Matter Learning outcomes Matter is composed of particles





















































- Slides: 65

Matter

Learning outcomes • Matter is composed of particles, which may be atoms, molecules or ions. • Atoms. Minute size of atoms. • Law of conservation of mass.


DIFFUSIONevidence for the existence of small particles • Spreading out of gases • Colour of ink sreading out when mixed with water • Hydrogen chloride and ammonia solution

AMMONIUM CHLORIDE formed because of ammonia’s and hydrogen chloride diffusion

Law of conservation of mass/matter • The law of conservation of mass/matter, also known as law of mass/matter conservation says that the mass of a closed system will remain constant, regardless of the processes acting inside the system. • Matter cannot be created/destroyed, although it may be rearranged. • For any chemical process in a closed system, the mass of the reactants must equal the mass of the products.



Learning Outcomes • Very brief outline of the historical development of atomic theory (outline principles only; mathematical treatment not required): Dalton: atomic theory; • Crookes: vacuum tubes, cathode rays; • Stoney: naming of the electron; • Thomson: negative charge of the electron; e/m for electrons (experimental details not required); • Millikan: magnitude of charge of electrons as shown by oil drop experiment (experimental details not required); • Rutherford: discovery of the nucleus as shown by the α−particle scattering experiment; • Discovery of protons in nuclei of various atoms; • Bohr: model of the atom; • Chadwick: discovery of the neutron.

HISTORY OF THE ATOM • GREEKS – Matter made of tiny indivisible particles

DALTON 1766 -1844 • All matter made of small particles called atoms • Atoms are indivisible • Atoms cannot be created or destroyed

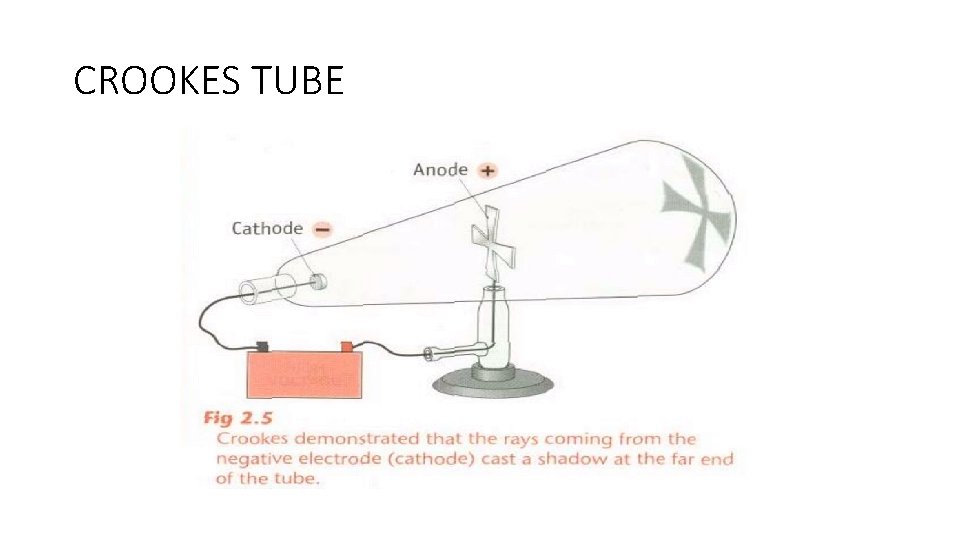
DISCOVERY OF THE ELECTRON • Crookes conducted experiments with a glass tube

CROOKES TUBE

CROOKES TUBES • Cathode connected to negative electrode • Anode connected to the positive electrode • CNAP

VACUUM TUBES • Gas at low pressure • Electric current passed through • Radiation came from the end of the tube connected to the negative(cathode) end of the battery • Cathode rays

