118 CONGRESSO NAZIONALE Societa Italiana di Medicina Interna



























- Slides: 58

118° CONGRESSO NAZIONALE Societa Italiana di Medicina Interna Best Strategies for HCV Eradication Cosimo Colletta, MD Chief of Hepatology Department of Medicine COQ Omegna Rome, 28 th October 2017

Disclosure Slide Cosimo Colletta, MD - Chief of Hepatology Department of Medicine, COQ Omegna • I DO NOT have any significant or other financial interests in the subject matter of this presentation and • I DO NOT intend to discuss an unlabeled, unapproved or investigative use of a commercial product or device • Please feel free to use, update, and share some or all of these slides in your noncommercial presentations to colleagues

A Call to Arms to Maximize HCV Cure Rates § The Tool Kit to Achieve Cure § The Path to 100% HCV Elimination § Key Targets to Reach 100% ØIncreasing Screening and Diagnosis ØExpanding Therapy to the Previously Excluded ØEvolving Options for DAA-Experienced Patients

The mission for HCV eradication is far from accomplished We should consider that disease elimination has required vaccination with all previously controlled infections

Not only have we not eradicated syphilis, rates of infection have increased in many places in recent years

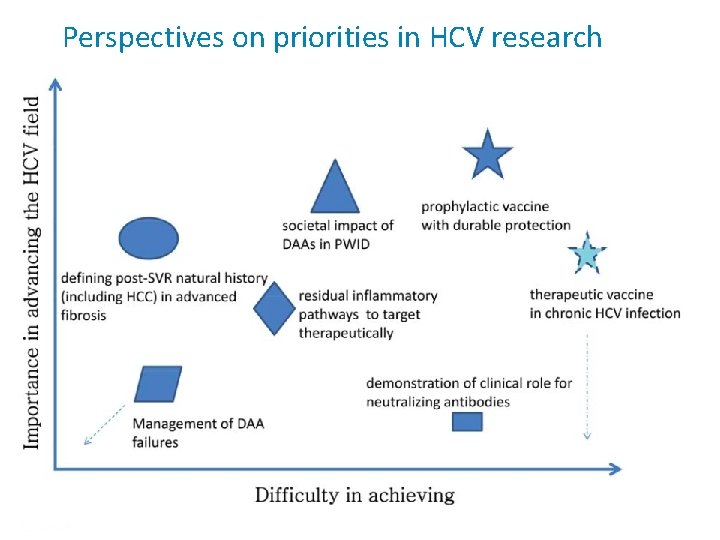
Perspectives on priorities in HCV research Presentation Title | Company Confidential © 2016 6

HCV burden: true prevalence is elusive • Asymptomatic nature of HCV (up to 50% of HCV-positive individuals are unaware of their infection) • Inadequate surveillance infrastructure • Reservoirs for further transmission § Improving surveillance: • Enhance epidemiologic understanding of HCV • Identify the highest priority groups for targeted prevention, and treatment towards eradication

Transmission opportunities must be eliminated • Screening for HCV • Treating those who test positive • Educating those who test positive about how to avoid infectng others • Changing behaviours to prevent new infections and reinfection

Focus on transmission § Strategy prioritization will differ depending on the specific transmission patterns in each target community § In high-prevalence developing countries, modifying injection practices and standardizing blood donation screening will have the greatest impact § Needle exchange programmes and education through substance abuse treatment clinics, primary care physicians and sex and drug education in middle and high schools will likely offer the greatest return in the other developed countries

Strategies to prevent new infections § Injection drug use replaced infected blood and tissue donations as the primary source of new infections § Although sexual transmission appears to be low, as much as a 23 -fold increase in HCV transmission among HIV-infected men who have sex with men has been associated with high-risk sexual behaviours § In addition to preventing new cases, existing infections must be effectively treated to achieve eradication

Implement universal screening • Risk-based screening is considered the most cost-effective strategy • Some infected individuals do not have a history of risk factors • Risk-based methods would identify only 85% of infections • HCV eradication cannot occur if clinicians continue to use a screening strategy that misses 15% of infections among those who seek care • No effective HCV vaccine exists but DAA treatments can work towards the same goal by eliminating the virus, one host at a time • In a recent analysis universal screening was cost-effective if 50% of those diagnosed initiated treatment

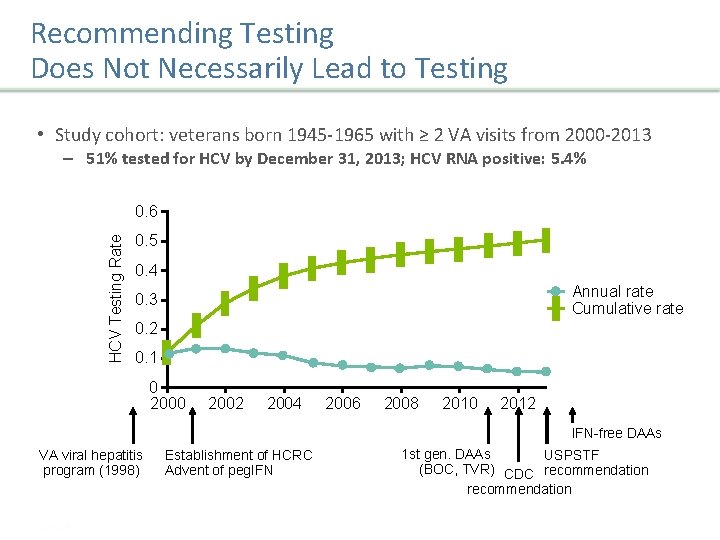
Recommending Testing Does Not Necessarily Lead to Testing • Study cohort: veterans born 1945 -1965 with ≥ 2 VA visits from 2000 -2013 – 51% tested for HCV by December 31, 2013; HCV RNA positive: 5. 4% HCV Testing Rate 0. 6 0. 5 0. 4 Annual rate Cumulative rate 0. 3 0. 2 0. 1 0 2002 2004 2006 2008 2010 2012 IFN-free DAAs VA viral hepatitis program (1998) Establishment of HCRC Advent of peg. IFN 1 st gen. DAAs USPSTF (BOC, TVR) CDC recommendation Sarkar S, et al. J Hepatol. 2016; 65: 259 -265. Presentation Title | Company Confidential © 2016 12

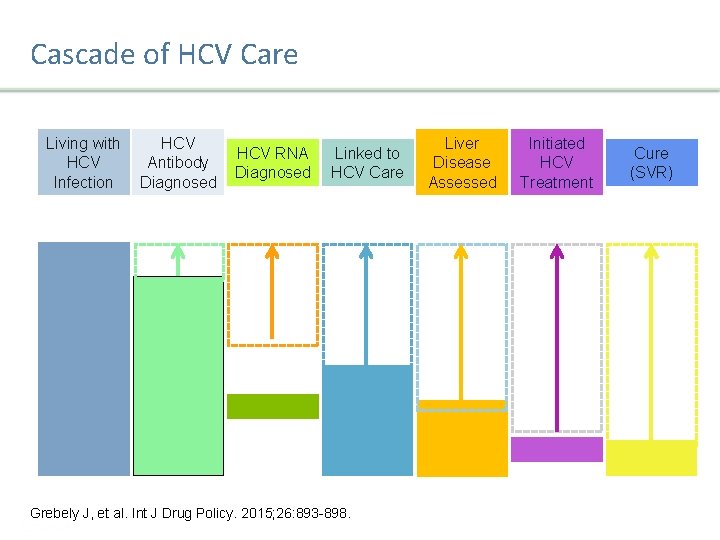
Cascade of HCV Care Living with HCV Antibody Infection Diagnosed HCV RNA Diagnosed Linked to HCV Care Liver Disease Assessed Initiated HCV Treatment Cure (SVR) Grebely J, et al. Int J Drug Policy. 2015; 26: 893 -898. Presentation Title | Company Confidential © 2016 13

