APL Pierre Fenaux Hopital Avicenne Paris 13 University























































- Slides: 94

APL Pierre Fenaux (Hopital Avicenne, Paris 13 University)

Etiology of APL About 10% of AML (about 130 new cases/year in France, probably more than 2000/year in India) n Incidence depending on ethnic (or environmental) factors n More and more often therapy related (after brest carcinoma) n

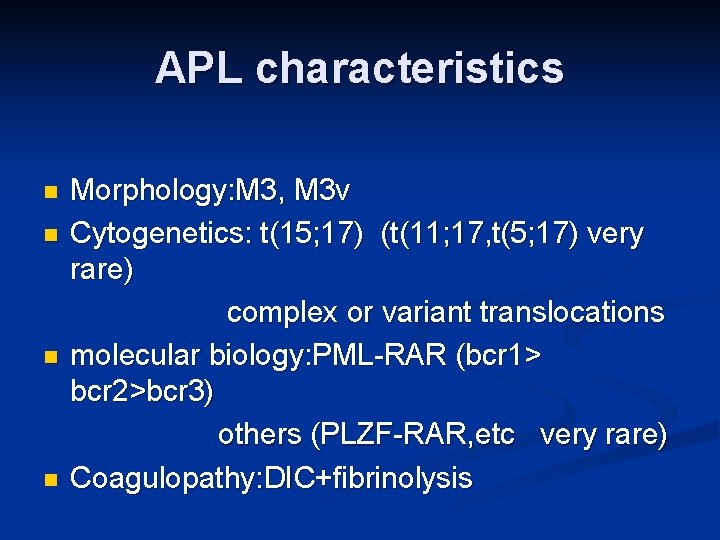
APL characteristics Morphology: M 3, M 3 v n Cytogenetics: t(15; 17) (t(11; 17, t(5; 17) very rare) complex or variant translocations n molecular biology: PML-RAR (bcr 1> bcr 2>bcr 3) others (PLZF-RAR, etc very rare) n Coagulopathy: DIC+fibrinolysis n

Prognostic factors in APL n WBC >10000/mm 3 (and platelets<40000/mm 3) (Sanz score) n RT-PCR analysis after consolidation treatment (but depends on sensitivity of the assay used) n other factors (M 3 v, bcr breakpoint…. : generally redundant with

Treatment of APL sensitive to n Anthracycline+/- Ara. C chemotherapy n ATRA n Arsenic derivatives

Treatment of APL n « classical » treatment n. Treatment with no or limited amount of chemotherapy?

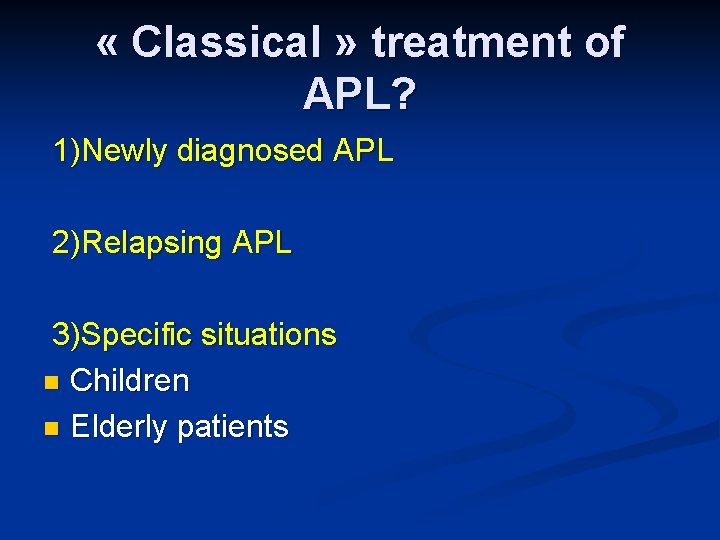
« Classical » treatment of APL? 1)Newly diagnosed APL 2)Relapsing APL 3)Specific situations n Children n Elderly patients

Newly diagnosed APL : Induction and consolidation treatment should combine ATRA and anthracycline based chemotherapy: n n Slightly increases the CR rate (from 80 to>90%) considerably reduces relapses (from 50 to 25%)

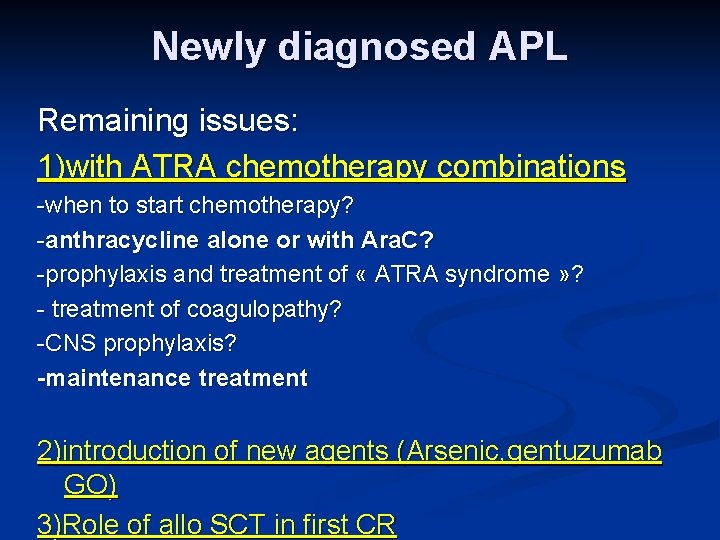
Newly diagnosed APL Remaining issues: 1)with ATRA chemotherapy combinations -when to start chemotherapy? -anthracycline alone or with Ara. C? -prophylaxis and treatment of « ATRA syndrome » ? - treatment of coagulopathy? -CNS prophylaxis? -maintenance treatment 2)introduction of new agents (Arsenic, gentuzumab GO) 3)Role of allo SCT in first CR

When should chemotherapy be started?

APL 93: INDUCTION TREATMENT. WBC 5000/mm 3 and age 65: R ATRA followed by chemotherapy ATRA CT group ATRA + chemotherapy started day 3 ATRA + CT group ATRA : 45 mg/m 2/d until CR chemotherapy : daunorubicin (DNR) 60 mg/m 2/d d 1 -3 Ara. C 200 mg/m 2/d d 1 -7

CONSOLIDATION AND MAINTENANCE TREATMENT no maintenance chemotherapy Ara. C 200 mg/m 2/d d 1 -7 Ara. C 1 g/m 2/12 h d 1 -4 DNR 60 mg/m 2/d d 1 -3 DNR 45 mg/m 2/d d 1 -3 . 6 mercaptopurine (90 mg/m 2/day). methotrexate (15 mg/m 2/week) R intermittent ATRA patients < 65 years 45 mg/m 2/d 15 days/ 3 months patients 66 -75 years both 2 years

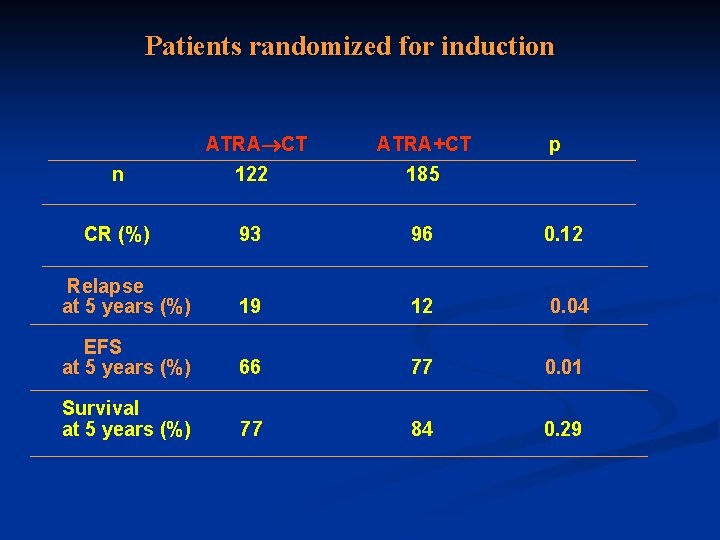
Patients randomized for induction ATRA CT ATRA+CT p n 122 185 CR (%) 93 96 0. 12 Relapse at 5 years (%) 19 12 0. 04 EFS at 5 years (%) 66 77 0. 01 Survival at 5 years (%) 77 84 0. 29

Prophylaxis and treatment of ATRA syndrome? High dose steroids or chemotherapy?

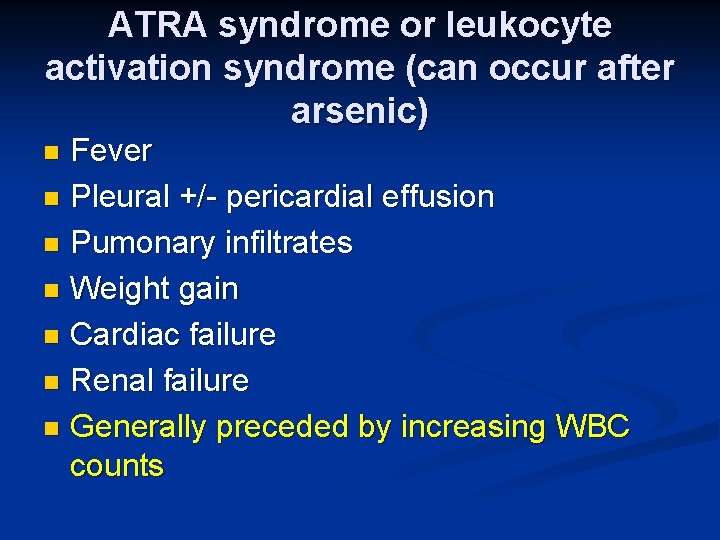
ATRA syndrome or leukocyte activation syndrome (can occur after arsenic) Fever n Pleural +/- pericardial effusion n Pumonary infiltrates n Weight gain n Cardiac failure n Renal failure n Generally preceded by increasing WBC counts n

Prophylaxis and treatment of ATRA syndrome 1)Treatment : high dose DXM (10 mg/12 H) 2)Prophylaxis increasing WBC: - Add chemotherapy? - Add high dose DXM?

