Unit 1 Matter is made of particles Matter






























- Slides: 50

Unit 1: Matter is made of particles

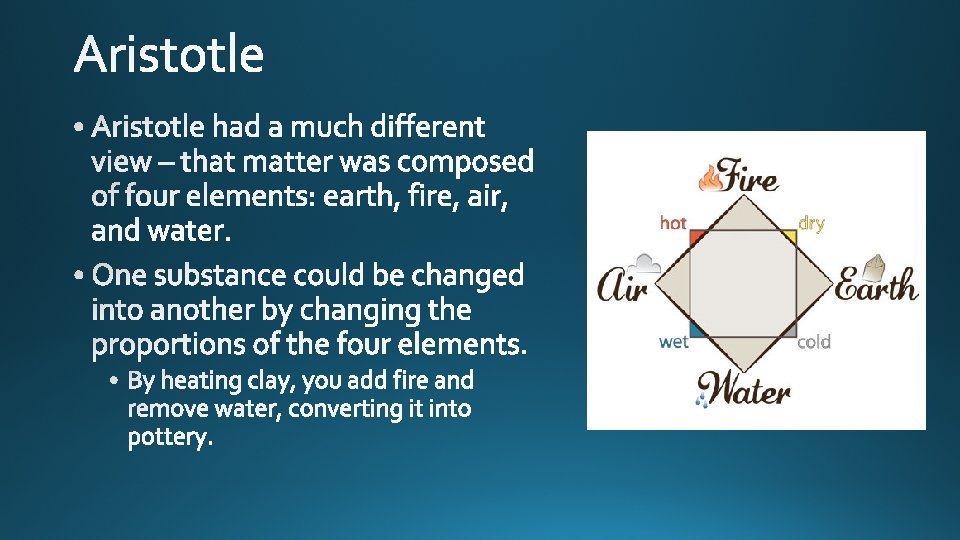
• Matter • Philosophy




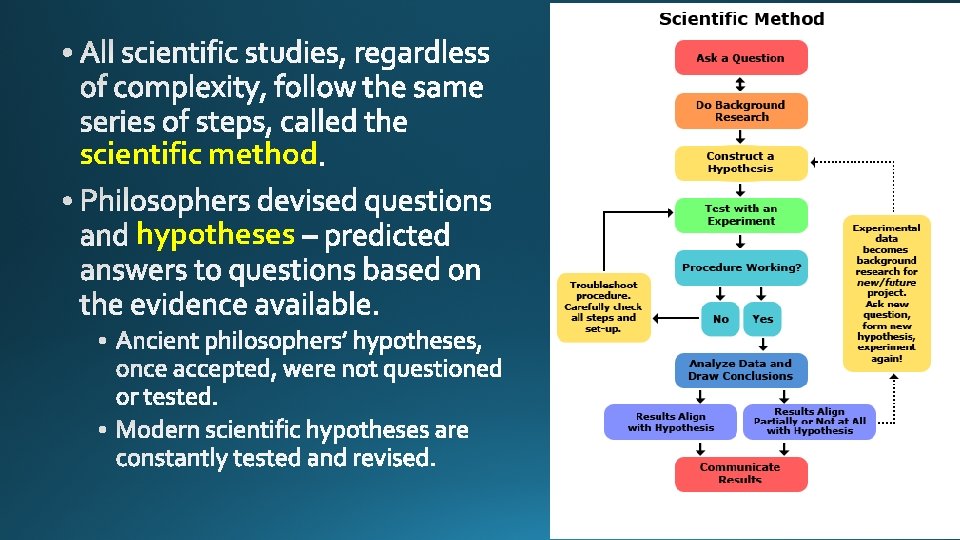
• Science reproducible

scientific method hypotheses

observation elements

Brownian motion

combustion

• Experiments independent variable experimental group control group constants

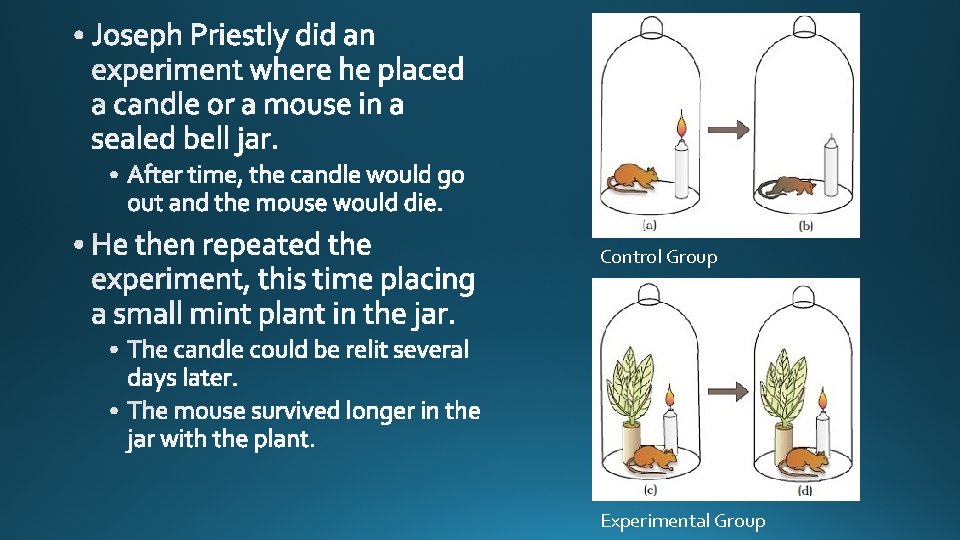
