APPROACH TO CONGENITAL HEART DISEASE Does the child



















- Slides: 61

APPROACH TO CONGENITAL HEART DISEASE

Does the child have congenital heart disease? ?

Nada’s Criteria Major Systolic murmur gr III or more in intensity Diastolic murmur Cyanosis Congestive heart failure

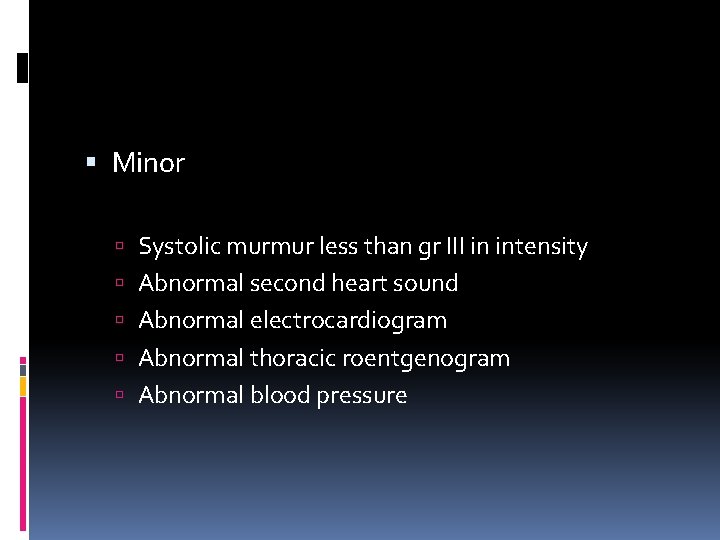
Minor Systolic murmur less than gr III in intensity Abnormal second heart sound Abnormal electrocardiogram Abnormal thoracic roentgenogram Abnormal blood pressure

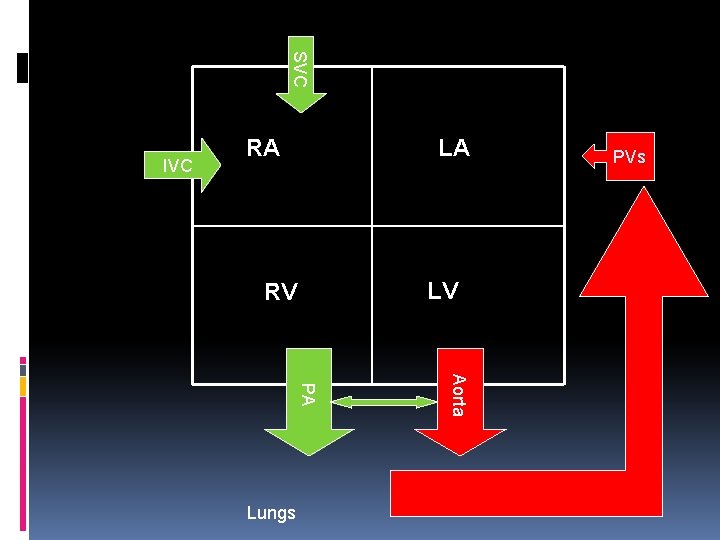
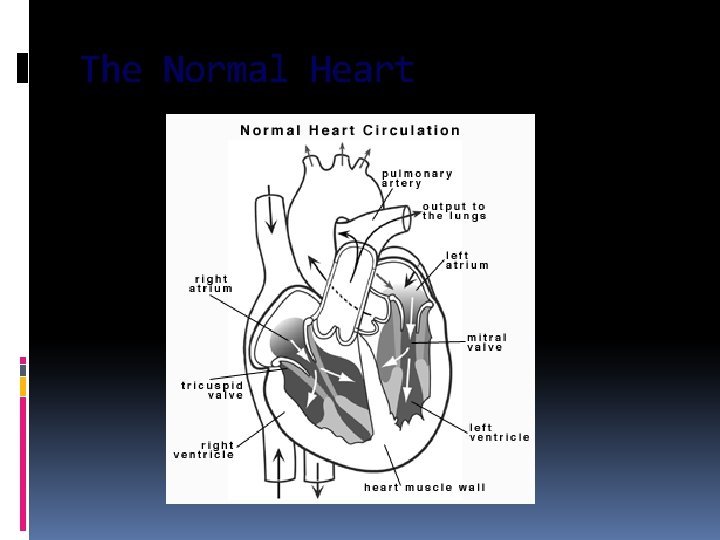
SVC IVC RA LA LV RV Aorta PA Lungs PVs

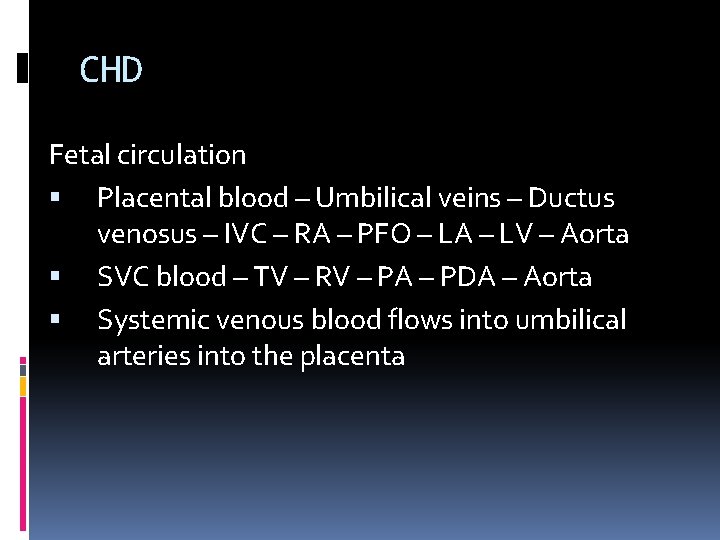
CHD Fetal circulation Placental blood – Umbilical veins – Ductus venosus – IVC – RA – PFO – LA – LV – Aorta SVC blood – TV – RV – PA – PDA – Aorta Systemic venous blood flows into umbilical arteries into the placenta