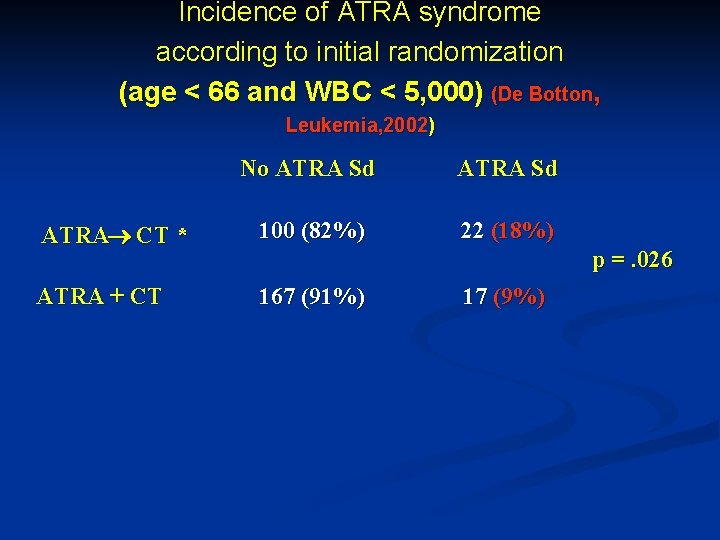
Incidence of ATRA syndrome according to initial randomization (age < 66 and WBC < 5, 000) (De Botton, Leukemia, 2002) No ATRA Sd ATRA CT * 100 (82%) 22 (18%) ATRA + CT 167 (91%) 17 (9%) ] p =. 026

Should Ara. C be added to anthracyclines?

PETHEMA LPA 96 PETHEMA LPA 99 INDUCTION Nov/96 - Oct/99 AIDA CONSOLIDATION Median follow up 70 mo. Nov/99 - Present CONSOLIDATION Risk-Adapted MAINTENANCE MTX + 6 -MP + ATRA Median follow up 30 mo.

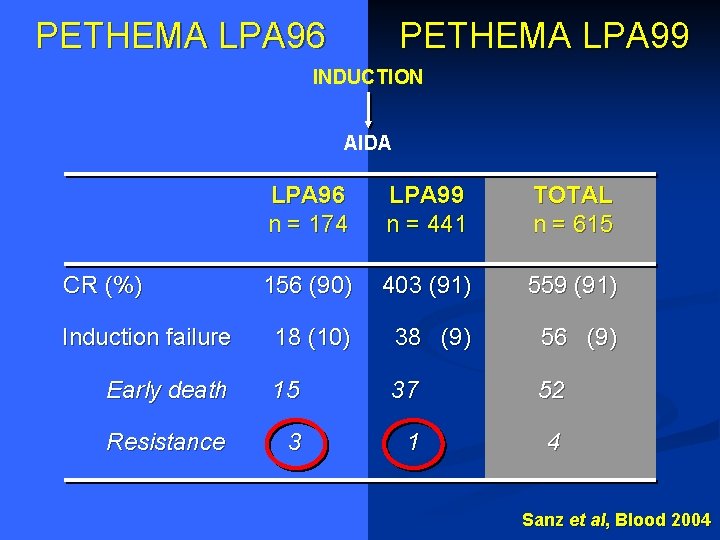
PETHEMA LPA 96 PETHEMA LPA 99 INDUCTION AIDA LPA 96 n = 174 LPA 99 n = 441 TOTAL n = 615 156 (90) 403 (91) 559 (91) 18 (10) 38 (9) 56 (9) Early death 15 37 52 Resistance 3 1 4 CR (%) Induction failure Sanz et al, Blood 2004

LPA 96 & LPA 99 Trials Clinical and molecular relapse LPA 96 LPA 99 N=403 N=156 Molecular persistence 5 Molecular relapse 7 Clinical relapse* CNS relapse P = 0. 03 28 16 5 (5 to 49 mo) 2 6 21 13 4 (8 to 28 mo)

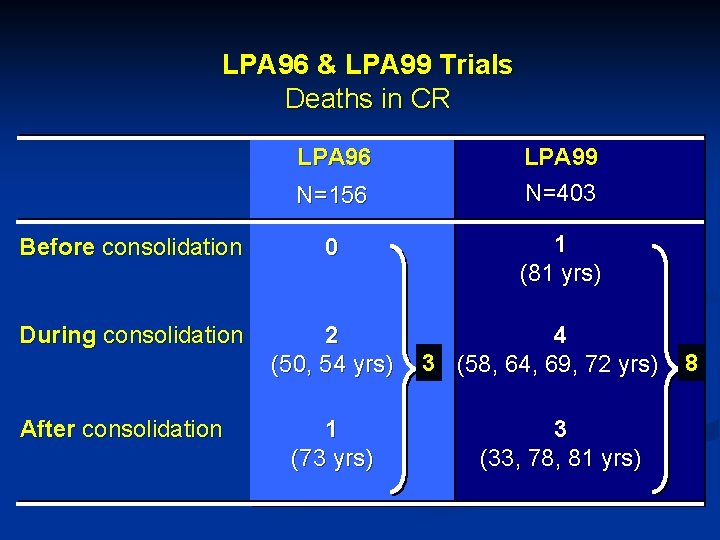
LPA 96 & LPA 99 Trials Deaths in CR LPA 96 N=156 LPA 99 N=403 Before consolidation 0 1 (81 yrs) During consolidation 2 (50, 54 yrs) 4 3 (58, 64, 69, 72 yrs) 8 1 (73 yrs) 3 (33, 78, 81 yrs) After consolidation

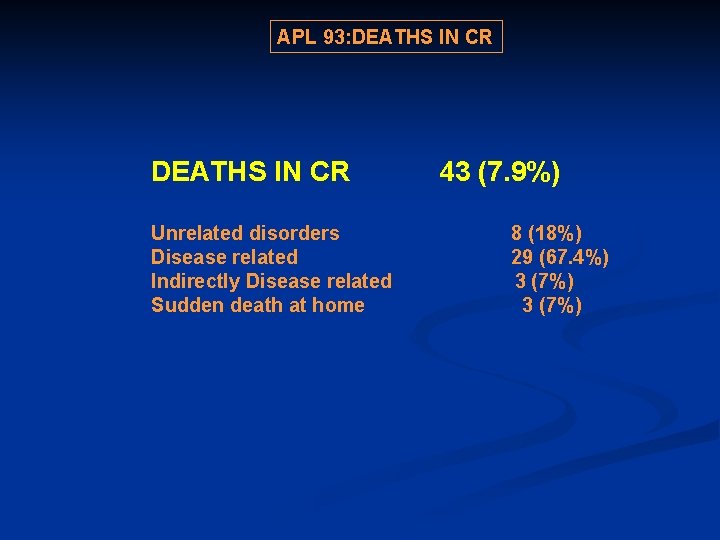
APL 93: DEATHS IN CR 43 (7. 9%) Unrelated disorders 8 (18%) Disease related 29 (67. 4%) Indirectly Disease related 3 (7%) Sudden death at home 3 (7%)

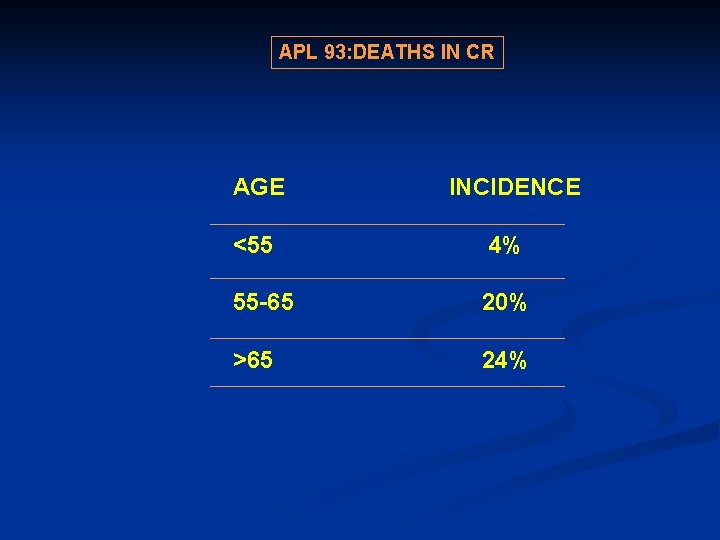
APL 93: DEATHS IN CR AGE INCIDENCE <55 4% 55 -65 20% >65 24%

APL 2000 trial Patients aged <60 with WBC<10000/mm 3: Reference arm (APL 93): ATRA+DNR+ Ara. C+ combined maintenance (ARA C+) VS same without Ara. C (ARA C -)

Chemotherapy: Ara. C + vs Ara. C- NO ARA C + n CR rate Leuke mic resist ance 87 94% 2 2 yr cum EFS OS relaps e 11. 9% 83. 4% 89. 9% 80 98% 0 3. 8% 93. 6% 97. 4%