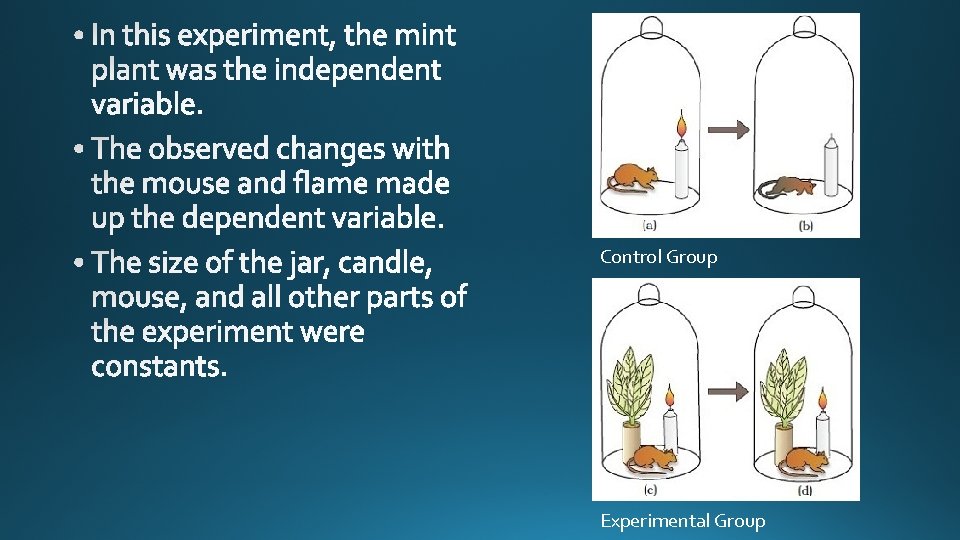
Control Group Experimental Group

Control Group Experimental Group

• Data conclusion


probability sample size

bias blind experiment • A double-blind experiment James Randi on the “medical psychic”.

atoms

element Copper blanks. These are stamped to make coins.

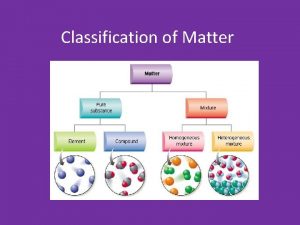
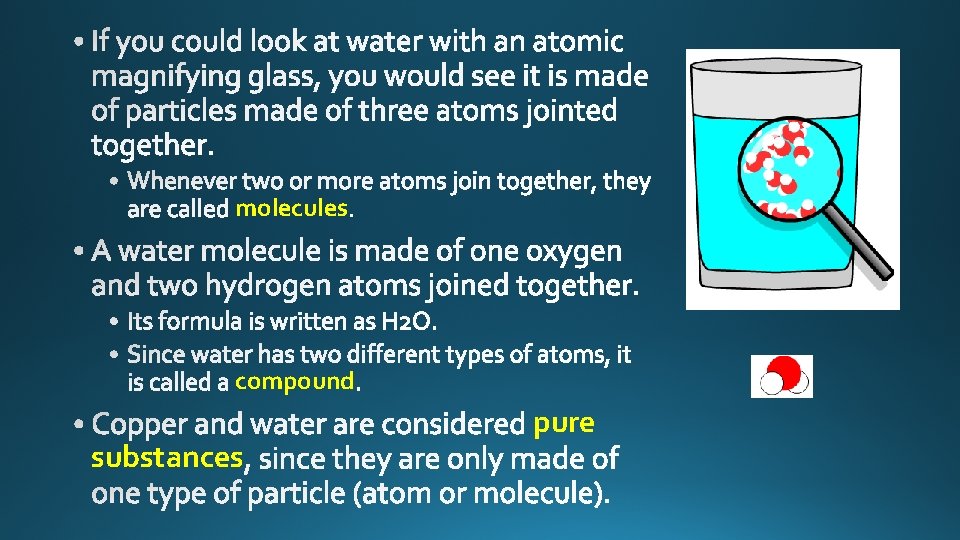
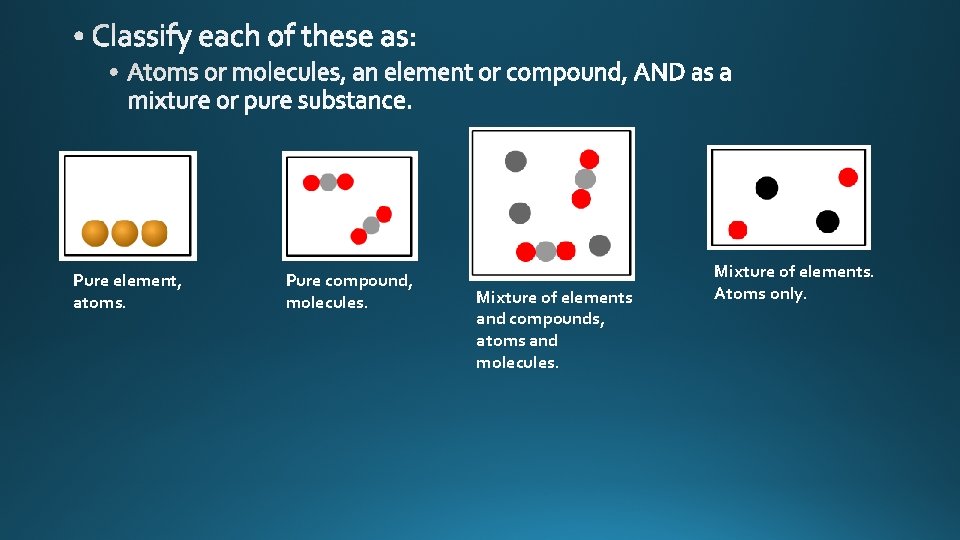
molecules compound substances pure