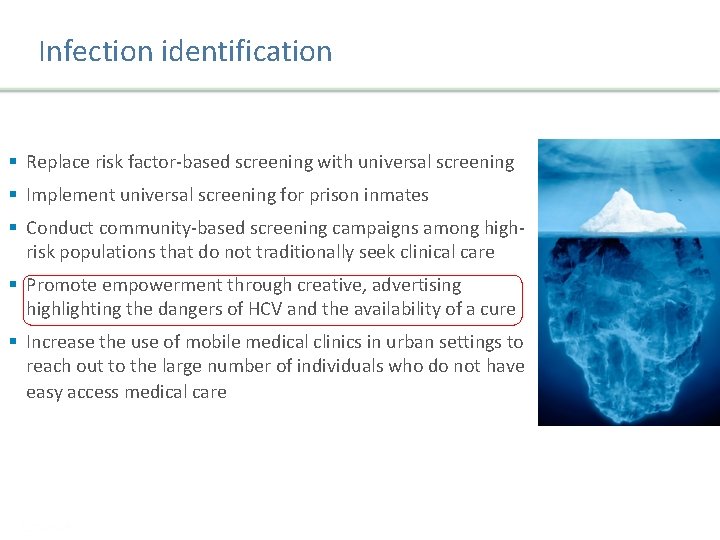
Infection identification § Replace risk factor-based screening with universal screening § Implement universal screening for prison inmates § Conduct community-based screening campaigns among highrisk populations that do not traditionally seek clinical care § Promote empowerment through creative, advertising highlighting the dangers of HCV and the availability of a cure § Increase the use of mobile medical clinics in urban settings to reach out to the large number of individuals who do not have easy access medical care Presentation Title | Company Confidential © 2016 14

Program Overview § Treating HCV Today § From Individual Cures to HCV Elimination § Addressing HCV in Primary Care § Overcoming Barriers to HCV Treatment § Engaging With Challenging Populations

What Are the Key Barriers to Successful HCV Therapy?

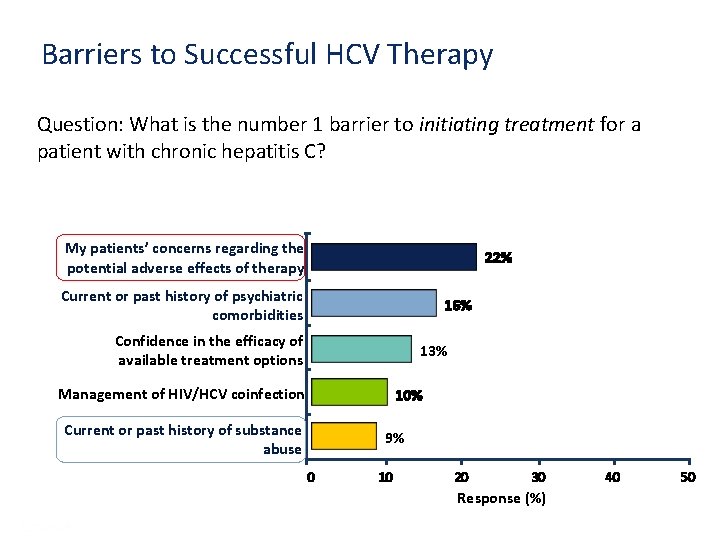
Barriers to Successful HCV Therapy Question: What is the number 1 barrier to initiating treatment for a patient with chronic hepatitis C? My patients’ concerns regarding the potential adverse effects of therapy 22% Current or past history of psychiatric comorbidities 16% Confidence in the efficacy of available treatment options 13% Management of HIV/HCV coinfection 10% Current or past history of substance abuse 9% 0 10 20 30 40 50 Response (%) Presentation Title | Company Confidential © 2016 17

Where HCV Therapy Stands Now § Interferon is gone; ribavirin. . . not quite § SVR in > 95% of pts § “Difficult-to-cure” populations no longer difficult – Black race – HIV coinfection – Cirrhosis § Genotype 3 remains more challenging (but not by as much) § Confidence that SVR 12 = cure – Late relapse beyond 12 wks after EOT exceedingly rare § Cost and access issues are improving

Treating Everyone § Encourages testing § Improves quality of life Presentation Title | Company Confidential © 2016 19

HCV Is Not JUST a Liver Virus § People with HCV infection are not just “livers on legs” § People with HCV infection have MANY health concerns Presentation Title | Company Confidential © 2016 20

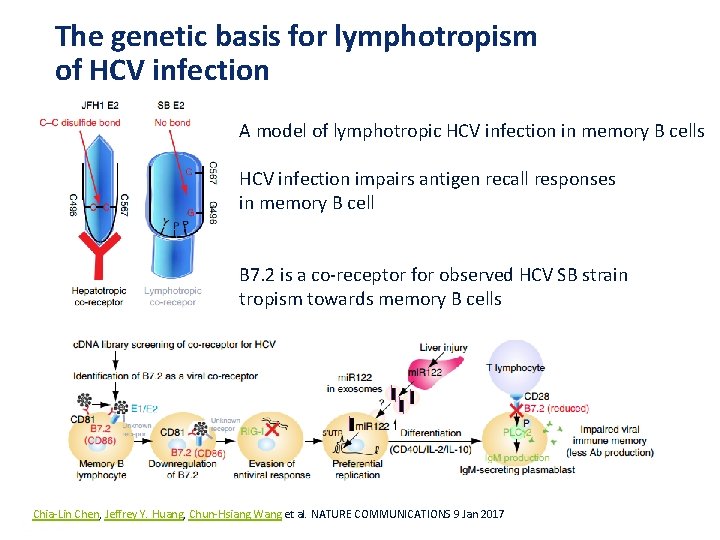
The genetic basis for lymphotropism of HCV infection A model of lymphotropic HCV infection in memory B cells HCV infection impairs antigen recall responses in memory B cell B 7. 2 is a co-receptor for observed HCV SB strain tropism towards memory B cells Chia-Lin Chen, Jeffrey Y. Huang, Chun-Hsiang Wang et al. NATURE COMMUNICATIONS 9 Jan 2017 Presentation Title | Company Confidential © 2016 21

