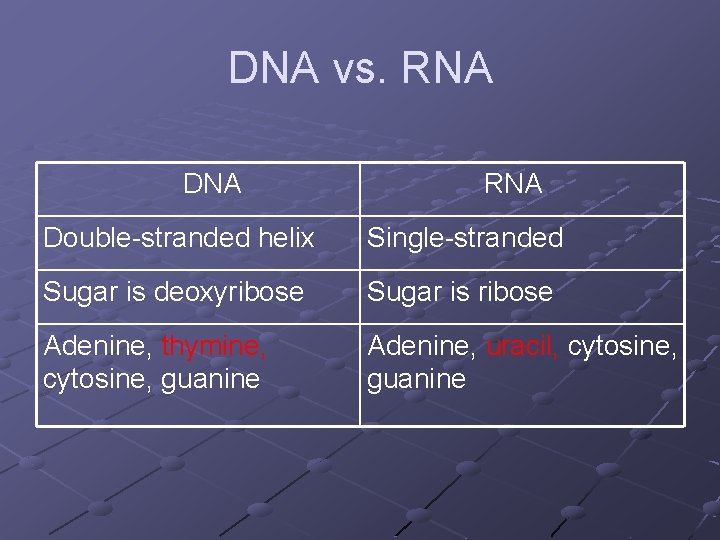
DNA vs. RNA DNA RNA Double-stranded helix Single-stranded Sugar is deoxyribose Sugar is ribose Adenine, thymine, cytosine, guanine Adenine, uracil, cytosine, guanine

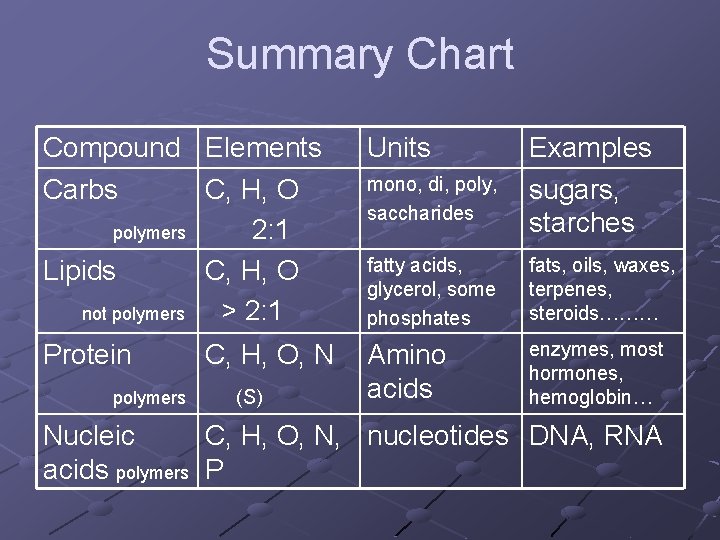
Summary Chart Compound Elements Carbs C, H, O polymers 2: 1 Lipids C, H, O not polymers > 2: 1 Units fatty acids, glycerol, some phosphates fats, oils, waxes, terpenes, steroids……… Protein Amino acids enzymes, most hormones, hemoglobin… polymers C, H, O, N (S) mono, di, poly, saccharides Examples sugars, starches Nucleic C, H, O, N, nucleotides DNA, RNA acids polymers P


3 other nucleotide containing molecules 1. Nicotinamide Adenine Dinucleotide (NAD+) 2. Flavin Adenine Dinucleotide (FAD) These 2 molecules are used as electron carriers in different cellular processes, which will be discussed in future units

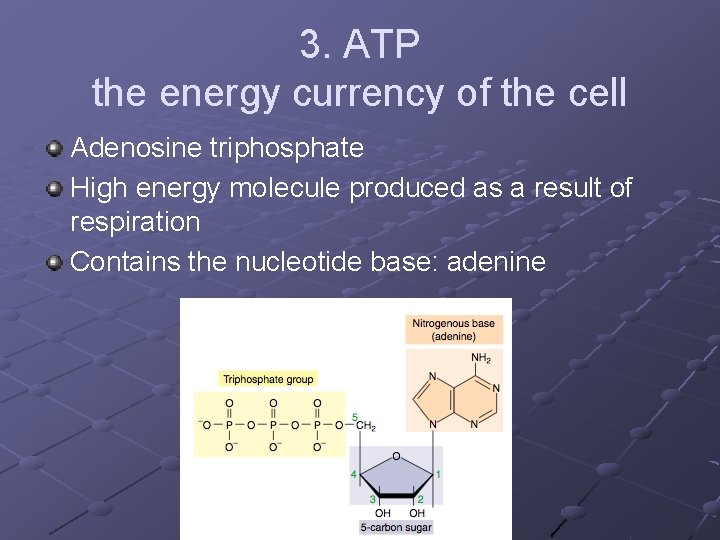
3. ATP the energy currency of the cell Adenosine triphosphate High energy molecule produced as a result of respiration Contains the nucleotide base: adenine

http: //www. bozemanscience. com/042 biologoical-molecules