Systolic murmurs Organic> gr III or associated with Thrill Pansystolic murmur-MR, TR and restrictive VSD ESM associated with thrill or click. ESM<gr III Hyperdynamic states, anaemia Still’s murmur

Diastolic murmur- always pathological Early- aortic or pulmonary Delayed- mitral or tricupid

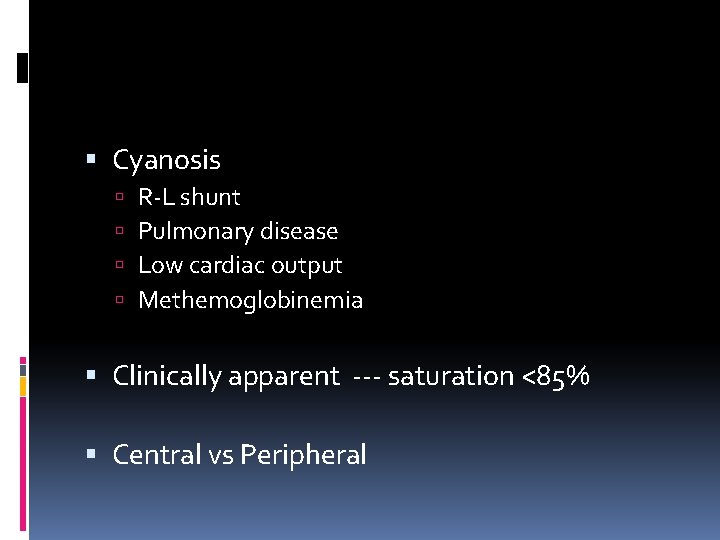
Cyanosis R-L shunt Pulmonary disease Low cardiac output Methemoglobinemia Clinically apparent --- saturation <85% Central vs Peripheral

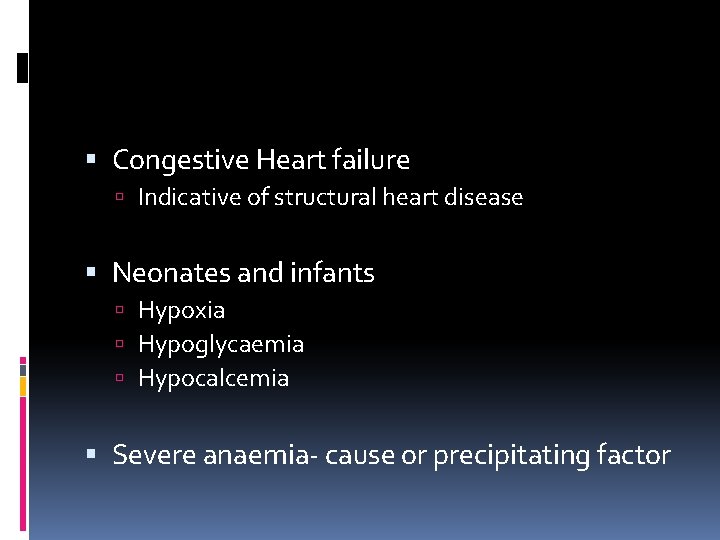
Congestive Heart failure Indicative of structural heart disease Neonates and infants Hypoxia Hypoglycaemia Hypocalcemia Severe anaemia- cause or precipitating factor

Minor Systolic murmur < gr III Does not rule out heart disease- severe heart disease can present without murmur-TGA, TAPVC PSM due MR/TR can be because of myocardial disease.

Abnormal second heart sound Two components A 2 and P 2 Split- normal A 2>P 2 Normal S 2 - All 3 components. Finding a normal S 2 in a significant structural heart disease is practically zero.