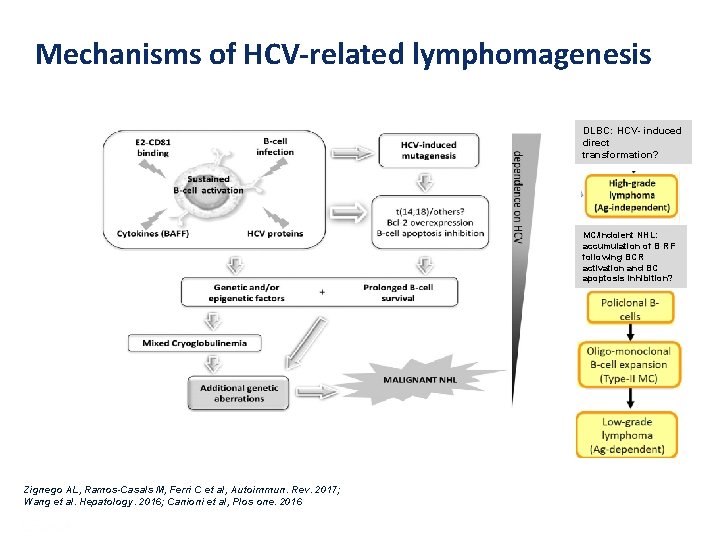
Mechanisms of HCV-related lymphomagenesis DLBC: HCV- induced direct transformation? MC/indolent NHL: accumulation of B RF following BCR activation and BC apoptosis inhibition? Zignego AL, Ramos-Casals M, Ferri C et al, Autoimmun. Rev. 2017; Wang et al. Hepatology. 2016; Canioni et al, Plos one. 2016 Presentation Title | Company Confidential © 2016 22

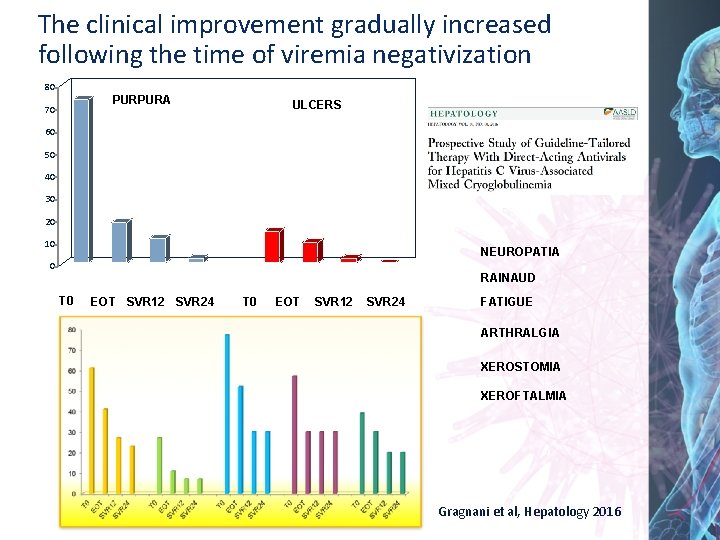
The clinical improvement gradually increased following the time of viremia negativization 80 PURPURA 70 ULCERS 60 50 40 30 20 10 NEUROPATIA 0 RAINAUD T 0 EOT SVR 12 SVR 24 FATIGUE ARTHRALGIA XEROSTOMIA XEROFTALMIA Gragnani et al, Hepatology 2016

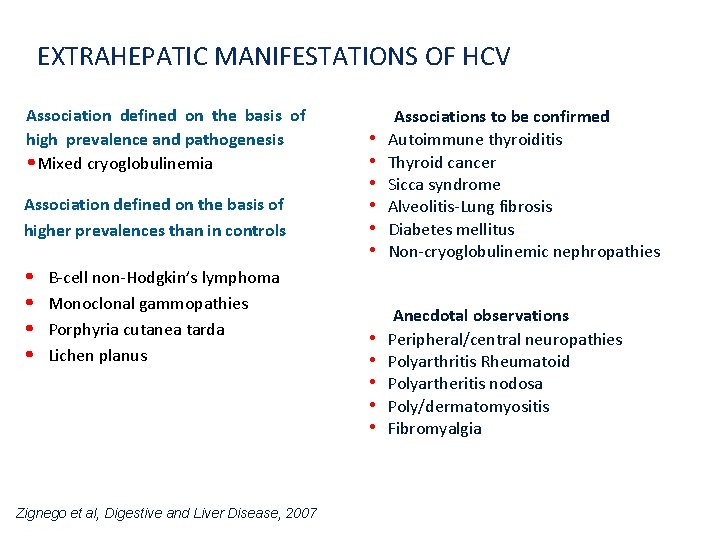
EXTRAHEPATIC MANIFESTATIONS OF HCV Association defined on the basis of high prevalence and pathogenesis • Mixed cryoglobulinemia Association defined on the basis of higher prevalences than in controls • • B-cell non-Hodgkin’s lymphoma Monoclonal gammopathies Porphyria cutanea tarda Lichen planus • • • Associations to be confirmed Autoimmune thyroiditis Thyroid cancer Sicca syndrome Alveolitis-Lung fibrosis Diabetes mellitus Non-cryoglobulinemic nephropathies • • • Anecdotal observations Peripheral/central neuropathies Polyarthritis Rheumatoid Polyartheritis nodosa Poly/dermatomyositis Fibromyalgia Zignego et al, Digestive and Liver Disease, 2007 Presentation Title | Company Confidential © 2016 24

Treating Everyone § Encourages testing § Improves quality of life § Reduces onward transmission Presentation Title | Company Confidential © 2016 25

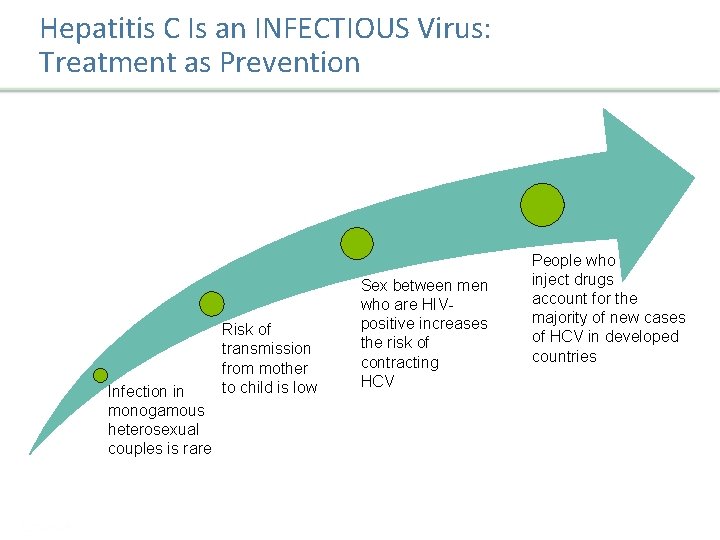
Hepatitis C Is an INFECTIOUS Virus: Treatment as Prevention Infection in monogamous heterosexual couples is rare Risk of transmission from mother to child is low Sex between men who are HIVpositive increases the risk of contracting HCV People who inject drugs account for the majority of new cases of HCV in developed countries 1. Terrault NA, et al. Hepatology. 2013; 57: 881 -899. 2. Thomas SL, et al. Int J Epidemiol. 1998; 27: 108 -117. 3. Larsen C, et al. PLo. S One. 2011; 6: 1 -9. 4. Shepard CW, et al. Lancet Infect Dis. 2005; 5: 558 -567. Presentation Title | Company Confidential © 2016 26