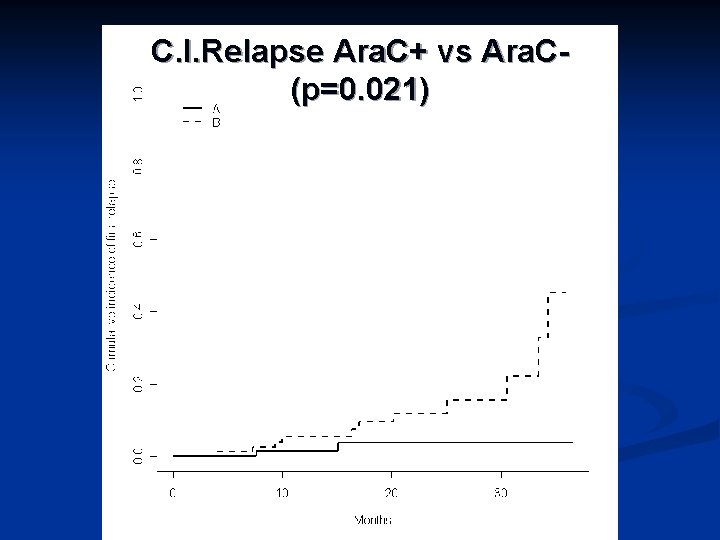
C. I. Relapse Ara. C+ vs Ara. C(p=0. 021)

Overall survival Ara. C+ vs Ara. C- (p=0. 085)

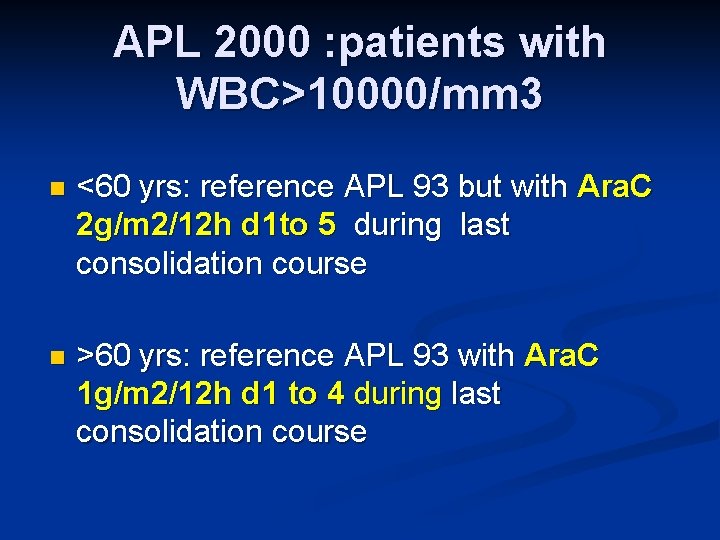
APL 2000 : patients with WBC>10000/mm 3 n <60 yrs: reference APL 93 but with Ara. C 2 g/m 2/12 h d 1 to 5 during last consolidation course n >60 yrs: reference APL 93 with Ara. C 1 g/m 2/12 h d 1 to 4 during last consolidation course

APL 2000 trial: patients with WBC>10000/mm 3

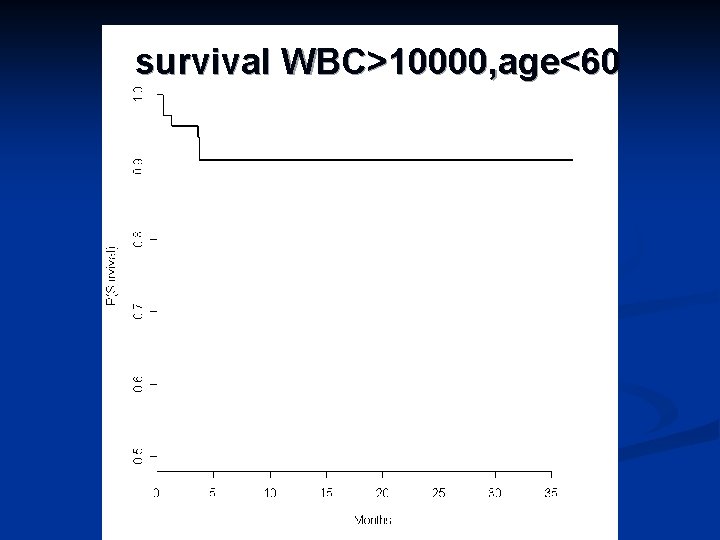
survival WBC>10000, age<60

High dose Ara. C in APL (Lengfelder, ASH 2003) 133 pts treated with ATRA and DAT-HAM -DAT -maintenance n 89% CR, 9% relapse n WBC<5000: 6% relapse n WBC>5000: 14% relapse n

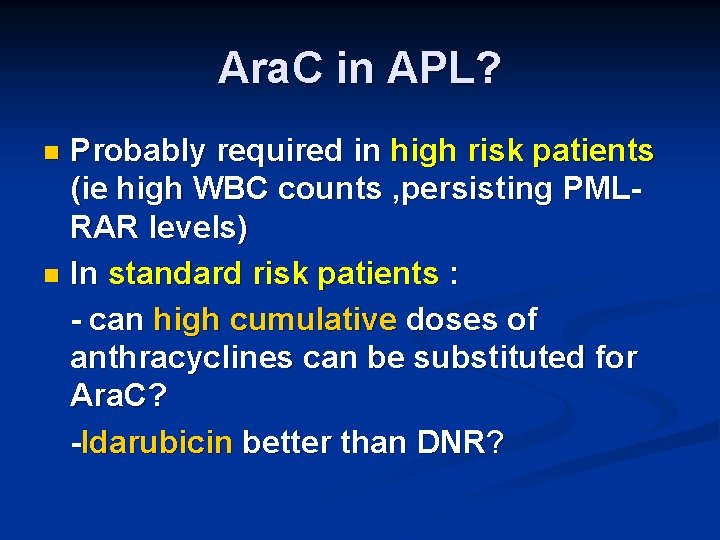
Ara. C in APL? Probably required in high risk patients (ie high WBC counts , persisting PMLRAR levels) n In standard risk patients : - can high cumulative doses of anthracyclines can be substituted for Ara. C? -Idarubicin better than DNR? n

Should CNS prophylaxis be made?

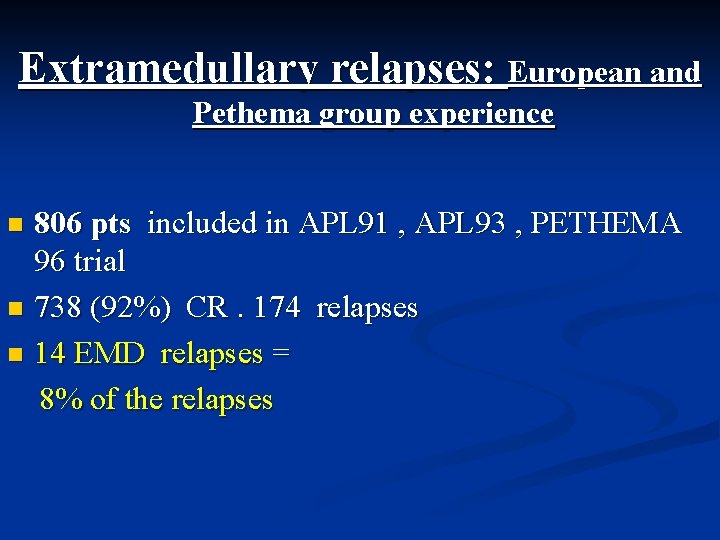
Extramedullary relapses: European and Pethema group experience 806 pts included in APL 91 , APL 93 , PETHEMA 96 trial n 738 (92%) CR. 174 relapses n 14 EMD relapses = 8% of the relapses n

EM site : CNS (n=10), skin (n=3), orbital (n=1) n Associated bone marrow (BM) relapse (n=9) (only molecular in 4 of them) n

Patients with EM relapse characterized, by n younger age (p=. 03) n higher WBC counts (p=. 007 ) n N 0 high dose Ara. C (p=0. 03)

Outcome of EMD relapses n n 4 (29%) pts still alive after 41+ to 53+ months. Median survival from EMD 13 months, Supports CNS treatment in pts with high WBC counts(>10000) -intratheca. I MTX+ Ara. C -high dose Ara. C?

Treatment of coagulopathy n Intensive platelet support (maintain plts>50000/mm 3) n 0 thers measures (heparin, antifibrinolytic agents, fibrinogen)?

Should APL patients receive maintenance therapy?