Abnormal Electrocardiogram


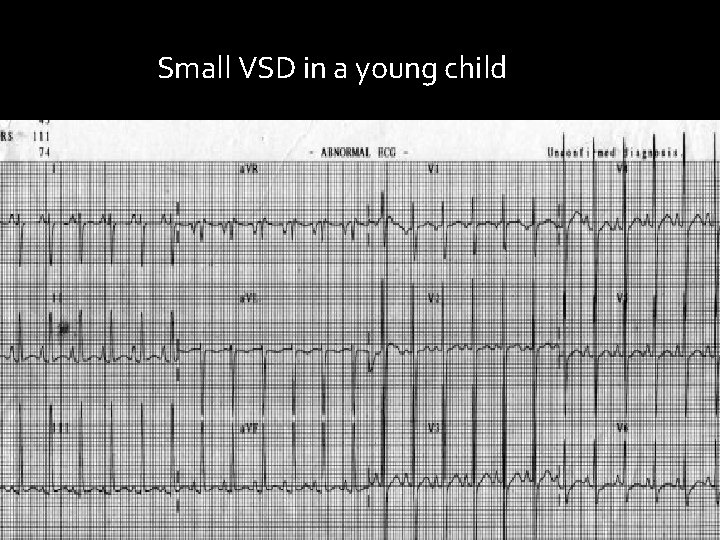
Small VSD in a young child

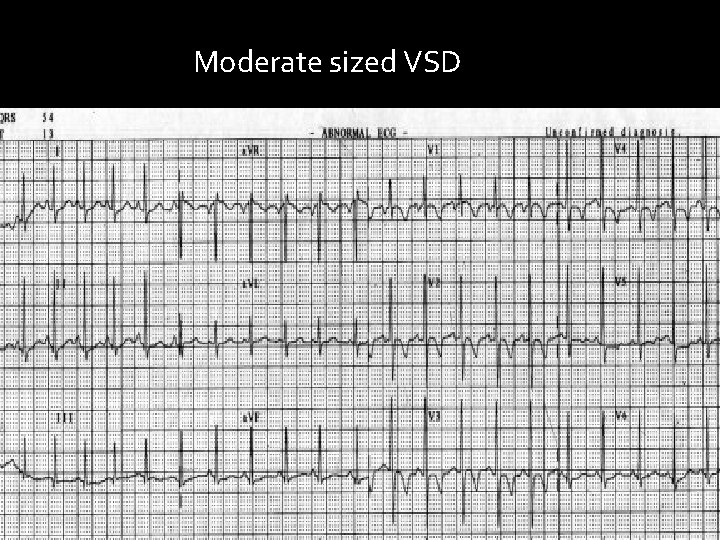
Moderate sized VSD


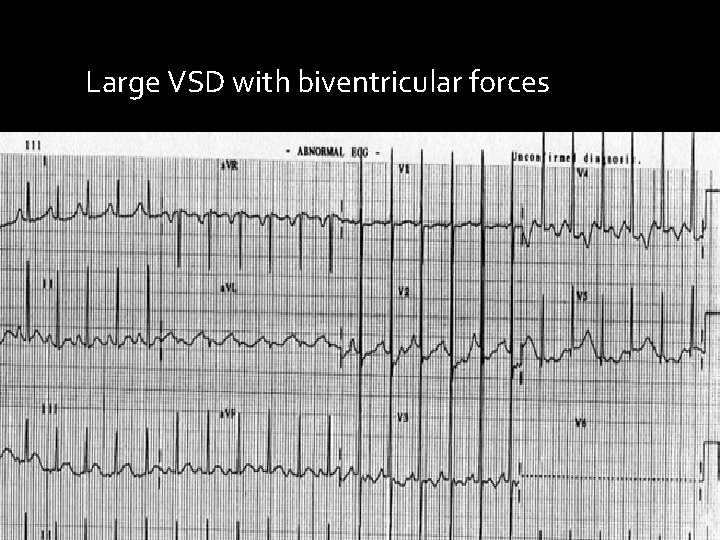
Large VSD with biventricular forces

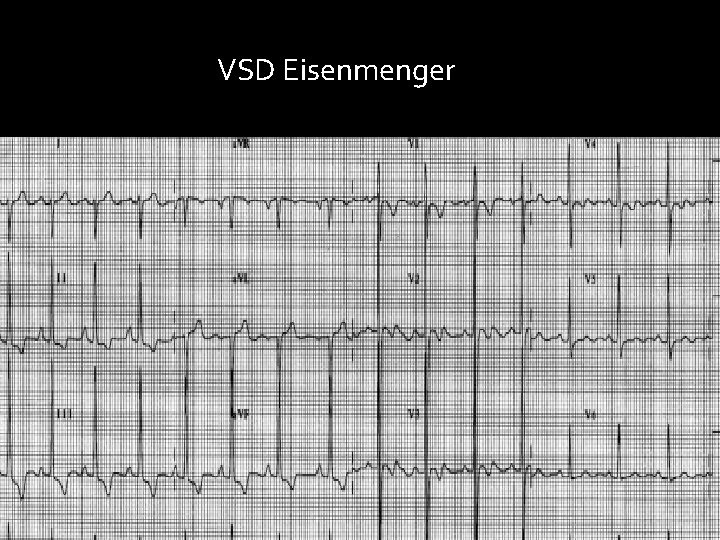
VSD Eisenmenger

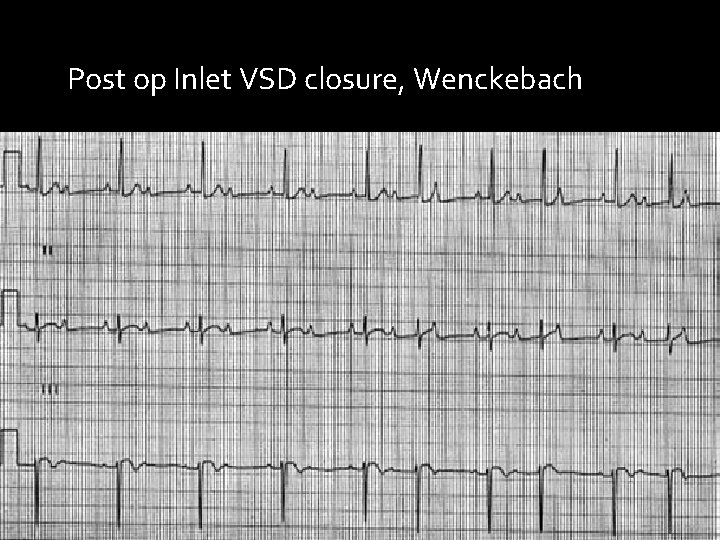
Post op Inlet VSD closure, Wenckebach

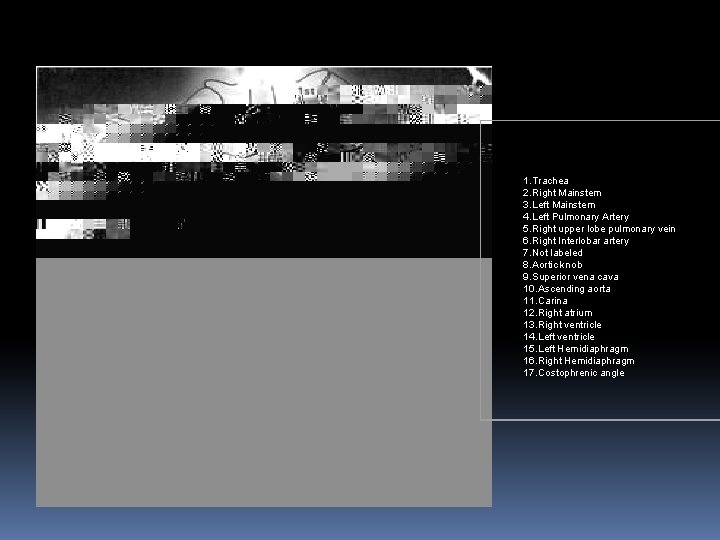
Abnormal chest roentgenogram


1. Trachea 2. Right Mainstem 3. Left Mainstem 4. Left Pulmonary Artery 5. Right upper lobe pulmonary vein 6. Right Interlobar artery 7. Not labeled 8. Aortic knob 9. Superior vena cava 10. Ascending aorta 11. Carina 12. Right atrium 13. Right ventricle 14. Left ventricle 15. Left Hemidiaphragm 16. Right Hemidiaphragm 17. Costophrenic angle