TUBES

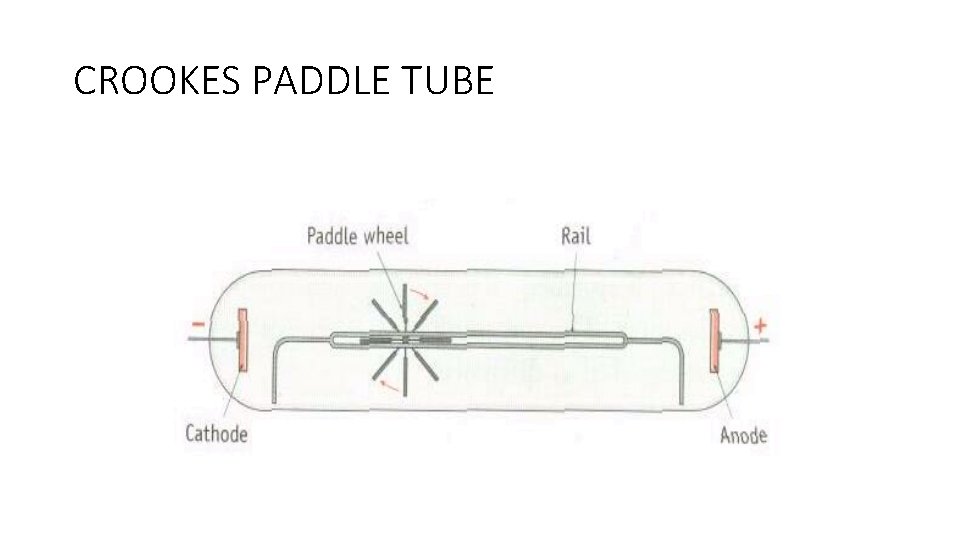
CROOKES PADDLE TUBE

CATHODE RAYS • Cast shadows • Cause glass to glow • Turn a paddle wheel • Rays are made of particles

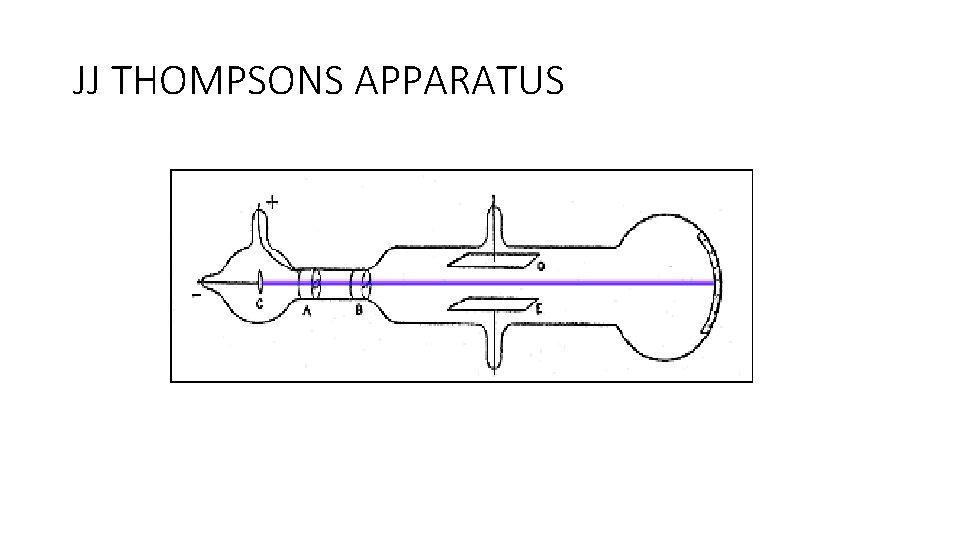
JJ THOMPSON • Hole in anode to allow beam of rays to pass through. • Beam could be deflected by electric plates. • Therefore beam is made of negative particles.