Maintenance treatment in APL Is it useful? n What should be used? n For how long? n

CONSOLIDATION AND MAINTENANCE TREATMENT no maintenance chemotherapy Ara. C 200 mg/m 2/d d 1 -7 Ara. C 1 g/m 2/12 h d 1 -4 DNR 60 mg/m 2/d d 1 -3 DNR 45 mg/m 2/d d 1 -3 . 6 mercaptopurine (90 mg/m 2/day). methotrexate (15 mg/m 2/week) R intermittent ATRA 2 patients < 65 years 45 mg/m /d 15 days/ 3 months patients 66 -75 years both 2 years

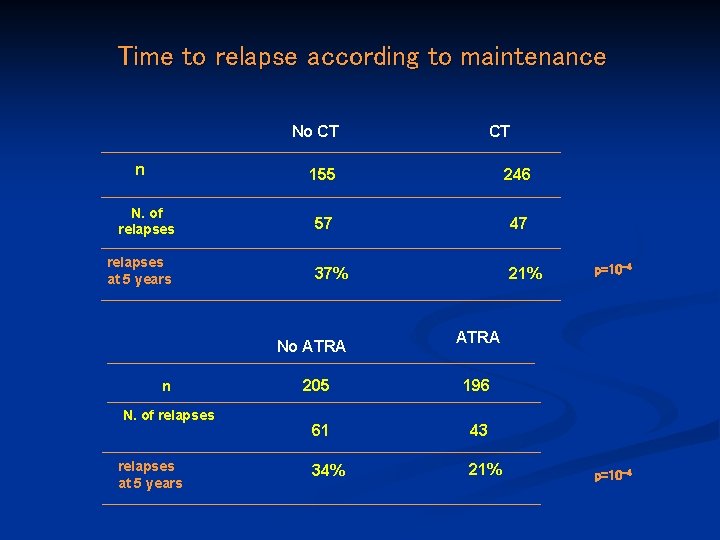
Time to relapse according to maintenance No CT n N. of relapses at 5 years 155 246 57 47 37% 21% No ATRA n N. of relapses at 5 years CT p=10 -4 ATRA 205 196 61 43 34% 21% p=10 -4

0. 4 0. 2 p = 0. 0004 no CT 0. 0 P(no relapse) 0. 6 0. 8 1. 0 Time to relapse according to Chemotherapy 0 500 1000 1500 days 2000 2500

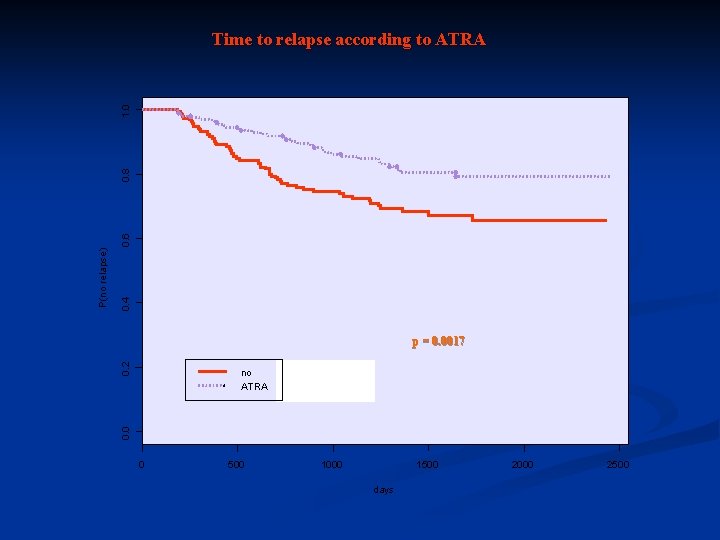
0. 4 0. 2 p = 0. 0017 no ATRA 0. 0 P(no relapse) 0. 6 0. 8 1. 0 Time to relapse according to ATRA 0 500 1000 1500 days 2000 2500

Survival according to maintenance No CT n CT 155 246 N. of deaths 36 32 Survival 77% 87% p= 0. 004 No ATRA n N. of deaths Survival ATRA 205 196 37 31 81% 85% p=0. 3

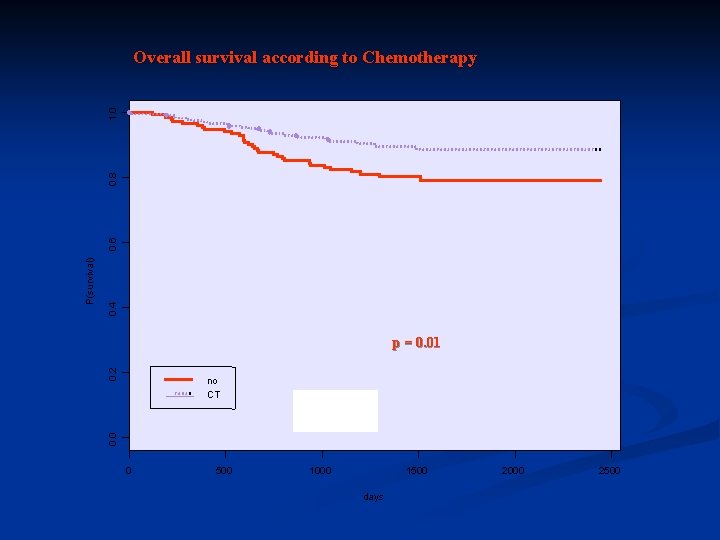
0. 4 0. 2 p = 0. 01 no CT 0. 0 P(survival) 0. 6 0. 8 1. 0 Overall survival according to Chemotherapy 0 500 1000 1500 days 2000 2500

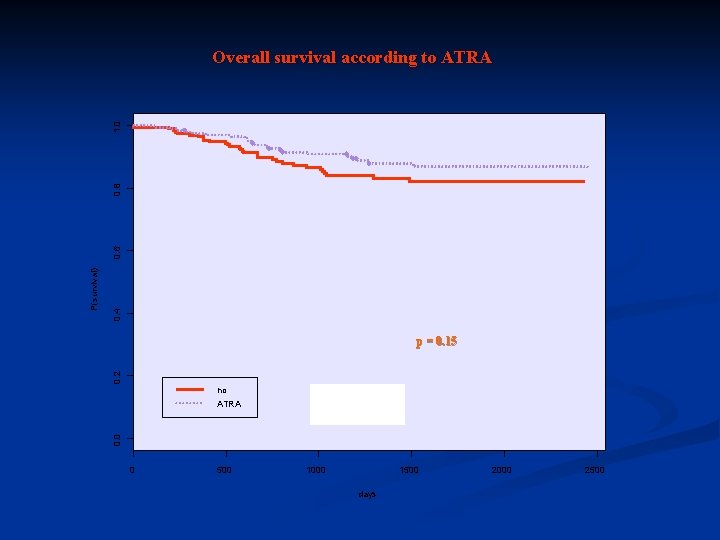
0. 4 0. 2 p = 0. 15 no ATRA 0. 0 P(survival) 0. 6 0. 8 1. 0 Overall survival according to ATRA 0 500 1000 1500 days 2000 2500

0. 8 no CT ATRA+CT 0 0. 2 0. 4 0. 6 p=10 -4 0 P P (no relpase) 1. 0 Time to relapse according to second randomization 500 1000 1500 days 2000 2500

Time to relapse according to second randomization in patients with WBC counts >5000/mm 3

Maintenance treatment in APL USEFUL: US intergroup (continuous ATRA during one year)

Duration of maintenance treatment (APL 93 trial): 46 patients < 1 year ( due to side effects): 21/46 (45%) relapses 313 patients > 1 year: 49/313 (16%) relapses Maintenance discontinuation <1 year may be deleterious

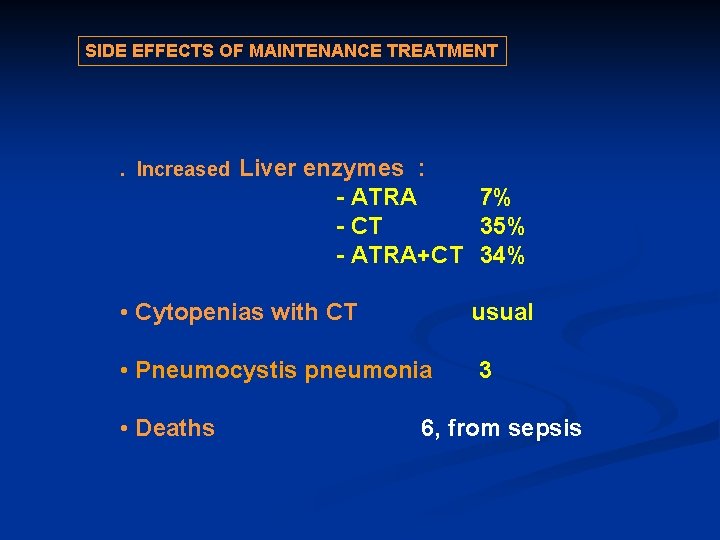
SIDE EFFECTS OF MAINTENANCE TREATMENT . Increased Liver enzymes : - ATRA 7% - CT 35% - ATRA+CT 34% • Cytopenias with CT usual • Pneumocystis pneumonia 3 • Deaths 6, from sepsis

Introduction of new agents (ATO, GO) in first line treatment -

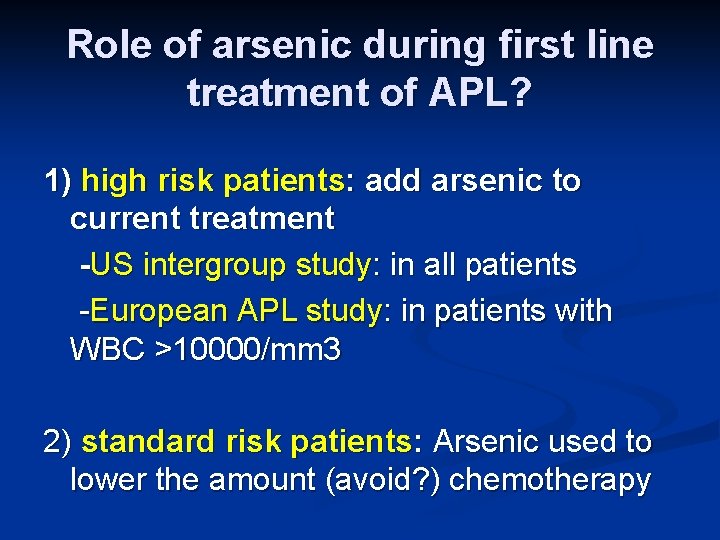
Role of arsenic during first line treatment of APL? 1) high risk patients: add arsenic to current treatment -US intergroup study: in all patients -European APL study: in patients with WBC >10000/mm 3 2) standard risk patients: Arsenic used to lower the amount (avoid? ) chemotherapy

A role for allo SCT in first CR?