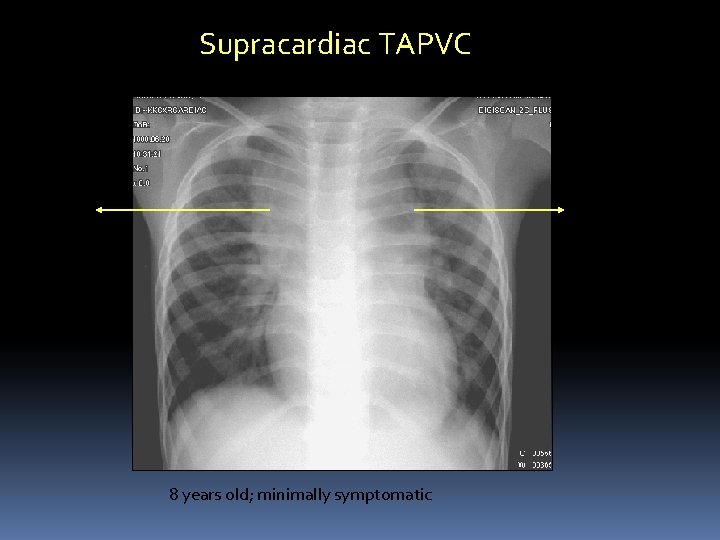
Supracardiac TAPVC Left vertical vein Dilated SVC 8 years old; minimally symptomatic

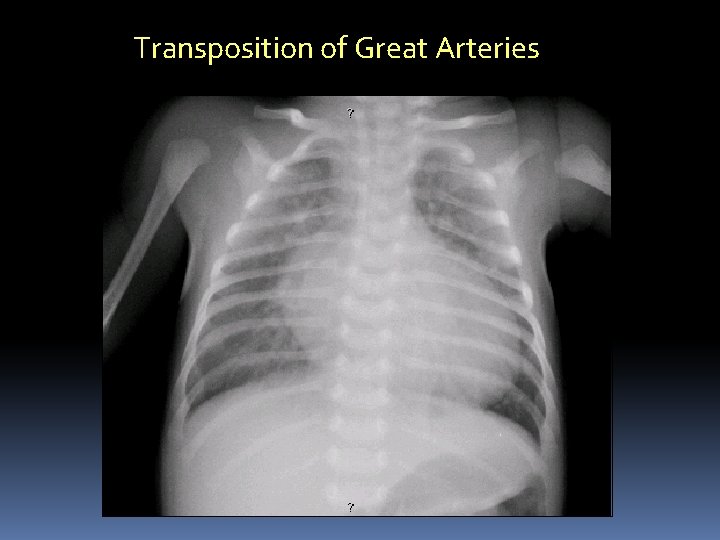
Transposition of Great Arteries


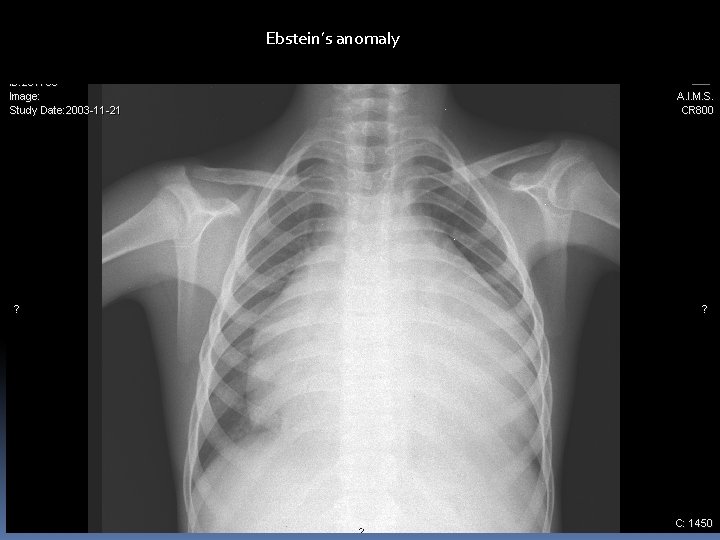
Ebstein’s anomaly

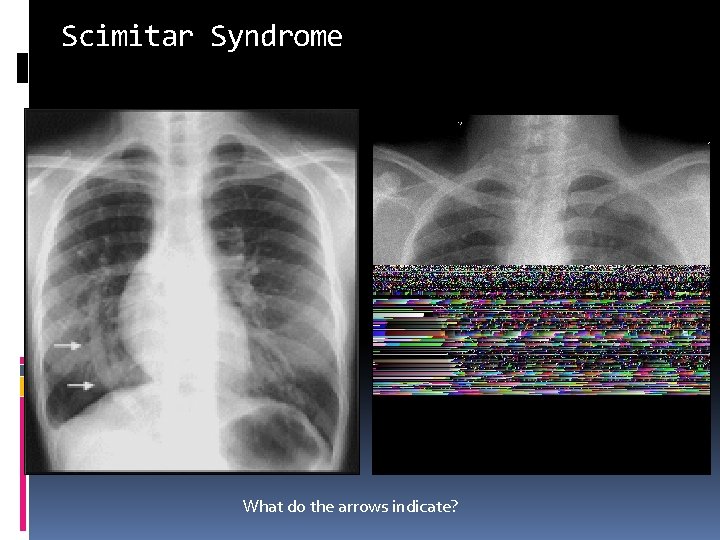
Scimitar Syndrome What do the arrows indicate?