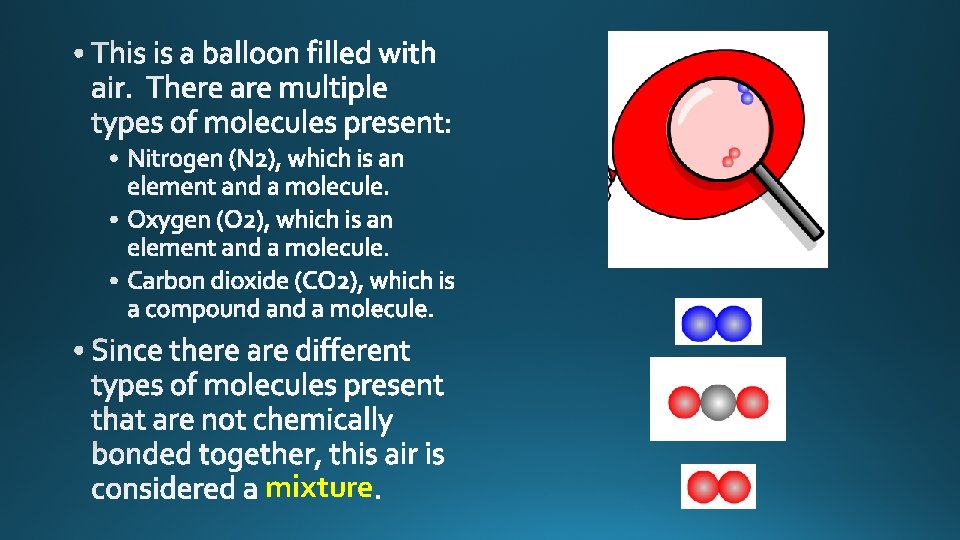
mixture

Pure element, atoms. Pure compound, molecules. Mixture of elements and compounds, atoms and molecules. Mixture of elements. Atoms only.

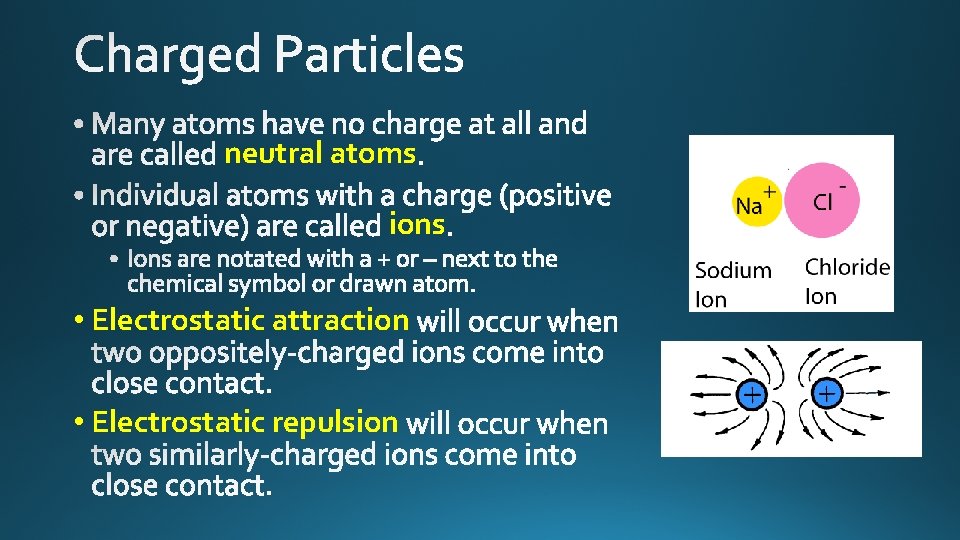
neutral atoms ions • Electrostatic attraction • Electrostatic repulsion