Allo SCT in first CR : rarely indicated For patients remaining RT-PCR positive after induction? n For patients with VERY high WBC counts (eg>50000/mm 3)? n

First line treatment of APL with limited (or no? ) chemotherapy

Treatment of APL with Arsenic and limited amounts of chemotherapy Lu et al (Blood, 2002) : As 4 S 4 1. 103 APL in CR after ATRA (n = 19) ATRA + CT (n = 84) - As 4 S 4 2 weeks on/2 weeks off during 4 years - 9 relapses 2. 19 newly diagnosed APL - As 4 S 4 for induction - 19 CR - As 4 S 4 for maintenance : 3/15 patients

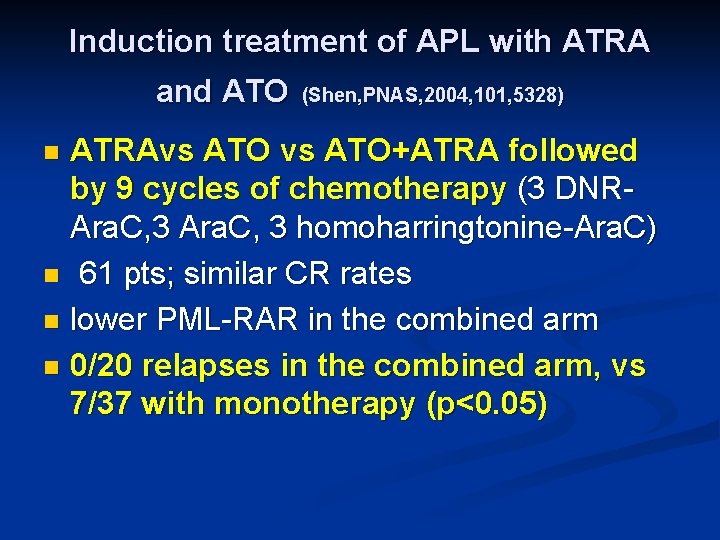
Induction treatment of APL with ATRA and ATO (Shen, PNAS, 2004, 101, 5328) ATRAvs ATO+ATRA followed by 9 cycles of chemotherapy (3 DNRAra. C, 3 homoharringtonine-Ara. C) n 61 pts; similar CR rates n lower PML-RAR in the combined arm n 0/20 relapses in the combined arm, vs 7/37 with monotherapy (p<0. 05) n

ATRA+ATO without chemotherapy in standard risk APL (Estey et al) ATRA 45 mg/m 2/d+ATO 0. 15 mg/kg. d (+GO or idarubicin if WBC>10000) n then ATRA 15 d/month +ATO 5 d/w 4 w/month during 6 months n 32 pts, 88% CR (including molecular CR) n Only 3 relapses n

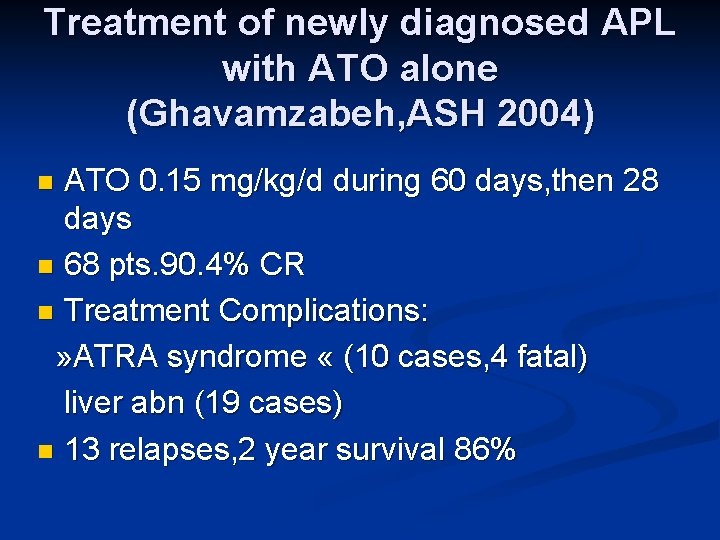
Treatment of newly diagnosed APL with ATO alone (Ghavamzabeh, ASH 2004) ATO 0. 15 mg/kg/d during 60 days, then 28 days n 68 pts. 90. 4% CR n Treatment Complications: » ATRA syndrome « (10 cases, 4 fatal) liver abn (19 cases) n 13 relapses, 2 year survival 86% n

Treatment of relapsing APL

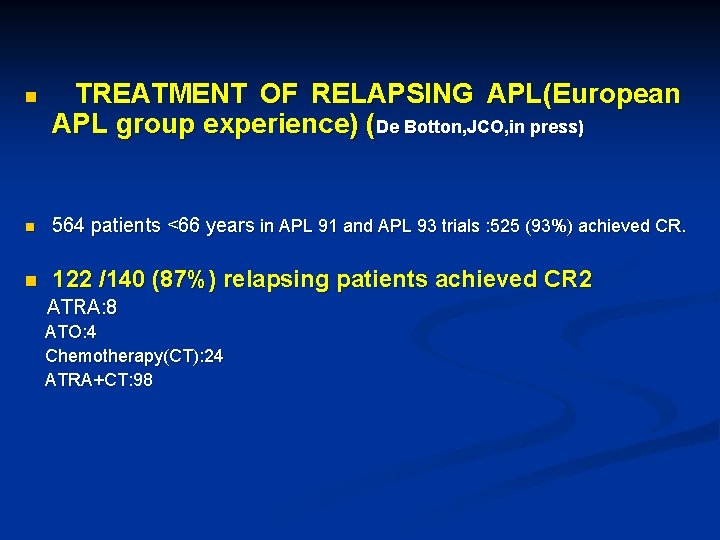
n TREATMENT OF RELAPSING APL(European APL group experience) (De Botton, JCO, in press) n 564 patients <66 years in APL 91 and APL 93 trials : 525 (93%) achieved CR. n 122 /140 (87%) relapsing patients achieved CR 2 ATRA: 8 ATO: 4 Chemotherapy(CT): 24 ATRA+CT: 98

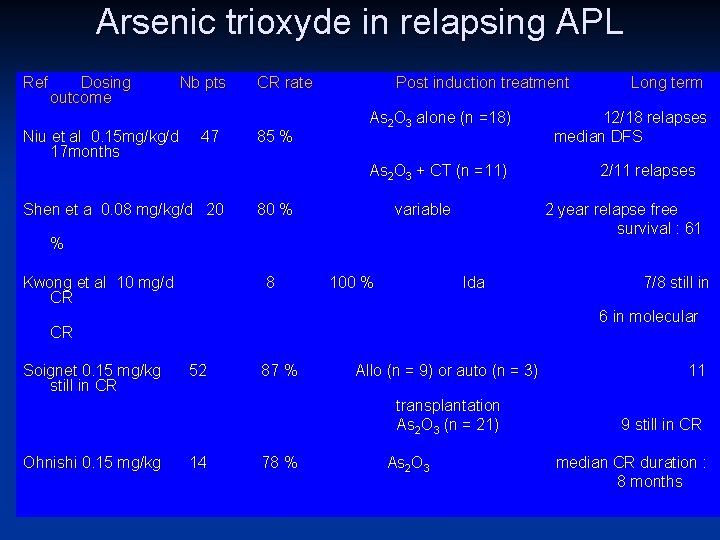
Arsenic trioxyde in relapsing APL Ref Dosing Nb pts CR rate Post induction treatment Long term outcome As 2 O 3 alone (n =18) 12/18 relapses Niu et al 0. 15 mg/kg/d 47 85 % median DFS 17 months As 2 O 3 + CT (n =11) 2/11 relapses Shen et a 0. 08 mg/kg/d 20 80 % variable % Kwong et al 10 mg/d CR 8 2 year relapse free survival : 61 100 % Ida 7/8 still in 6 in molecular CR Soignet 0. 15 mg/kg 52 still in CR 87 % Allo (n = 9) or auto (n = 3) transplantation As 2 O 3 (n = 21) Ohnishi 0. 15 mg/kg 14 78 % As 2 O 3 11 9 still in CR median CR duration : 8 months

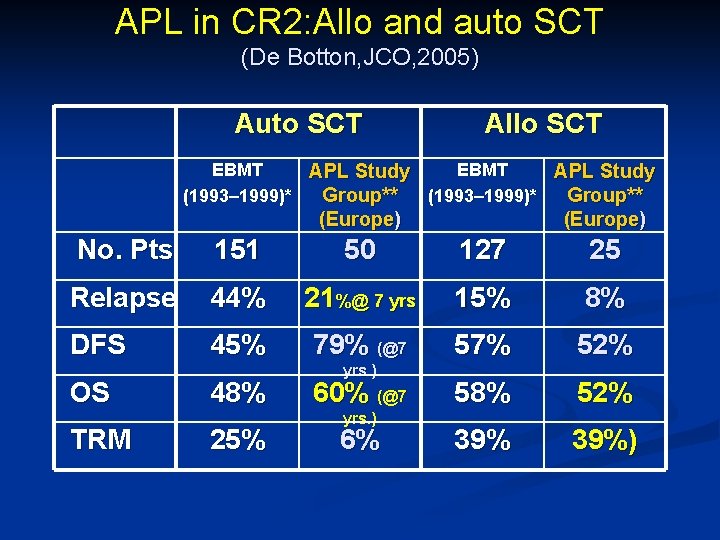
APL in CR 2: Allo and auto SCT (De Botton, JCO, 2005) Auto SCT Allo SCT EBMT APL Study (1993– 1999)* Group** (Europe) No. Pts 151 50 127 25 Relapse 44% 21%@ 7 yrs 15% 8% DFS 45% 79% (@7 57% 52% 58% 52% 39%) OS TRM 48% 25% yrs. ) 60% (@7 yrs. ) 6%