Situs inversus, Dextrocardia, left arch

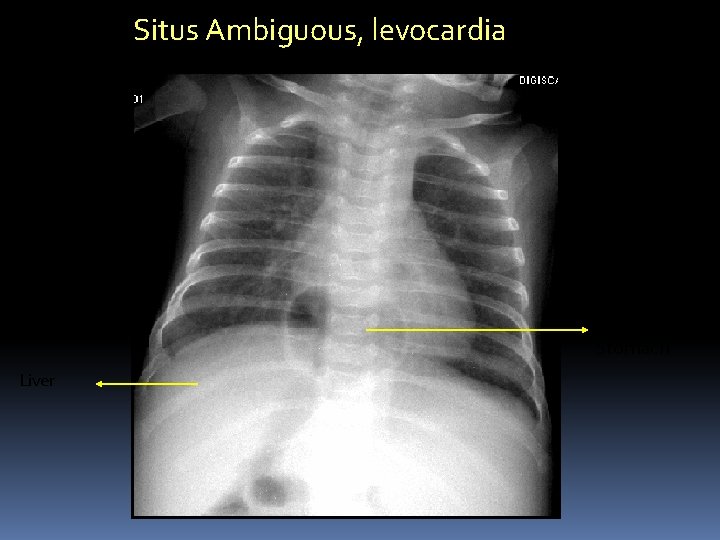
Situs Ambiguous, levocardia Mid line Stomach Liver

Device closure of ASD

Device closure of VSD

The Normal Heart

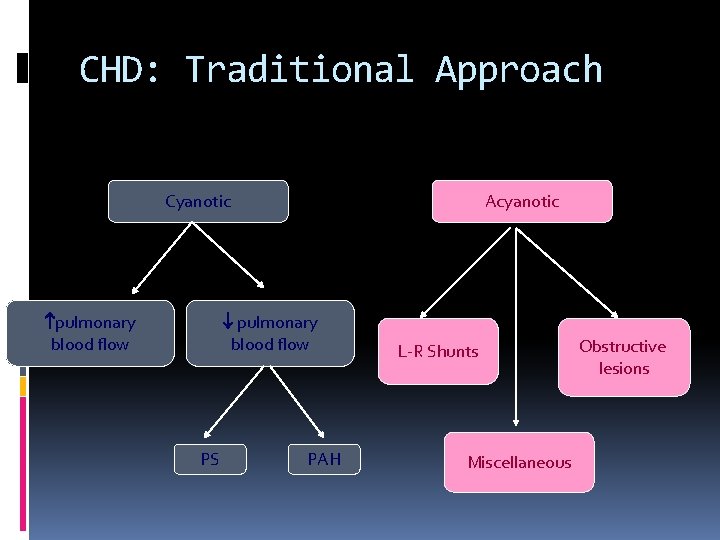
CHD: Traditional Approach Acyanotic Cyanotic pulmonary blood flow PS PAH L-R Shunts Miscellaneous Obstructive lesions

CHD - Classification Acyanotic CHD – where the shunting of blood is from left side to right side (pressure on the left is higher than right) �Pink �Breathlessness, esp on exertion �Recurrent respiratory tract infections �Failure to thrive Acyanotic CHD without shunt – malformations in the heart

CHD Classification Cyanotic CHD – Where shunting is from right side to left side (pressure in the right side is higher than on the left side) �Blue child – cyanosis �Clubbing �Cyanotic Spells �May occasionally be “pink”

Acyanotic CHD Without shunts Malformations of the heart on right side; on left side With shunts Level of shunt �Atrial - ASD �Ventricular - VSD �Great arteries – PDA, AP window �Multiple levels – AV canal defects �Misc – ALCAPA, RSOV, Coronary AV fistula

Cyanotic CHD With increased pulm blood flow TGA TAPVC Tricuspid atresia with non-restrictive VSD Common atrium Truncus arteriosus TOF with PA and MAPCAs

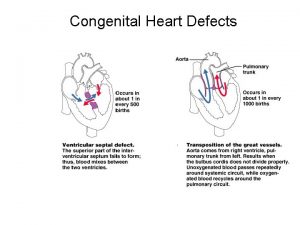
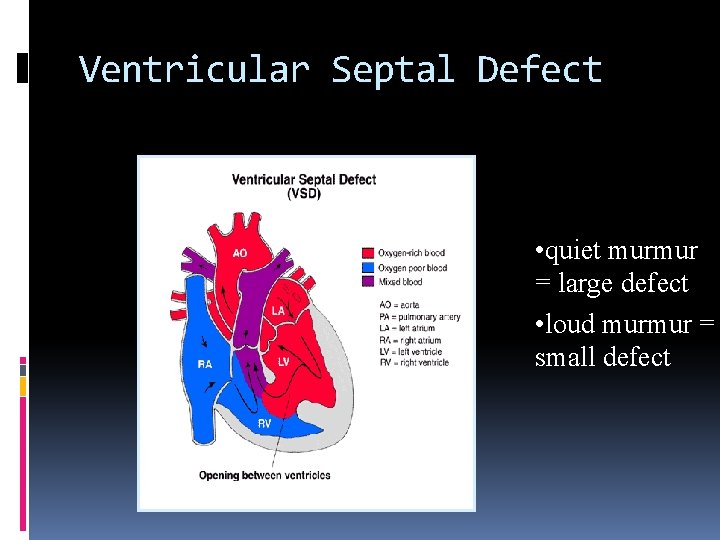
Ventricular Septal Defects 25 -30% of the cardiac defects Natural history depends on size and location of the defect Perimembranous or ‘subaortic’ (80%) Subpulmonic or outlet VSD Inlet VSD Trabecular or muscular VSD

Ventricular Septal Defect • quiet murmur = large defect • loud murmur = small defect