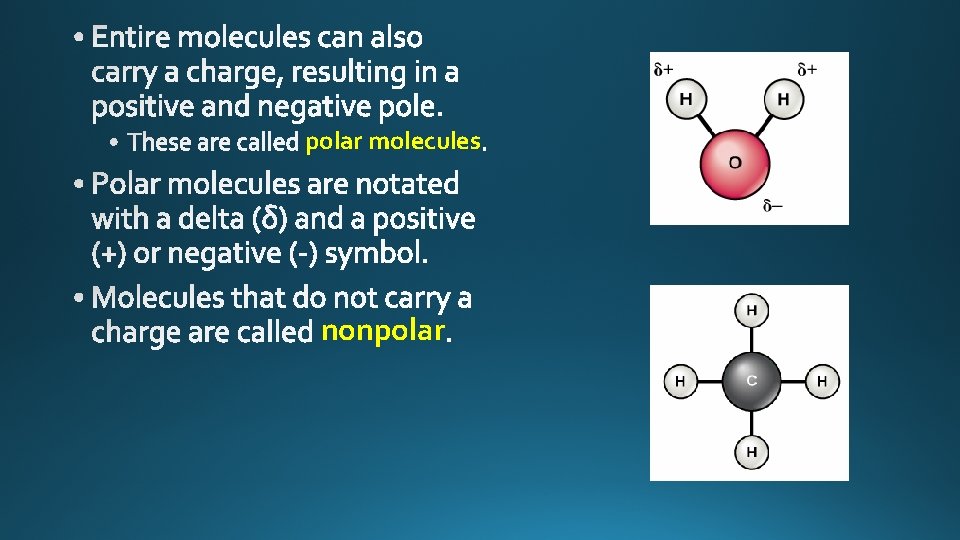
polar molecules nonpolar

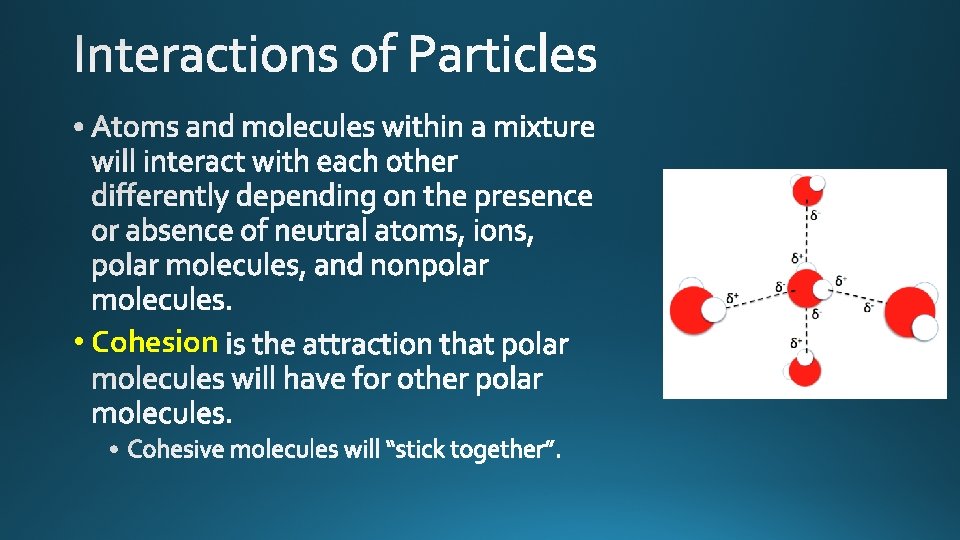
• Cohesion


• Adhesion meniscus

solubility solute solvent solution

soluble insoluble like dissolves like” rule


magnetic • Conductivity