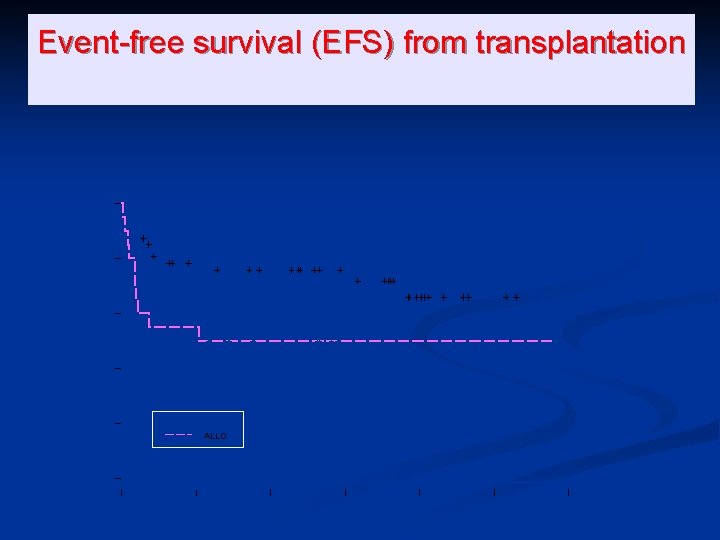
0. 6 0. 4 0. 2 AUTO ALLO 0. 0 P(No event) 0. 8 1. 0 Event-free survival (EFS) from transplantation 0 500 1000 1500 Days from transplant 2000 2500 3000

Allo SCT after ATO Salvage Post-transplant survival (Soignet, Dombret) 100 % 1 0 0 % 8 0 % 6 0 % At risk deaths 2 -year est. 22 4 86% 40 % 4 0 % 2 0 % 0 % 0 12 24 1 st rel. 36 48 Months Median follow-up: 30 months post SCT (range, 9. 5 to 45) > 2 nd rel. 0 12 At risk deaths 2 -year est. 16 3 88% 6 1 83% 24 36 48 Months

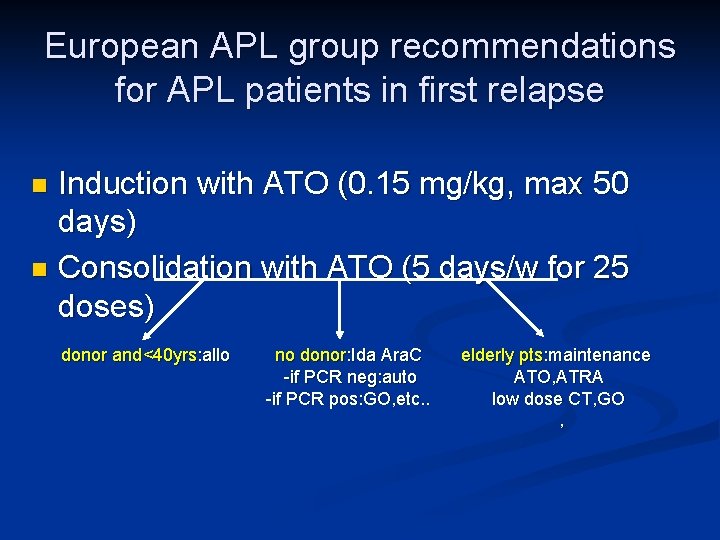
European APL group recommendations for APL patients in first relapse Induction with ATO (0. 15 mg/kg, max 50 days) n Consolidation with ATO (5 days/w for 25 doses) n donor and<40 yrs: allo no donor: Ida Ara. C -if PCR neg: auto -if PCR pos: GO, etc. . elderly pts: maintenance ATO, ATRA low dose CT, GO ,

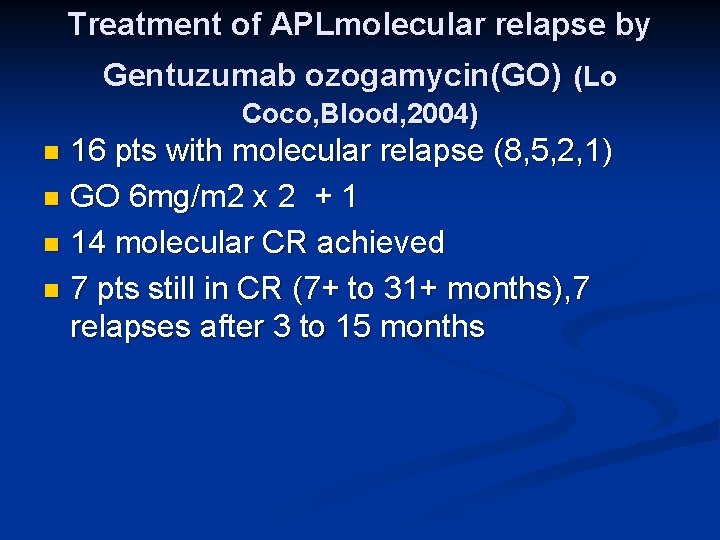
Treatment of APLmolecular relapse by Gentuzumab ozogamycin(GO) (Lo Coco, Blood, 2004) 16 pts with molecular relapse (8, 5, 2, 1) n GO 6 mg/m 2 x 2 + 1 n 14 molecular CR achieved n 7 pts still in CR (7+ to 31+ months), 7 relapses after 3 to 15 months n

Treatment of APL in children and elderly patients

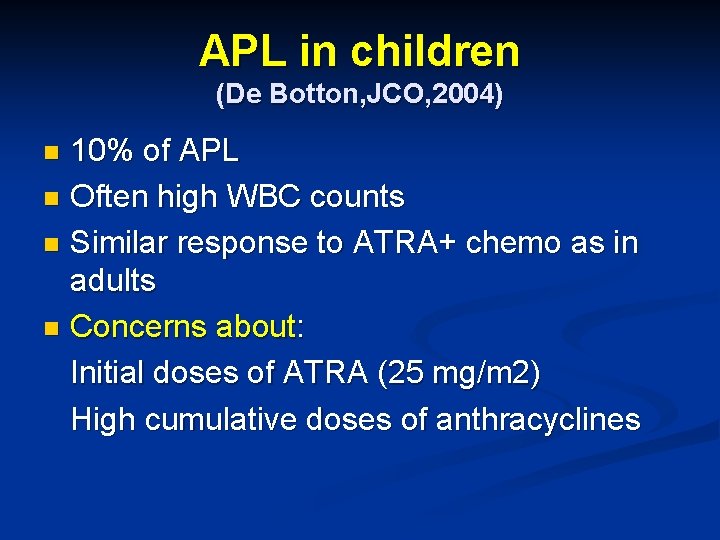
APL in children (De Botton, JCO, 2004) 10% of APL n Often high WBC counts n Similar response to ATRA+ chemo as in adults n Concerns about: Initial doses of ATRA (25 mg/m 2) High cumulative doses of anthracyclines n

5 -year OS 0. 6 0. 4 0. 2 0. 0 P (Survival) 0. 8 1. 0 Children 91% Adults 77% 0 500 1000 1500 Days 2000 2500 3000

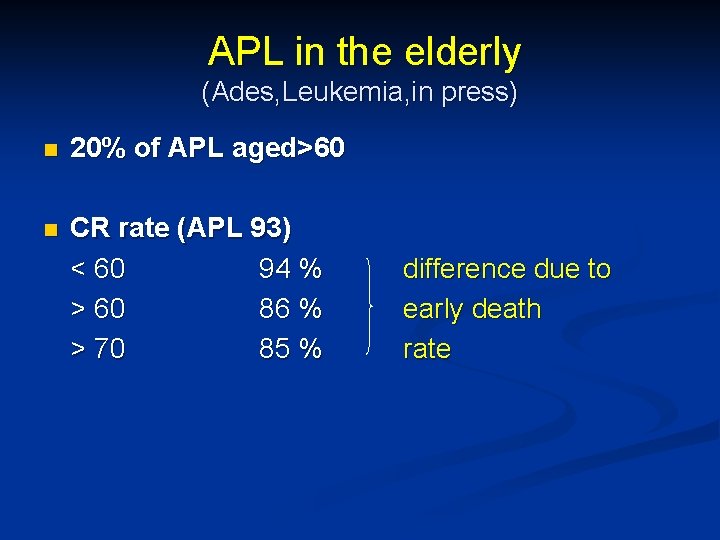
APL in the elderly (Ades, Leukemia, in press) n 20% of APL aged>60 n CR rate (APL 93) < 60 94 % > 60 86 % > 70 85 % difference due to early death rate

APL in the elderly (2) Relapse at 4 years - 26 % in pts < 60 - 20 % in pts > 60 n Survival at 4 years - 79 % in pts < 60 - 55 % in pts > 60 due to deaths in CR n