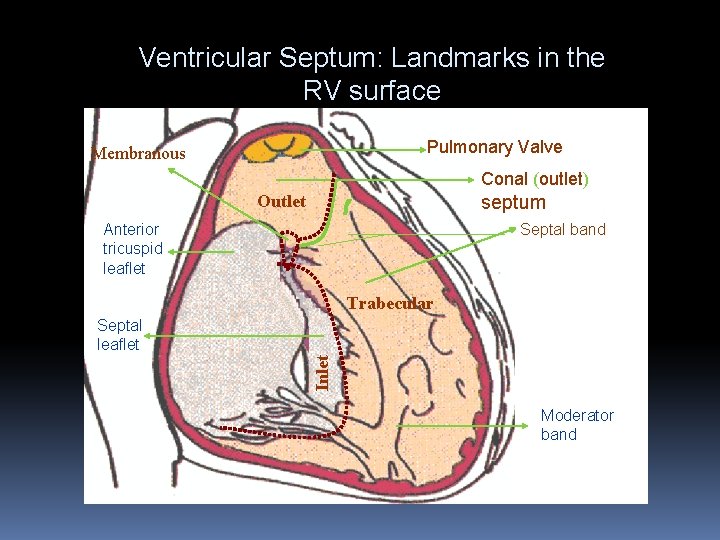
Ventricular Septum: Landmarks in the RV surface Pulmonary Valve Membranous Conal (outlet) Outlet septum Anterior tricuspid leaflet Septal band Trabecular Inlet Septal leaflet Moderator band

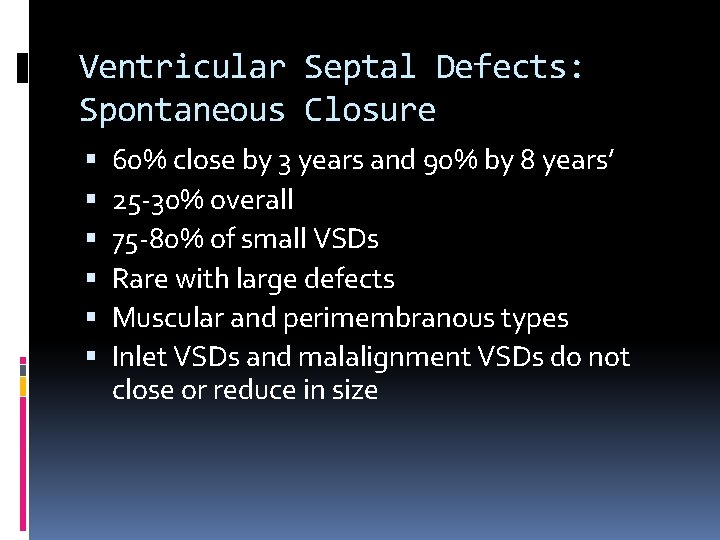
Ventricular Septal Defects: Spontaneous Closure 60% close by 3 years and 90% by 8 years’ 25 -30% overall 75 -80% of small VSDs Rare with large defects Muscular and perimembranous types Inlet VSDs and malalignment VSDs do not close or reduce in size

Large VSDs Heart Failure Pneumonia, can be life threatening Does not respond like usual community acquired pneumonias Failure to thrive Pulmonary vascular changes, can become inoperable fast

Timing Guidelines: Large Perimembranous VSD No symptoms: Anytime after 3 months Symptoms: Earlier Associated conditions & Trisomy 21 Age at operation beyond 1 -2 months does not impact surgical outcome

Timing Guidelines: Large Muscular VSD Higher likelihood of spontaneous closure Close watch for signs of spontaneous closure Medical management of CHF may be tried until the age of 6 months At times PA banding

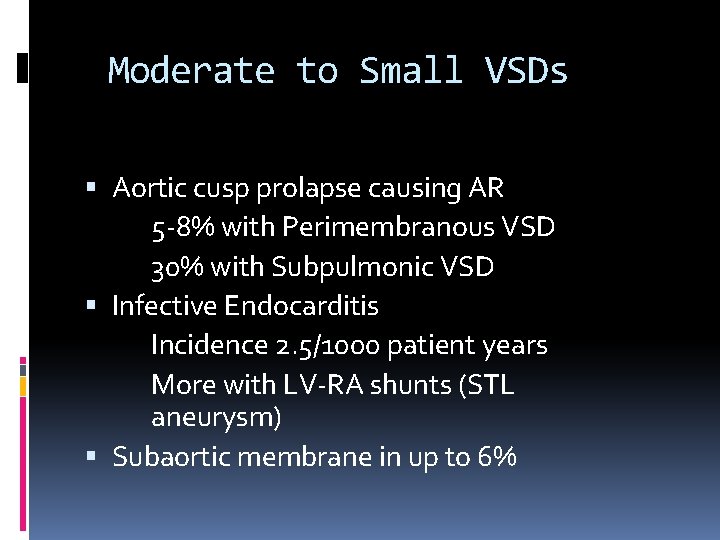
Moderate to Small VSDs Aortic cusp prolapse causing AR 5 -8% with Perimembranous VSD 30% with Subpulmonic VSD Infective Endocarditis Incidence 2. 5/1000 patient years More with LV-RA shunts (STL aneurysm) Subaortic membrane in up to 6%

Moderate VSD If symptomatic, operate If asymptomatic, decision based on the shunt Clinical assessment Echo: Significant LA and LV enlargement and degree of restriction

Small VSD Medical follow up Infective Endocarditis Prophylaxis Echo at regular intervals Operate if AR develops

Ventricular Septal Defects 25 -30% of the cardiac defects Natural history depends on size and location of the defect Perimembranous or ‘subaortic’ (80%) Subpulmonic or outlet VSD Inlet VSD Trabecular or muscular VSD