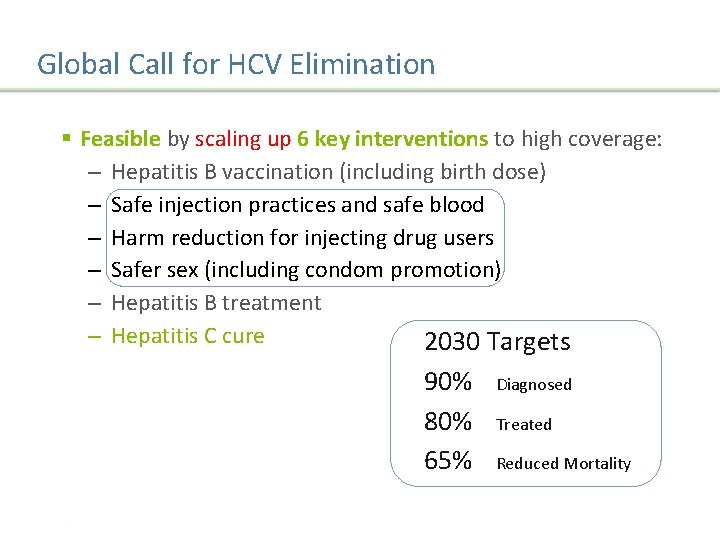
Global Call for HCV Elimination § Feasible by scaling up 6 key interventions to high coverage: – Hepatitis B vaccination (including birth dose) – Safe injection practices and safe blood – Harm reduction for injecting drug users – Safer sex (including condom promotion) – Hepatitis B treatment – Hepatitis C cure 2030 Targets 90% 80% 65% Diagnosed Treated Reduced Mortality WHO. Towards the elimination of hepatitis B and C by 2030. Draft WHO Global Hepatitis Strategy, 2016 -2021. Presentation Title | Company Confidential © 2016 27

Eliminating HCV Is Everyone’s Job • Identify undiagnosed HCV – HCV rapid testing – Opiate replacement therapy – Syringe access • Preventing infection and reinfection – Outreach and education – Safer injection counseling – Reinfection prevention counseling • Treatment access and delivery – Linkage to HCV care – Access to HCV drugs – Primary care–based therapy – Methadone-based directly observed therapy – Access to specialty care End Hep C SF. Presentation Title | Company Confidential © 2016 28

Multiple Models of HCV Care Are Needed • Primary care • Community health centers • Tertiary care clinics • Drug and alcohol clinics • Prisons Presentation Title | Company Confidential © 2016 29

HCV Treatment in Primary Care: Why? § Multiple models of care will reach different populations § Number of prescribers a determinant of treatment uptake § Empowerment of PCPs through HCV cure § Quality care can be delivered by PCPs Presentation Title | Company Confidential © 2016 30

What Tools Are Required § Pathology services • HCV disease staging • HCV prescribing capacity • Adherence education • Drug–drug interaction assessment § Specialist referral pathway Presentation Title | Company Confidential © 2016 31

Strategies for Increasing HCV Testing and Diagnosis § Free on-site counseling and testing § Dried blood spot testing § Risk-based assessment § Targeted case-finding § Hepatitis Care Coordination with case management § 1. Meyer JP, et al. Int J Drug Policy. 2015; 26: 922 -935. § 2. Sahajian F, et al. J Public Health (Oxf). 2011; 33: 182 -192. § 3. Morano JP, et al. J Community Health. 2014; 39: 922 -934. § 4. Cullen BL, et al. J Public Health (Oxf). 2012; 34: 14 -23. Presentation Title | Company Confidential © 2016 32

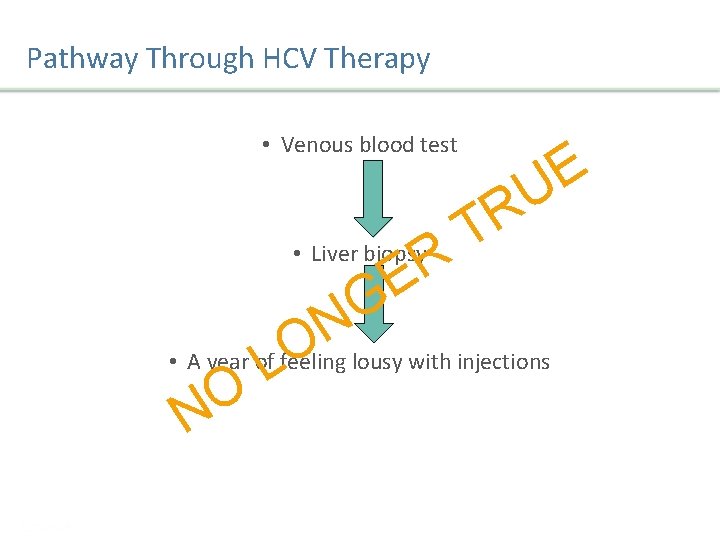
Pathway Through HCV Therapy • Venous blood test R E G • Liver biopsy E U R T N O • A year of feeling lousy with injections L O N Presentation Title | Company Confidential © 2016 33

Current Pathway Through HCV Therapy • Easy diagnostics (dried blood spots) • Easy staging (Fibro. Scan, etc) • Easy, effective therapy • We HAVE to get the right messages out—HOW? Presentation Title | Company Confidential © 2016 34

Getting the Right Message Out § Your pts are your best advocates § Get pts to tell their friends and to bring their friends in for treatment § Teaching sessions, in drug services, are run by former patients from the Hepatitis C Trust Presentation Title | Company Confidential © 2016 35

What considerations need to be made for special patient groups in the era of DAAs?

Additional Reasons Why Pts May Not Engage § Practical problems for immigrants • Language barriers: “How can I go for a test? • Practical barriers: “We work more than 10 hrs a day, six days a wk. Even if we are not feeling well, we don’t have time to see a doctor” § Apathy about HCV among baby boomers: “I feel fine” Sweeney L, et al. BMC Health Serv Res. 2015; 15: 97. Presentation Title | Company Confidential © 2016 37

Barriers for the Engaged Pt • “I want treatment now” • BUT. . . – Access to drugs remains problematic – In many countries, pts with mild disease wait Presentation Title | Company Confidential © 2016 38

Keeping the Engaged Pt Engaged • How can we make waiting easier? • Stay interested • Avoid duplicate appointments • Keep people informed Presentation Title | Company Confidential © 2016 39

HCV Treatment for Marginalized Populations § Defining marginalized (underserved) populations § Linkage to HCV care and treatment § Strategies to enhance DAA uptake in PWID § Personal perspective on initial DAA scale-up § HCV reinfection Presentation Title | Company Confidential © 2016 40