density volume and mass

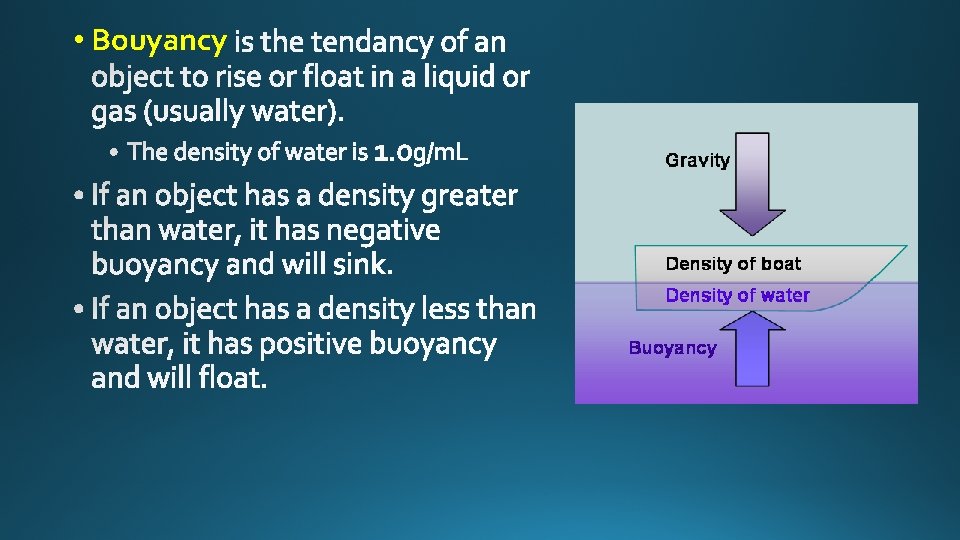
• Bouyancy

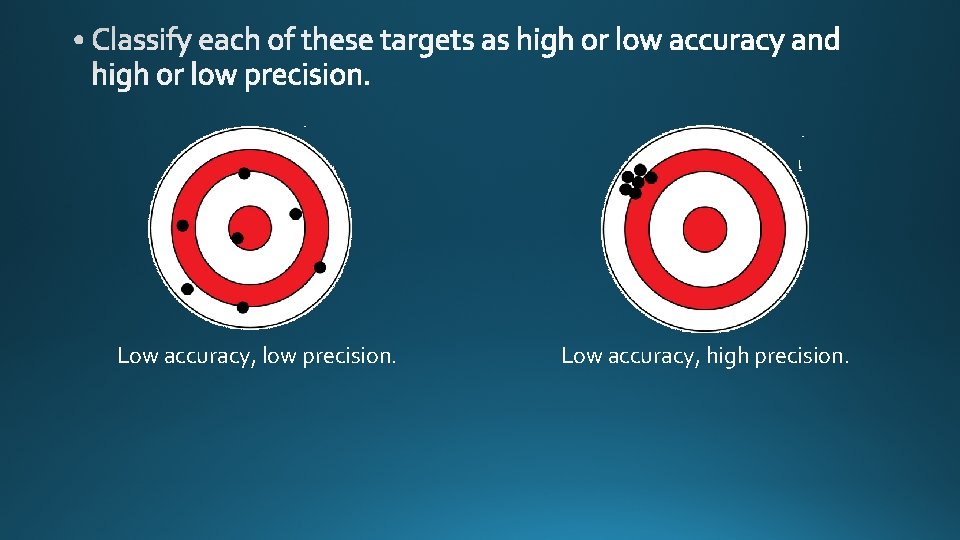
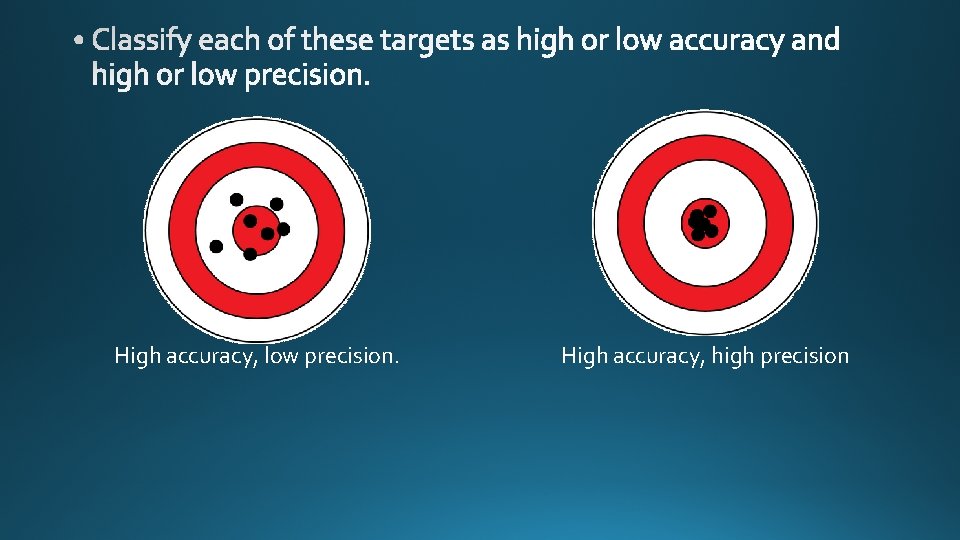
• Qualitative • Quantitative • Accuracy • Precision

Low accuracy, low precision. Low accuracy, high precision.