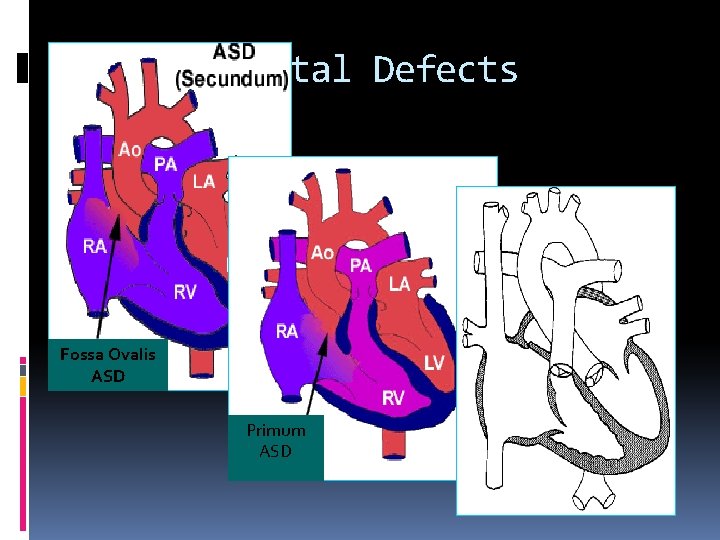
Atrial Septal Defect Incidence: Accounts for 14% of all CHD with female predominance (4: 1) Types: Sinus venosus Fossa ovalis or ostium secundum Ostimum primum

Atrial Septal Defects Fossa Ovalis ASD Primum ASD

ASD Wide fixed second sound P 2 is generally loud even without PAH Short Ejection murmur at pulmonary area = Small defect (<2: 1 shunt) 3/6 ESM + RV S 3 or Tricuspid MDM = Large defect (>2: 1 shunt)

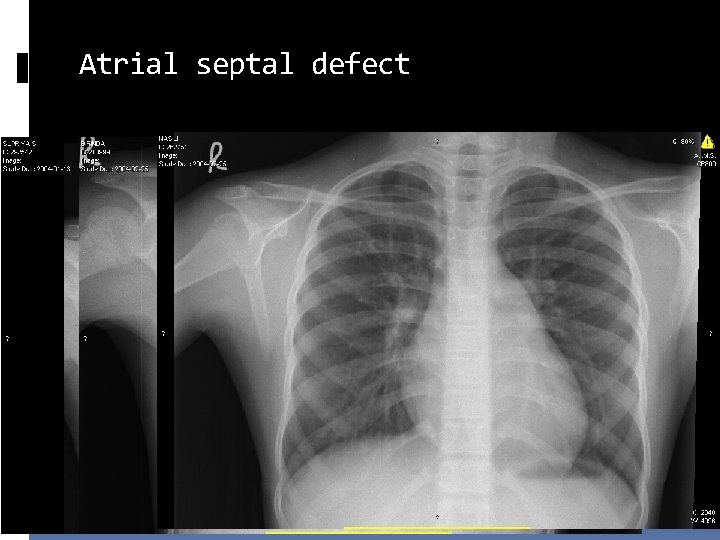
Atrial septal defect

Timing Guidelines: Large ASD No symptoms: Preschool age If symptomatic (510%): Intervene earlier, infant repair not associated with increased risk

Early closure of ASD Advisable if the child is symptomatic July 1998 – March 2004, 43 children aged <2 years 2. 3% of ASD repairs Indications: FTT or RTI ( 2 admissions) Follow up: No LRTI or admission Significant weight gain

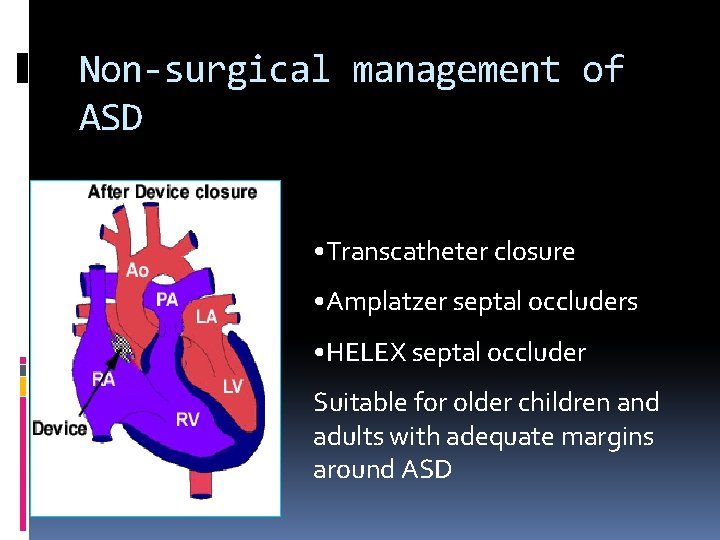
Non-surgical management of ASD • Transcatheter closure • Amplatzer septal occluders • HELEX septal occluder Suitable for older children and adults with adequate margins around ASD

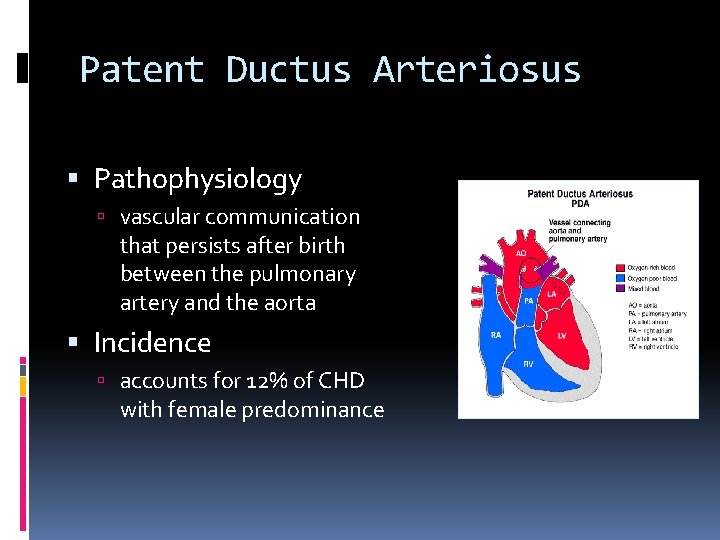
Patent Ductus Arteriosus Pathophysiology vascular communication that persists after birth between the pulmonary artery and the aorta Incidence accounts for 12% of CHD with female predominance