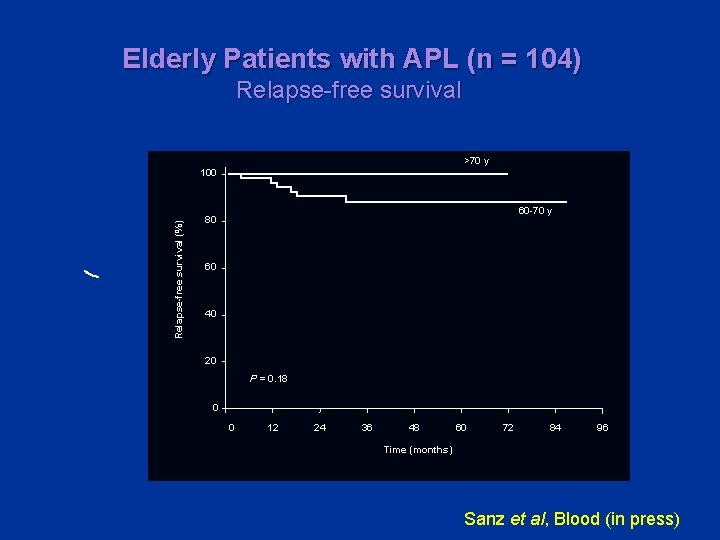
Elderly Patients with APL (n = 104) Cumulative incidence of relapse 100 Probability of relapse 80 60 40 20 8. 5% 0 0 12 24 36 48 60 72 84 96 Time (months) Sanz et al, Blood (in press)

Elderly Patients with APL (n = 104) Relapse-free survival >70 y Relapse-free survival (%) 100 60 -70 y 80 60 40 20 P = 0. 18 0 0 12 24 36 48 60 72 84 96 Time (months) Sanz et al, Blood (in press)

Arsenic derivatives for APL in children and elderly patients? Children: add Arsenic to reduce the cumu. Iative dose of anthracyclines? n Elderly patients (or cardiac problems…): add arsenic to reduce the total amount of chemotherapy n


Question 1: All but one of the following parameters are prognostic factors in APL A: pretreatment WBC count n B: age n C: hemoglobin level n D: PML-RAR alpha transcript after consolidation treatment n

Question 2: all but one of the following symptoms are hallmarks of the ATRA syndrome n A: pulmonary infiltrates n B: pleural effusion n C: clinical bleeding n D: unexplained fever

Question 3: what is the major component of treatment of initial coagulopathy in APL? A: heparin n B: platelet transfusions n C: tranexamic acid or other antifibrinolytic drugs n D: fibrinogen infusions n

Question 4: APL patient with WBC=15000/mm 3. First line treatment? A: ATRA followed by anthracycline based CT n B: anthracycline based CT` n C: ATRA plus anthracycline based CT n D: arsenic derivative alone n

Question 5: what is your induction treatment for first relapse APL (WBC< 10000/mm 3) A: ATRA alone n B: Arsenic derivative alone` n C: ATRA+ anthracycline based CT n D: Gentuzumab (mylotarg) n


LUNG PNEUMOCYSTIS INFECTION 3 patients ATRA+CT at 5 months ATRA+CT at 13 months CT at 19 months maintenance prematurely stopped 1 relapse Prophylaxis required

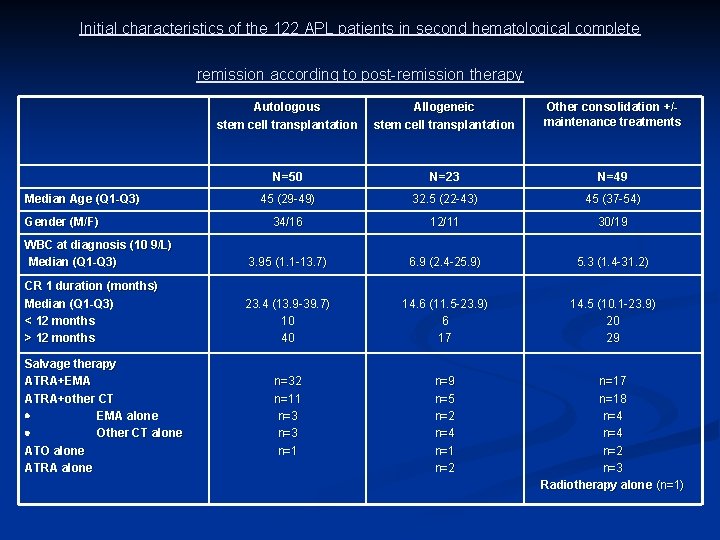
Initial characteristics of the 122 APL patients in second hematological complete remission according to post-remission therapy Autologous stem cell transplantation Allogeneic stem cell transplantation Other consolidation +/- maintenance treatments N=50 N=23 N=49 45 (29 -49) 32. 5 (22 -43) 45 (37 -54) 34/16 12/11 30/19 WBC at diagnosis (10 9/L) Median (Q 1 -Q 3) 3. 95 (1. 1 -13. 7) 6. 9 (2. 4 -25. 9) 5. 3 (1. 4 -31. 2) CR 1 duration (months) Median (Q 1 -Q 3) < 12 months > 12 months 23. 4 (13. 9 -39. 7) 10 40 14. 6 (11. 5 -23. 9) 6 17 14. 5 (10. 1 -23. 9) 20 29 n=32 n=11 n=3 n=1 n=9 n=5 n=2 n=4 n=1 n=2 n=17 n=18 n=4 n=2 n=3 Radiotherapy alone (n=1) Median Age (Q 1 -Q 3) Gender (M/F) Salvage therapy ATRA+EMA ATRA+other CT EMA alone Other CT alone ATO alone ATRA alone

Pretransplant characteristics and transplantation procedures in the 73 APL patients autografted (n=50) or allografted (n=23) in second complete remission Time to SCT after CR 2 achievement Median (Q 1 -Q 3) (days) < 6 months > 6 months RT-PCR analysis before SCT Positive Negative Not assessed Conditioning regimen for SCT Stem cell source Peripheral Blood Bone marrow Donor Autologous stem cell transplantation Allogeneic stem cell transplantation N=50 N=23 112 (82 -137) 44 6 80. 5 (55 -125) 19 4 30 2 28 20 9 6 3 14 *Cy-TBI (n=28) *Bu-Cy (n=17) Bu-Melphalan (n=4) Cy-Melphalan (n=1) *Cy-TBI (n=17) *Bu-Cy (n=6) 43 7 3 20 HLA identical sibling (n=18) Unrelated donor (n=5)

n ; n 7 -year RFS, EFS and OS in the auto SCT group were 79. 4%, 60. 6% and 59. 8%, respectively, with a TRM of 6%. 7 -year RFS, EFS and OS in the allo SCT group were 92. 3%, 52. 2% and 51. 8%, respectively, with a TRM of 39%. No significant differences in RFS and EFS were seen between auto and allo SCT (p=. 19 and p=. 11, respectively) but was OS significantly better in the auto SCT group than in the allo SCT group (p=. 04). In non-transplanted patients, 7 -year RFS, EFS and OS were 38%, 30. 4% and 39. 5%, respectively. n n n

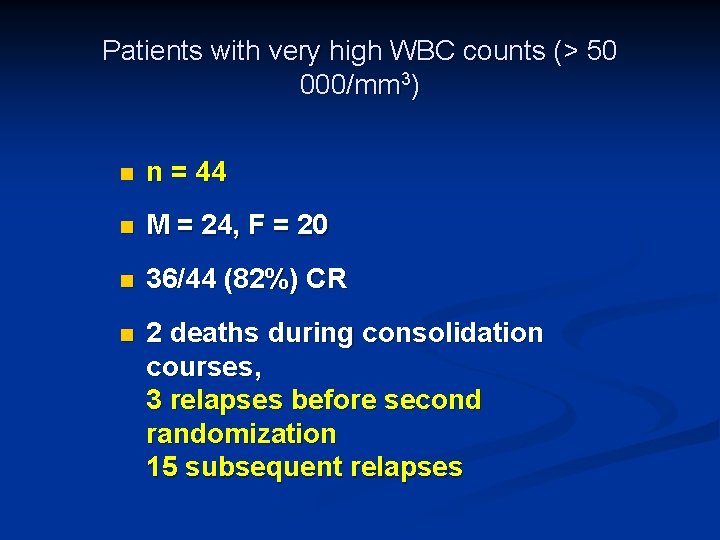
Patients with very high WBC counts (> 50 000/mm 3) n n = 44 n M = 24, F = 20 n 36/44 (82%) CR n 2 deaths during consolidation courses, 3 relapses before second randomization 15 subsequent relapses

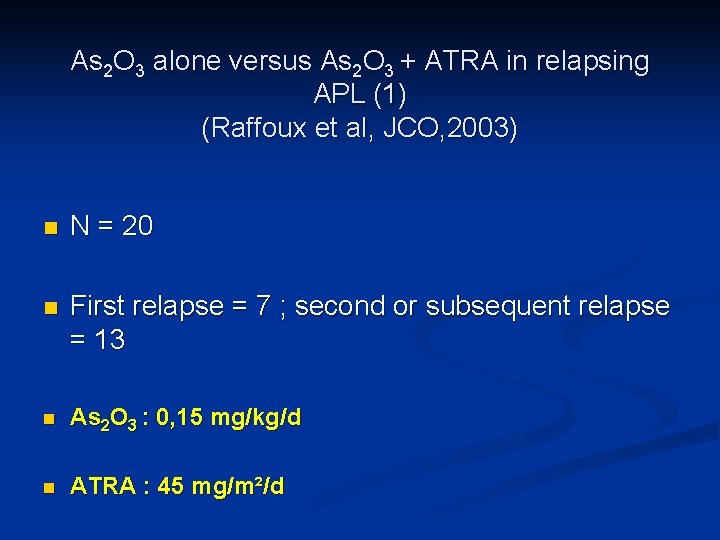
As 2 O 3 alone versus As 2 O 3 + ATRA in relapsing APL (1) (Raffoux et al, JCO, 2003) n N = 20 n First relapse = 7 ; second or subsequent relapse = 13 n As 2 O 3 : 0, 15 mg/kg/d n ATRA : 45 mg/m²/d