High accuracy, low precision. High accuracy, high precision

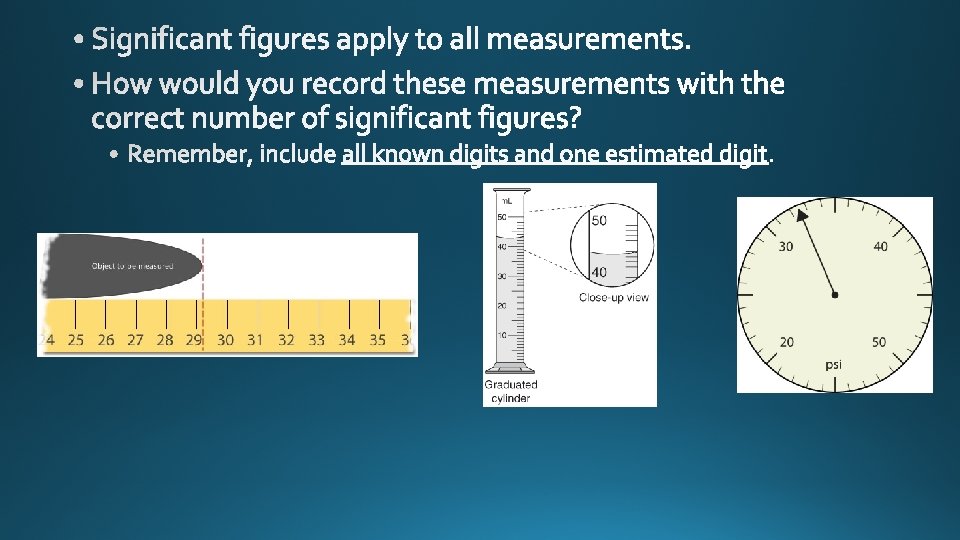
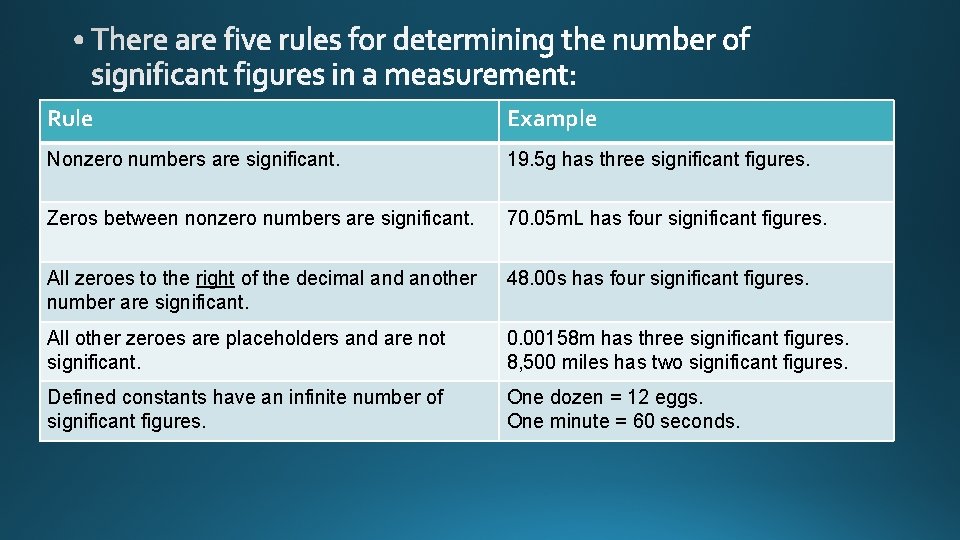
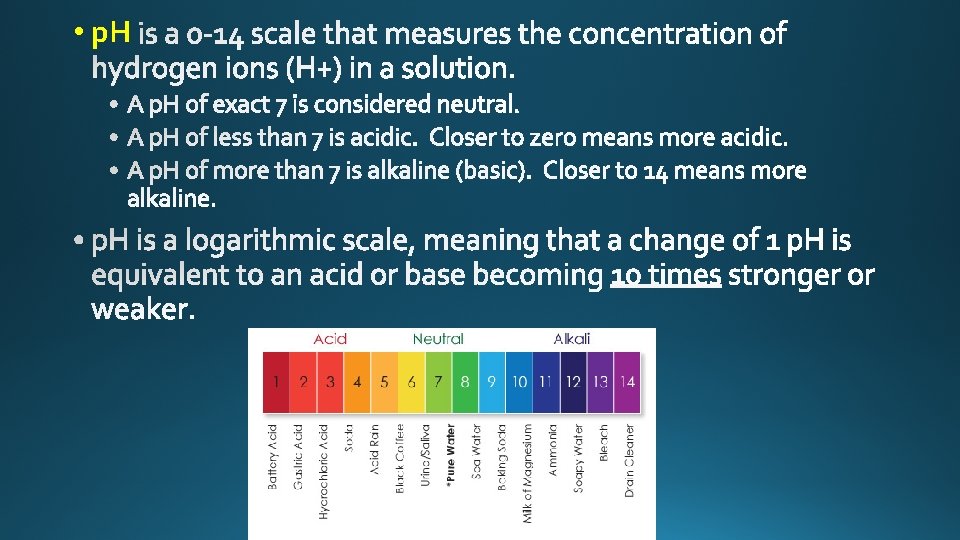
significant figures


Rule Example Nonzero numbers are significant. 19. 5 g has three significant figures. Zeros between nonzero numbers are significant. 70. 05 m. L has four significant figures. All zeroes to the right of the decimal and another number are significant. 48. 00 s has four significant figures. All other zeroes are placeholders and are not significant. 0. 00158 m has three significant figures. 8, 500 miles has two significant figures. Defined constants have an infinite number of significant figures. One dozen = 12 eggs. One minute = 60 seconds.