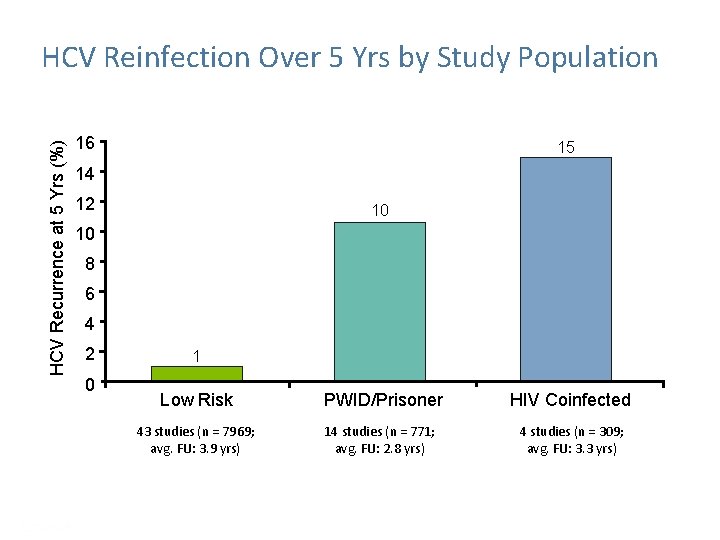
HCV Recurrence at 5 Yrs (%) HCV Reinfection Over 5 Yrs by Study Population 16 15 14 12 10 10 8 6 4 2 0 1 Low Risk PWID/Prisoner HIV Coinfected 43 studies (n = 7969; avg. FU: 3. 9 yrs) 14 studies (n = 771; avg. FU: 2. 8 yrs) 4 studies (n = 309; avg. FU: 3. 3 yrs) Simmons B, et al. Clin Infect Dis. 2016: 62: 683 -694. Presentation Title | Company Confidential © 2016 41

Specific Issues Related to HCV Reinfection § Acknowledgement: There will be cases of HCV reinfection § Harm reduction access (NEP, OST): To reduce risk of reinfection § Individual-level strategies: Treatment of injecting partners § Rapid scale-up: Initial increase in reinfections, then control § Access to retreatment: without stigma and discrimination Presentation Title | Company Confidential © 2016 42

Take Home Points • Treatment in 2017 is effective, becoming simplified, and provides all-oral options for every patient – New generation of therapy is pangenotypic, effective for previous DAA failure • Goals in HCV therapy expanding beyond individual cure to HCV elimination; keys include: – Involving primary care – Addressing barriers to treatment – Providing effective therapy to challenging patient groups Presentation Title | Company Confidential © 2016 43


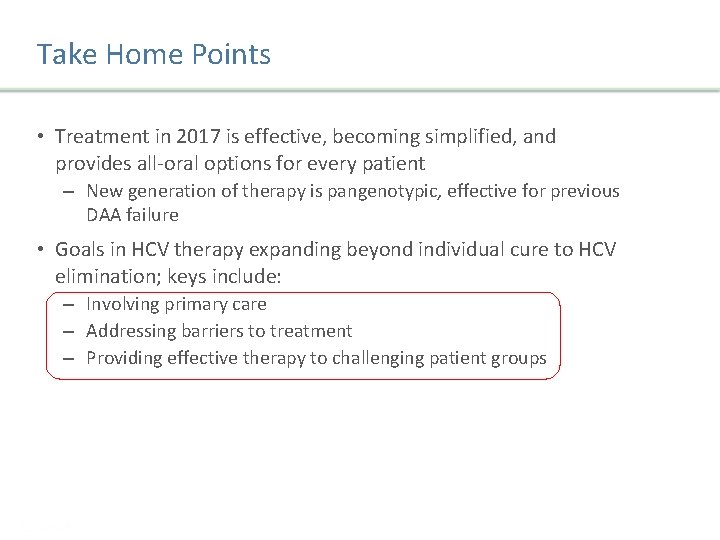
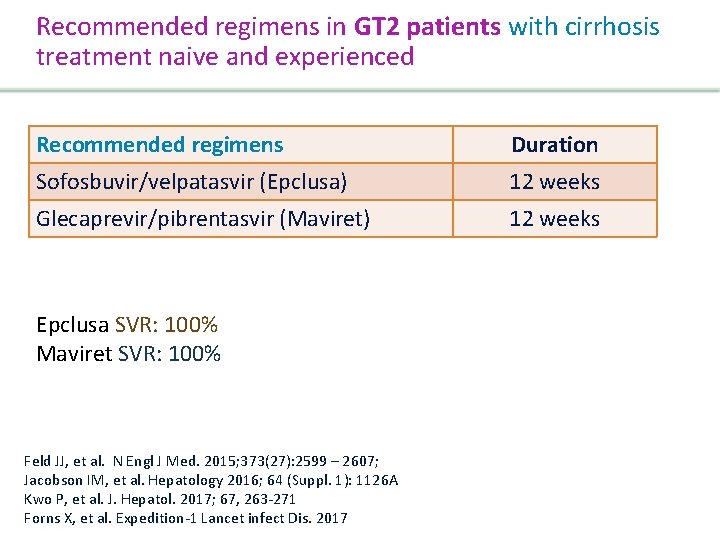
Recommended regimens in GT 1/4 patients without cirrhosis, treatment naive and experienced Recommended regimens Duration Elbasvir/Grazoprevir (Zepatier)§ 12 weeks Sofosbuvir/velpatasvir (Epclusa) 12 weeks Glecaprevir/pibrentasvir (Maviret) 8 weeks • §Zepatier single tablet, once daily SVR: 97% (C-EDGE) • Epclusa single tablet, once daily SVR: 98% (ASTRAL-1) • Maviret, 3 tablet, once daily SVR: 99%. (ENDURANCE-1) Zeuzem S, et al. J. Hepatol. 2017; 66 (5) 910 -918 Sulkowski MS, et al. Lancet 2015; 285(9973): 1087 -97 Feld JJ et al. The New England Journal of Medicine. 2015; 373(27): 2599 – 2607 Zeuzem S, et al. Hepatology 2016; 64(Suppl. 1) 132 A Jacobson IM, et al. Hepatology 2016; 64 (Suppl. 1): 1126 A Presentation Title | Company Confidential © 2016 45