Patent Ductus Arteriosus Newborn – Preterm Vs Term Baby Role of Indomethacin or ibuprofen/ surgery Unlikely to close or reduce in size after a month in term babies Preterms may take longer

Patent Ductus Arteriosus Large Duct – Usually symptomatic Surgical intervention or transcatheter closure Asymptomatic PDA Risk of Infective endocarditis Large ducts develop Eisenmenger syndrome Silent PDA can be left alone

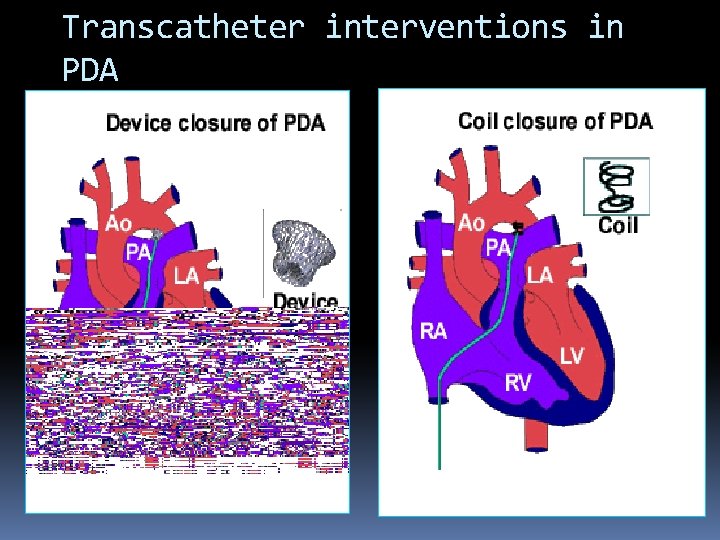
Transcatheter interventions in PDA

Patent Ductus Arteriosus Surgical Management VATS Ligation Division and suturing Transpulmonary closure
 Cyanotic vs acyanotic
Cyanotic vs acyanotic Tetralogy of fallot murmur
Tetralogy of fallot murmur Tet spell
Tet spell Congenital heart disease pda
Congenital heart disease pda Canadian congenital heart alliance
Canadian congenital heart alliance Congenital heart
Congenital heart Congenital heart defect
Congenital heart defect Communicable disease and non communicable disease
Communicable disease and non communicable disease Modern treatment of heart disease
Modern treatment of heart disease Pico question examples heart disease
Pico question examples heart disease Ronaldo heart disease
Ronaldo heart disease Hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy Heart disease data
Heart disease data Rheumatic heart disease
Rheumatic heart disease Mitral regurgitation symptoms
Mitral regurgitation symptoms Lvvo heart
Lvvo heart Terrible t's cyanotic heart disease
Terrible t's cyanotic heart disease Upper lobe blood diversion
Upper lobe blood diversion Pathophysiology of ischemic heart disease
Pathophysiology of ischemic heart disease Dopamine uses
Dopamine uses Pathophysiology of valvular heart disease
Pathophysiology of valvular heart disease Nursing assessment for congestive heart failure
Nursing assessment for congestive heart failure Pathophysiology of valvular heart disease
Pathophysiology of valvular heart disease Causes of valvular heart disease
Causes of valvular heart disease Ahd heart
Ahd heart Causes of valvular heart disease
Causes of valvular heart disease Heart disease
Heart disease Rhuematic
Rhuematic Coronary heart disease
Coronary heart disease Antianginal drugs classification
Antianginal drugs classification Rheumatic heart disease
Rheumatic heart disease Rheumatic heart disease causes
Rheumatic heart disease causes Heart disease
Heart disease Emedu ecg
Emedu ecg Nyha class
Nyha class Heart disease and stroke are the world's biggest killers
Heart disease and stroke are the world's biggest killers Vijaya's echo criteria
Vijaya's echo criteria кагами-огата
кагами-огата Congenital pneumonia
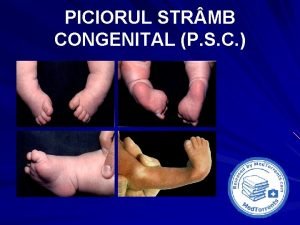
Congenital pneumonia Picior stramb congenital
Picior stramb congenital Trabeculodysgenesis
Trabeculodysgenesis Congenital rubella syndrome
Congenital rubella syndrome Congenital malformations
Congenital malformations Congenital voice disorders
Congenital voice disorders Hormone type 4
Hormone type 4 Congenital fibrosis of the extraocular muscles
Congenital fibrosis of the extraocular muscles Choanal atresia,
Choanal atresia, Most common congenital anomalies
Most common congenital anomalies Dr dawn lim
Dr dawn lim Congenital rubella syndrome triad
Congenital rubella syndrome triad Congenital cardiologist near me
Congenital cardiologist near me Congenital rubella syndrome triad
Congenital rubella syndrome triad Congenital adrenal hyperplasia characteristics
Congenital adrenal hyperplasia characteristics Potter face oligohydramnios
Potter face oligohydramnios Congenital hydronephrosis
Congenital hydronephrosis Congenital limb deficiency
Congenital limb deficiency Site:slidetodoc.com
Site:slidetodoc.com Michael addidle
Michael addidle Kode icd 10 clubfoot
Kode icd 10 clubfoot Congenital toxoplasmosis
Congenital toxoplasmosis Choledocolithaisis
Choledocolithaisis Congenital glaucoma
Congenital glaucoma