• Density



• Flammability • Reactivity

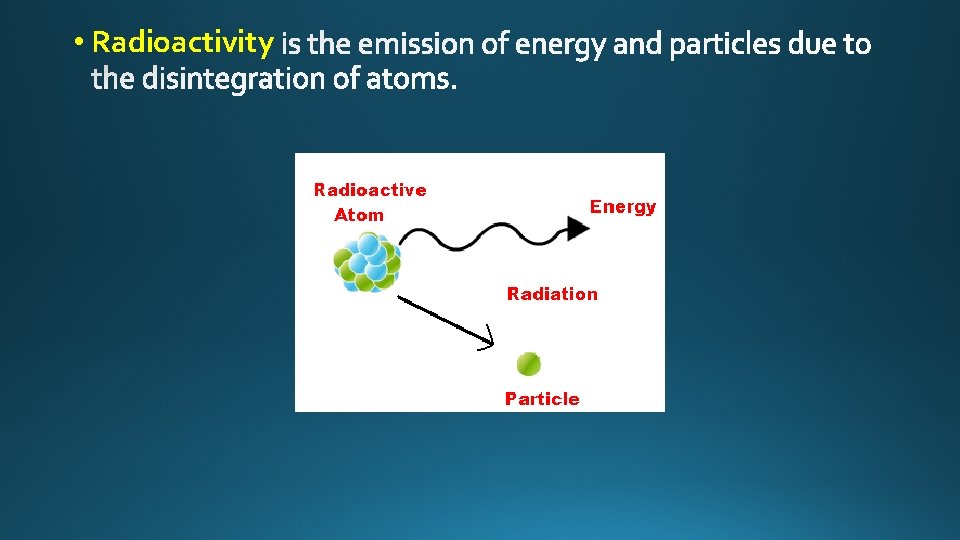
• Radioactivity


physical change

• Chemical changes

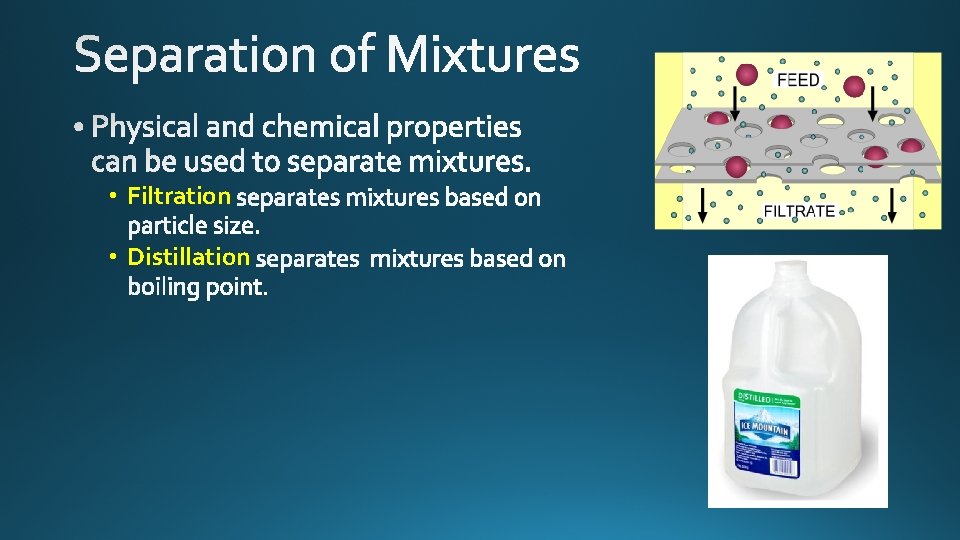
• Filtration • Distillation

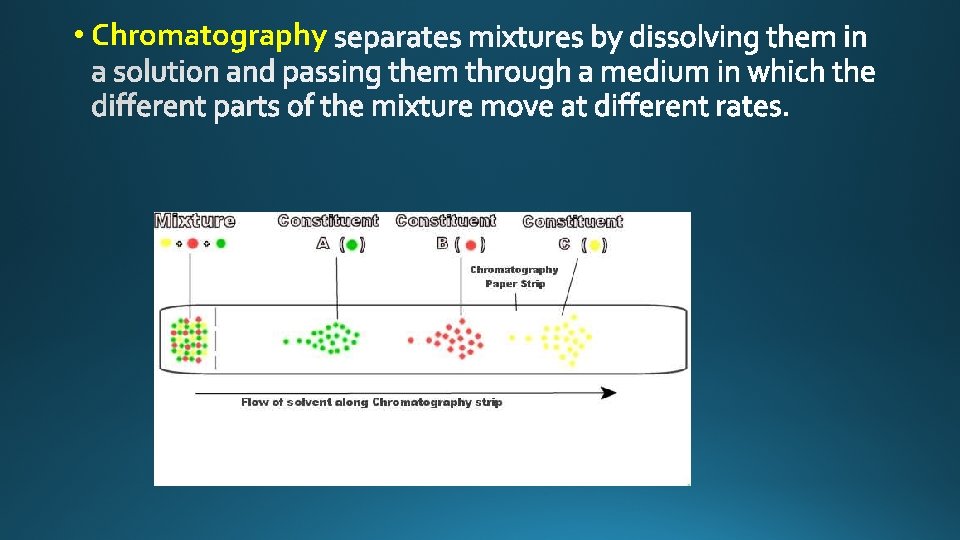
• Chromatography
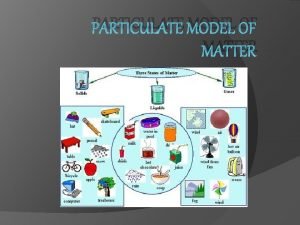
 The kinetic theory explains how particles in matter behave
The kinetic theory explains how particles in matter behave Building block of matter which contains subatomic particles
Building block of matter which contains subatomic particles Mutual force
Mutual force Unit 6 review questions
Unit 6 review questions All matter is made up of
All matter is made up of All matter is made up of
All matter is made up of What is the difference between gray and grey
What is the difference between gray and grey All matter is made up of
All matter is made up of Matter is made up of
Matter is made up of What is everything around us made of
What is everything around us made of Section 1 composition of matter
Section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Brain falx
Brain falx Section 1 composition of matter
Section 1 composition of matter Gray matter and white matter
Gray matter and white matter Gray matter
Gray matter Energy naturally flows from warmer matter to cooler matter.
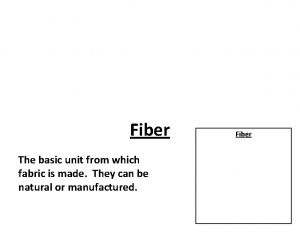
Energy naturally flows from warmer matter to cooler matter. Is the basic unit of textile.
Is the basic unit of textile. Plasma particles
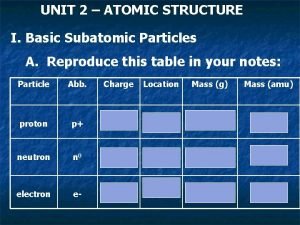
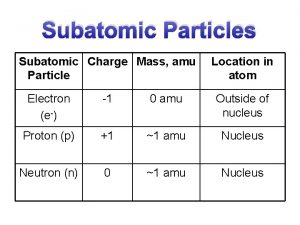
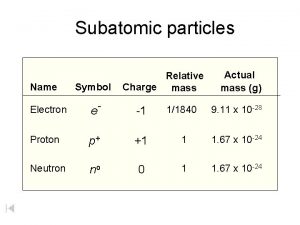
Plasma particles Subatomic particles table
Subatomic particles table Point like particles
Point like particles Arrangement of subatomic particles