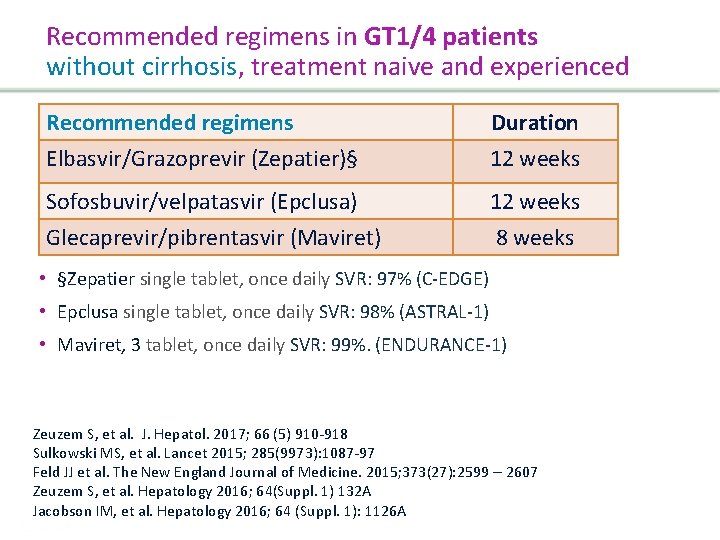
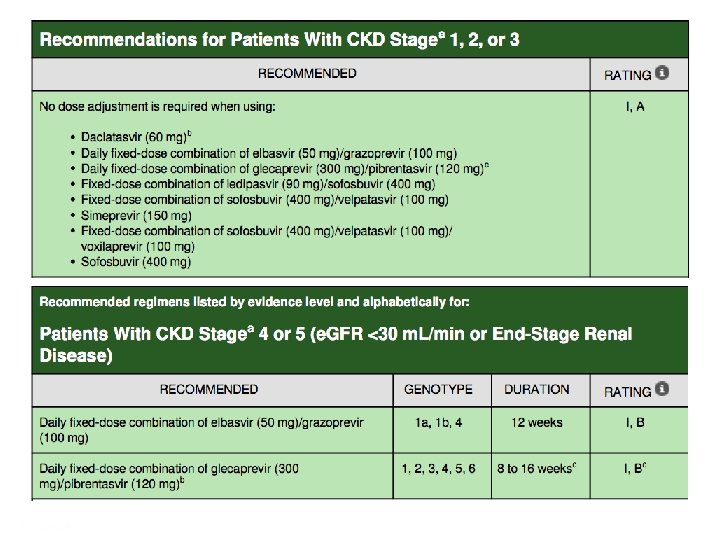
Recommended regimens in GT 1/4 patients with cirrhosis, treatment naive and experienced Recommended regimens Duration Elbasvir/Grazoprevir (Zepatier) 12 weeks Sofosbuvir/velpatasvir 100 (Epclusa) Glecaprevir 300/pibrentasvir (Maviret 3 c die) 12 weeks • Zepatier single tablet, once daily SVR: 97% (C-EDGE) • Epclusa single tablet, once daily SVR: 100% (ASTRAL-1) • Maviret 3 tablet, once daily SVR: 99% (EXPEDITION-1) Zeuzem et al. J. Hepatol. 2017; 66 (5) 910 -918 Lawitz EJ, et al. Lancet. 2015; 385(9973): 1075 -86 Feld et al. N Engl J Med. 2015; 373(27): 2599 – 2607 Forns X, et al. Expedition-1 Lancet infect Dis. 2017 Jacobson IM, et al. Hepatology 2016; 64 (Suppl. 1): 1126 A Presentation Title | Company Confidential © 2016 46

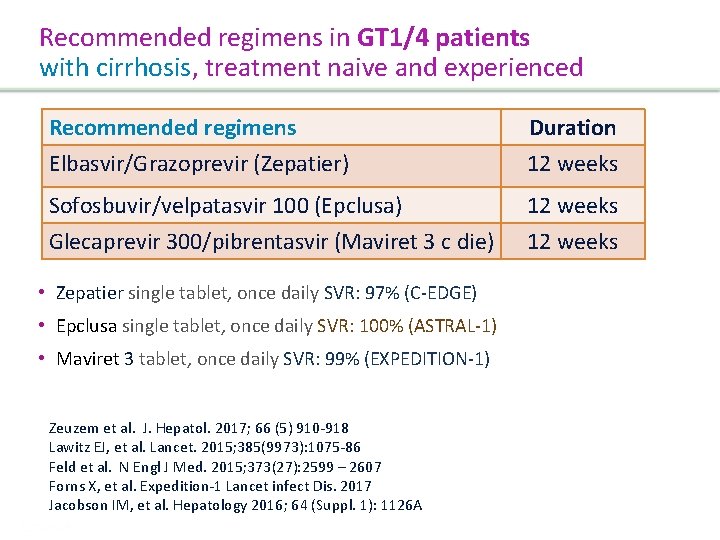
Recommended regimens in GT 2 patients without cirrhosis, treatment naive and experienced Recommended regimens Sofosbuvir/velpatasvir (Epclusa) Glecaprevir/pibrentasvir (Maviret) Duration 12 weeks 8 weeks SVR: 98 -100% Feld JJ, et al. N Engl J Med. 2015; 373(27): 2599 – 2607; Jacobson IM, et al. Hepatology 2016; 64 (Suppl. 1): 1126 A Kwo P, et al. J. Hepatol. 2017; 67, 263 -271 Forns X, et al. Expedition-1 Lancet infect Dis. 2017 Presentation Title | Company Confidential © 2016 47

Recommended regimens in GT 2 patients with cirrhosis treatment naive and experienced Recommended regimens Duration Sofosbuvir/velpatasvir (Epclusa) 12 weeks Glecaprevir/pibrentasvir (Maviret) 12 weeks Epclusa SVR: 100% Maviret SVR: 100% Feld JJ, et al. N Engl J Med. 2015; 373(27): 2599 – 2607; Jacobson IM, et al. Hepatology 2016; 64 (Suppl. 1): 1126 A Kwo P, et al. J. Hepatol. 2017; 67, 263 -271 Forns X, et al. Expedition-1 Lancet infect Dis. 2017 Presentation Title | Company Confidential © 2016 48

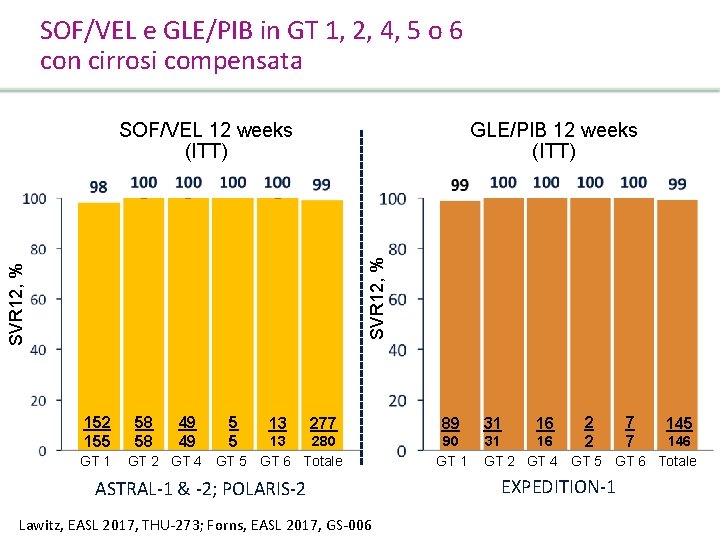
SOF/VEL e GLE/PIB in GT 1, 2, 4, 5 o 6 con cirrosi compensata SOF/VEL 12 weeks (ITT) GLE/PIB 12 weeks (ITT) SVR 12, % 152 155 GT 1 58 58 49 49 5 5 13 277 13 280 GT 2 GT 4 GT 5 GT 6 Totale ASTRAL-1 & -2; POLARIS-2 Lawitz, EASL 2017, THU-273; Forns, EASL 2017, GS-006 89 90 GT 1 31 16 2 2 7 7 145 31 16 146 GT 2 GT 4 GT 5 GT 6 Totale EXPEDITION-1 Presentation Title | Company Confidential © 2016 49