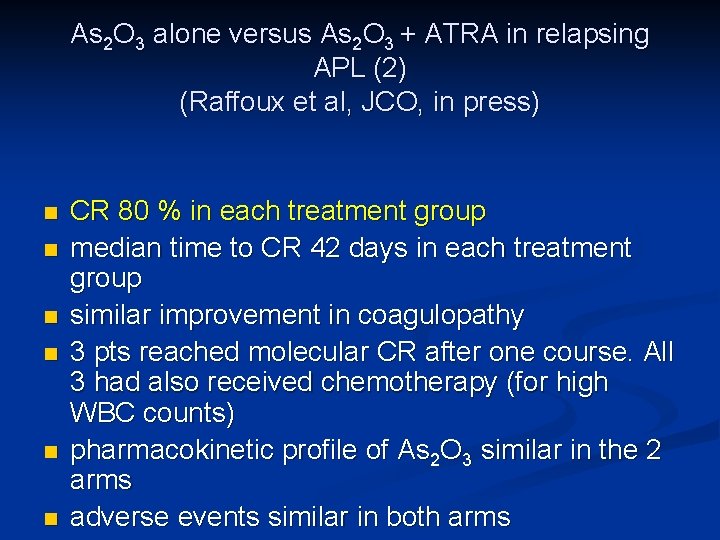
As 2 O 3 alone versus As 2 O 3 + ATRA in relapsing APL (2) (Raffoux et al, JCO, in press) n n n CR 80 % in each treatment group median time to CR 42 days in each treatment group similar improvement in coagulopathy 3 pts reached molecular CR after one course. All 3 had also received chemotherapy (for high WBC counts) pharmacokinetic profile of As 2 O 3 similar in the 2 arms adverse events similar in both arms

As 2 O 3 alone versus As 2 O 3 + ATRA in relapsing APL (3) (Raffoux et al, JCO, in press) n 10 pts received As 2 O 3 ± ATRA consolidation (2 courses). Only 2/8 PCR negative after 2 consolidation courses n 8 allo or autologous SCT Similar survival n

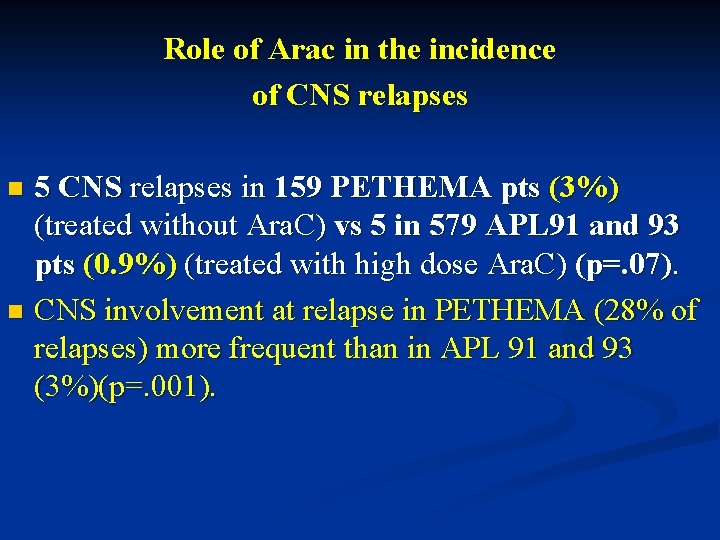
Role of Arac in the incidence of CNS relapses 5 CNS relapses in 159 PETHEMA pts (3%) (treated without Ara. C) vs 5 in 579 APL 91 and 93 pts (0. 9%) (treated with high dose Ara. C) (p=. 07). n CNS involvement at relapse in PETHEMA (28% of relapses) more frequent than in APL 91 and 93 (3%)(p=. 001). n
 Mesenteric root
Mesenteric root The judgement of paris
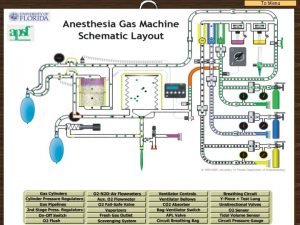
The judgement of paris Anesthesia machine checkout
Anesthesia machine checkout Apl muscle
Apl muscle Apl
Apl Laptop apl
Laptop apl Apl campus
Apl campus Apl machinery pvt ltd
Apl machinery pvt ltd Johns hopkins applied physics lab internship
Johns hopkins applied physics lab internship Apl excel
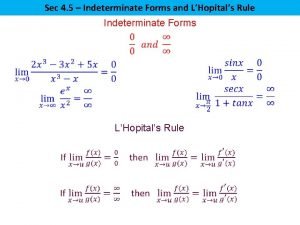
Apl excel Lhospitals rule
Lhospitals rule L hopital's rule
L hopital's rule L'hôpital's rule
L'hôpital's rule Hopital mohamed seghir nekkache
Hopital mohamed seghir nekkache Code lilas hopital
Code lilas hopital Partial sum of an infinite series
Partial sum of an infinite series Kvocient matematika
Kvocient matematika Eprd hopital
Eprd hopital Etiquette hopital
Etiquette hopital De l'hopital teorema
De l'hopital teorema Hopital militaire constantine
Hopital militaire constantine Organigramme d'une mutuelle
Organigramme d'une mutuelle What is l hospital rule
What is l hospital rule Hopital militaire constantine
Hopital militaire constantine 沈榮麟
沈榮麟 Pierre elliott trudeau high school courses
Pierre elliott trudeau high school courses Wybitny astronom i fizyk z pizy
Wybitny astronom i fizyk z pizy Pierre robin syndrome pictures
Pierre robin syndrome pictures Jean-pierre houdin
Jean-pierre houdin Inspection saint pierre 1
Inspection saint pierre 1 Pierre de coubertin and plato
Pierre de coubertin and plato Pierre thuillier
Pierre thuillier Queensmount school bournemouth
Queensmount school bournemouth Ronsard biographie pdf
Ronsard biographie pdf Biografia de pierre de ronsard
Biografia de pierre de ronsard Pierre de fermat family
Pierre de fermat family Pierre de fermat
Pierre de fermat Sermaye türleri bourdieu
Sermaye türleri bourdieu Pierre bourdieu distinction
Pierre bourdieu distinction Pierre bachelet décès
Pierre bachelet décès Pierre anschutz
Pierre anschutz Broca neuropsicologia
Broca neuropsicologia Pierre schillinger
Pierre schillinger Pierre yared
Pierre yared Jean-pierre bely
Jean-pierre bely Jean-pierre bely
Jean-pierre bely Jean pierre bravo
Jean pierre bravo Jean pierre augier sculpteur
Jean pierre augier sculpteur Jean pierre paoli
Jean pierre paoli Formation pierre vermersch
Formation pierre vermersch Contracautela
Contracautela Pierre seban
Pierre seban Tamara pierre louis
Tamara pierre louis Pierre bjurhager
Pierre bjurhager Pierre hoogenboom
Pierre hoogenboom Pierre mai
Pierre mai Rimbaud contexte historique
Rimbaud contexte historique Definition of determinant in maths
Definition of determinant in maths Pierre sikivie
Pierre sikivie Pierre sikivie
Pierre sikivie Trois feuilles mortes poeme
Trois feuilles mortes poeme Bonne annee story answers
Bonne annee story answers Pierre bottero bibliographie
Pierre bottero bibliographie Career cruising yrdsb
Career cruising yrdsb Dr pierre michaud
Dr pierre michaud Pierre girier
Pierre girier Pierre budin
Pierre budin Pierre feugier nancy
Pierre feugier nancy Neurochirurgien clinique du bois lille
Neurochirurgien clinique du bois lille Marie curiová pierre curie
Marie curiová pierre curie Pierre yves marie
Pierre yves marie Jean pierre pintor
Jean pierre pintor Pierre hermé dubai
Pierre hermé dubai Pierre auguste renoir biographie
Pierre auguste renoir biographie Cread rennes
Cread rennes Choderlos de laclos mouvement
Choderlos de laclos mouvement Pierre henri bertoye
Pierre henri bertoye International pierre de coubertin committee
International pierre de coubertin committee Eglise saint pierre aumaitre angouleme
Eglise saint pierre aumaitre angouleme Pierre elliott trudeau high school courses
Pierre elliott trudeau high school courses Pierre bertieaux
Pierre bertieaux Audrey pierre
Audrey pierre Pierre dusein
Pierre dusein Kedarie pierre johnson
Kedarie pierre johnson Pierre de blanchefort, french nobleman, diary entry, 1576
Pierre de blanchefort, french nobleman, diary entry, 1576 Tipos de signos zodiacales
Tipos de signos zodiacales Pierre bauche
Pierre bauche Bruno jougla
Bruno jougla Pr innocent pierre guissou
Pr innocent pierre guissou Pierre giraud html
Pierre giraud html Pierre theuma
Pierre theuma Giovanna giardino
Giovanna giardino Pierre ferruit
Pierre ferruit Jaccard matlab
Jaccard matlab Jean-pierre bely
Jean-pierre bely