Arrangement of subatomic particles Number of particles and volume relationship
Number of particles and volume relationship Connected particles a level maths
Connected particles a level maths Mass of subatomic particles in amu
Mass of subatomic particles in amu Nucleon number
Nucleon number Definite arrangement
Definite arrangement Kinetic theory of solids
Kinetic theory of solids Solid liquid gas plasma
Solid liquid gas plasma Solid
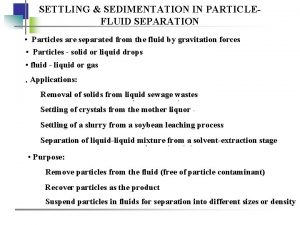
Solid Settling velocity stokes law
Settling velocity stokes law The search for fractionally charged particles has
The search for fractionally charged particles has Conduction convection radiation venn diagram
Conduction convection radiation venn diagram Photochromic particles
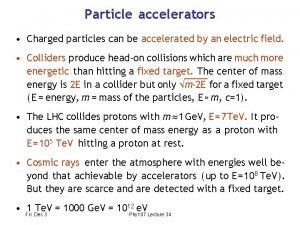
Photochromic particles Charged particles can be accelerated by
Charged particles can be accelerated by Plasma to gas
Plasma to gas 20 examples of liquids
20 examples of liquids Chapter 6 ions charged particles in solution
Chapter 6 ions charged particles in solution Point like particles
Point like particles Postulate 1
Postulate 1 Elementary particles
Elementary particles Classification of elementary particles
Classification of elementary particles Loose solid particles
Loose solid particles Arithmetic mean diameter formula
Arithmetic mean diameter formula Condensation particle theory
Condensation particle theory Polonium reactivity
Polonium reactivity How are particles arranged in an atom
How are particles arranged in an atom The smallest particle of an element
The smallest particle of an element A heterogeneous mixture of intermediate-sized particles
A heterogeneous mixture of intermediate-sized particles Spaces between particles of liquid
Spaces between particles of liquid