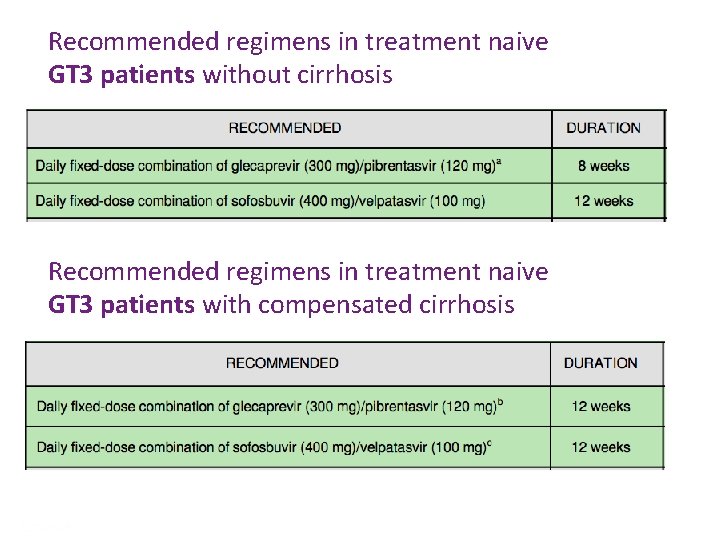
Recommended regimens in treatment naive GT 3 patients without cirrhosis Recommended regimens in treatment naive GT 3 patients with compensated cirrhosis Presentation Title | Company Confidential © 2016 50

Recommended regimens in treatment experienced GT 3 patients with and without compensated cirrhosis Presentation Title | Company Confidential © 2016 51

Special Patient Groups Need Special Attention HCV patients co-infected with HIV Presentation Title | Company Confidential © 2016 52

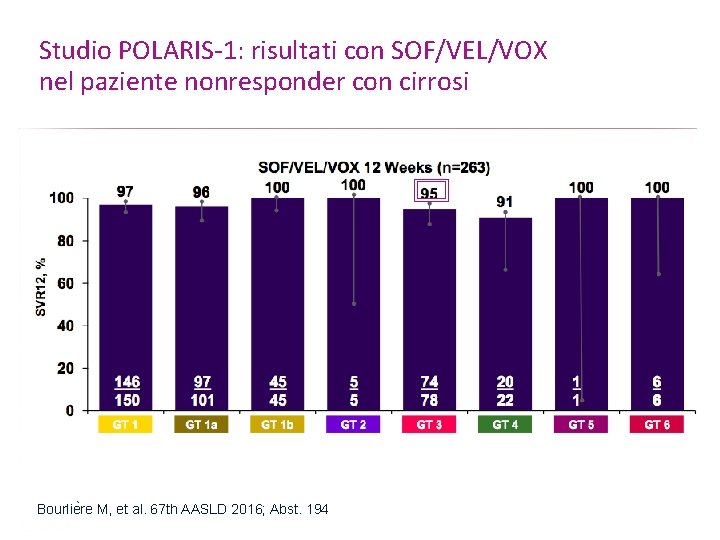
Studio POLARIS-1: risultati con SOF/VEL/VOX nel paziente nonresponder con cirrosi Bourlie re M, et al. 67 th AASLD 2016; Abst. 194 Presentation Title | Company Confidential © 2016 53

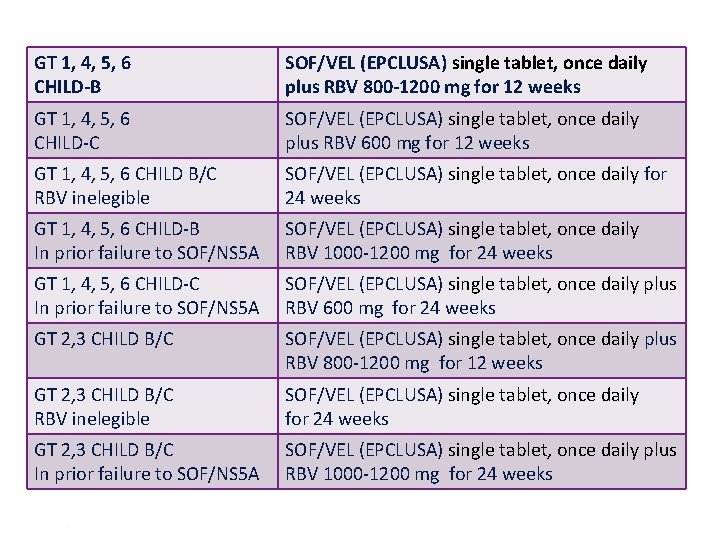
GT 1, 4, 5, 6 CHILD-B SOF/VEL (EPCLUSA) single tablet, once daily plus RBV 800 -1200 mg for 12 weeks GT 1, 4, 5, 6 CHILD-C SOF/VEL (EPCLUSA) single tablet, once daily plus RBV 600 mg for 12 weeks GT 1, 4, 5, 6 CHILD B/C RBV inelegible SOF/VEL (EPCLUSA) single tablet, once daily for 24 weeks GT 1, 4, 5, 6 CHILD-B In prior failure to SOF/NS 5 A SOF/VEL (EPCLUSA) single tablet, once daily RBV 1000 -1200 mg for 24 weeks GT 1, 4, 5, 6 CHILD-C In prior failure to SOF/NS 5 A SOF/VEL (EPCLUSA) single tablet, once daily plus RBV 600 mg for 24 weeks GT 2, 3 CHILD B/C SOF/VEL (EPCLUSA) single tablet, once daily plus RBV 800 -1200 mg for 12 weeks GT 2, 3 CHILD B/C RBV inelegible SOF/VEL (EPCLUSA) single tablet, once daily for 24 weeks GT 2, 3 CHILD B/C In prior failure to SOF/NS 5 A SOF/VEL (EPCLUSA) single tablet, once daily plus RBV 1000 -1200 mg for 24 weeks Presentation Title | Company Confidential © 2016 54

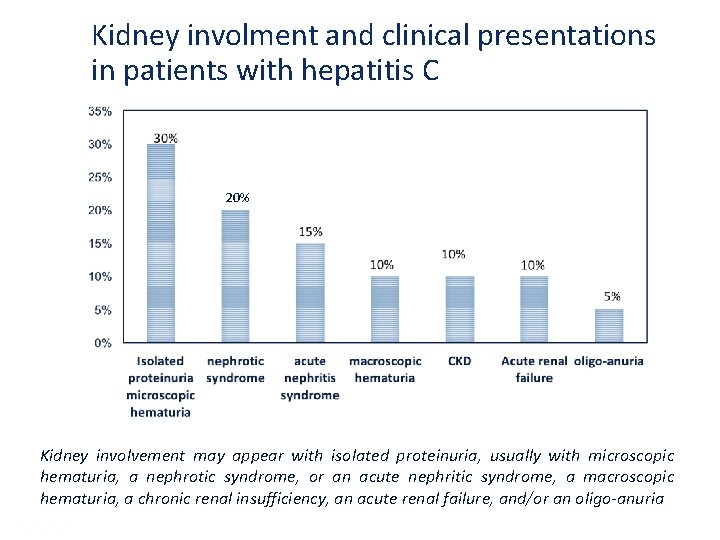
Kidney involment and clinical presentations in patients with hepatitis C 20% Kidney involvement may appear with isolated proteinuria, usually with microscopic hematuria, a nephrotic syndrome, or an acute nephritic syndrome, a macroscopic hematuria, a chronic renal insufficiency, an acute renal failure, and/or an oligo-anuria Presentation Title | Company Confidential © 2016 55

Presentation Title | Company Confidential © 2016 56

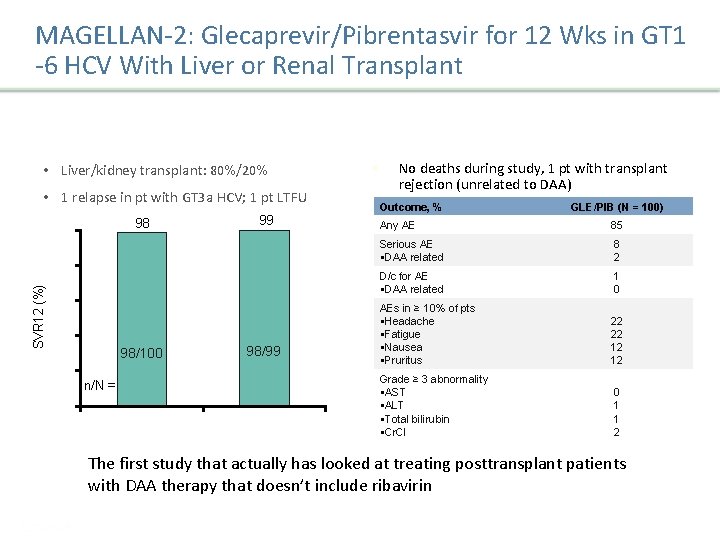
MAGELLAN-2: Glecaprevir/Pibrentasvir for 12 Wks in GT 1 -6 HCV With Liver or Renal Transplant • Liver/kidney transplant: 80%/20% • 1 relapse in pt with GT 3 a HCV; 1 pt LTFU 98 100 99 SVR 12 (%) 80 60 40 98/100 20 98/99 n/N = 0 ITT m. ITT § No deaths during study, 1 pt with transplant rejection (unrelated to DAA) Outcome, % GLE/PIB (N = 100) Any AE 85 Serious AE §DAA related 8 2 D/c for AE §DAA related 1 0 AEs in ≥ 10% of pts §Headache §Fatigue §Nausea §Pruritus 22 22 12 12 Grade ≥ 3 abnormality §AST §ALT §Total bilirubin §Cr. Cl 0 1 1 2 Reau N, et al. EASL 2017. Abstract LBO-03. The first study that actually has looked at treating posttransplant patients with DAA therapy that doesn’t include ribavirin Presentation Title | Company Confidential © 2016 57

Conclusions § The introduction of new therapeutic agents does not detract from the importance of preventive strategies § It seems that an effective vaccine is achievable in the near future, and as Bill Gates has said, “Treatment without prevention is simply unsustainable. ” Studies on vaccine development should be prioritized. § On the other hand, reduction of harmful behaviors should be the main strategy to reduce the prevalence of HCV infection in certain high-risk groups, such as IVDUs and inmates. § Furthermore, public knowledge and awareness are vital to the eradication of every disease, and ignorance will prevent future eradication of HCV Presentation Title | Company Confidential © 2016 58
 Società italiana di medicina interna
Società italiana di medicina interna Società italiana di medicina ambientale
Società italiana di medicina ambientale Registro nazionale società sportive dilettantistiche
Registro nazionale società sportive dilettantistiche Società italiana di psichiatria
Società italiana di psichiatria Gastroscopia celiachia
Gastroscopia celiachia Sociedad venezolana de medicina interna
Sociedad venezolana de medicina interna Congresso sicob cagliari
Congresso sicob cagliari 6 congresso brasileiro de psicologia
6 congresso brasileiro de psicologia Congresso mv
Congresso mv Congresso mineiro de nefrologia
Congresso mineiro de nefrologia Ciam congresso
Ciam congresso Foram decisões do congresso de viena exceto
Foram decisões do congresso de viena exceto Investire in agricoltura in albania
Investire in agricoltura in albania Clistene riforme
Clistene riforme I sumeri praticavano una religione monoteista
I sumeri praticavano una religione monoteista Snc società
Snc società 2247 cc
2247 cc Costituzione società per azioni
Costituzione società per azioni Womi test
Womi test Società in accomandita semplice
Società in accomandita semplice Piramide sociale egizia
Piramide sociale egizia Società di vita apostolica
Società di vita apostolica Acquisto di partecipazioni rilevanti in società quotate
Acquisto di partecipazioni rilevanti in società quotate Le api scuola primaria
Le api scuola primaria Descolarizzare la società
Descolarizzare la società Impresa individuale e collettiva
Impresa individuale e collettiva Autonomia patrimoniale
Autonomia patrimoniale Associazione segreta piemontese risorgimento
Associazione segreta piemontese risorgimento Sas società
Sas società Comuneros società segreta
Comuneros società segreta Bergson slancio vitale
Bergson slancio vitale Oligarchia spartana scuola primaria
Oligarchia spartana scuola primaria Parafrasi inno di mameli
Parafrasi inno di mameli Borsa nazionale del lavoro
Borsa nazionale del lavoro Istituto nazionale di urbanistica
Istituto nazionale di urbanistica Direzione nazionale antimafia organigramma
Direzione nazionale antimafia organigramma Classificazione nazionale dei dispositivi medici
Classificazione nazionale dei dispositivi medici Associazione nazionale bed and breakfast
Associazione nazionale bed and breakfast Centro nazionale trapianti
Centro nazionale trapianti Infn lab nazionale di legnaro, legnaro
Infn lab nazionale di legnaro, legnaro Festa nazionale inghilterra
Festa nazionale inghilterra Organigramma sistema sanitario nazionale
Organigramma sistema sanitario nazionale Direzione nazionale antimafia e antiterrorismo
Direzione nazionale antimafia e antiterrorismo Classificazione cnd dispositivi medici
Classificazione cnd dispositivi medici Istituto gemmologico nazionale
Istituto gemmologico nazionale Assdi nazionale
Assdi nazionale Consolidato mondiale
Consolidato mondiale Tema nazionale fidapa
Tema nazionale fidapa Rete accelerometrica nazionale
Rete accelerometrica nazionale Sport in germania
Sport in germania Carta nazionale professioni museali
Carta nazionale professioni museali Centro nazionale trapianti
Centro nazionale trapianti Immagini inno di mameli
Immagini inno di mameli Pnsd schema
Pnsd schema Il reddito nazionale
Il reddito nazionale Olga
Olga Rete accelerometrica nazionale
Rete accelerometrica nazionale Galleria nazionale delle marche
Galleria nazionale delle marche Centro nazionale trapianti
Centro nazionale trapianti