JJ THOMPSONS APPARATUS

JJ THOMPSON • Used a magnetic field from an electromagnet to deflect the electrons • Calculated the ratio of charge to mass for electron

GEORGE STONEY • Named these negative particles electrons

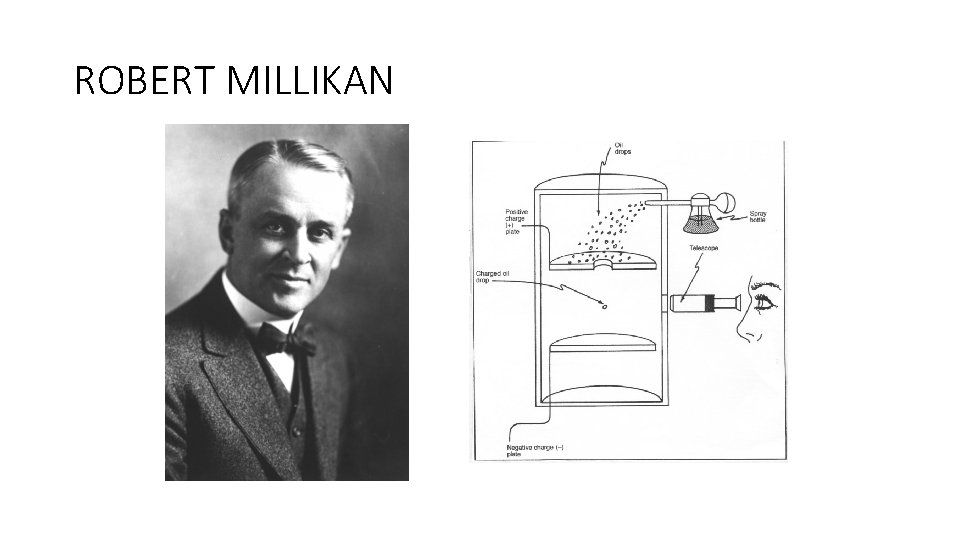
ROBERT MILLIKAN • Famous oil-drop experiment • It measured the charge on the electron • X-rays ionised air molecules by striping electrons off their atoms. • Oil droplets picked up electrons became negative • Increased the + charge until the droplet hovered. • Took measurements and calculated the charge on the electron.

ROBERT MILLIKAN

ROBERT MILLIKAN

THOMPSON’S ATOM • Atom a sphere of positive charges with negative electons embedded

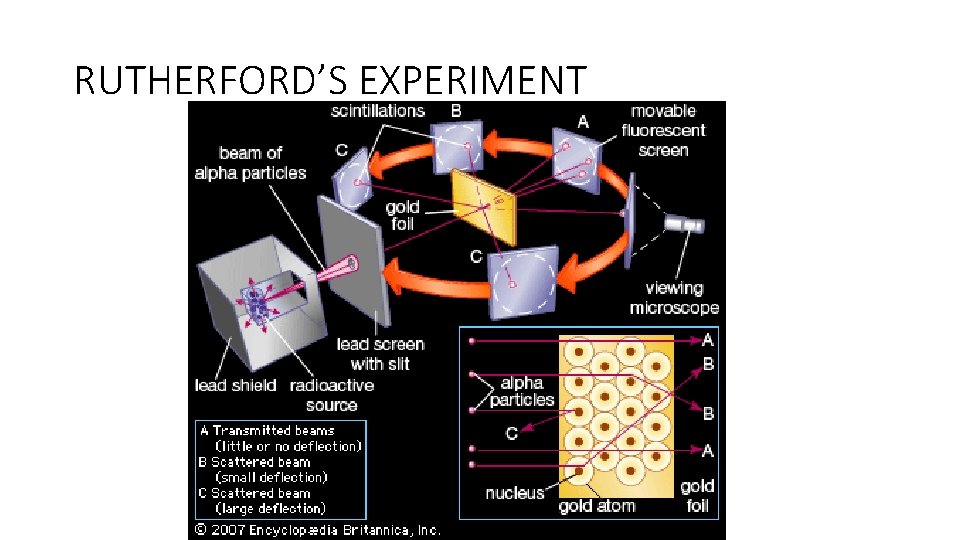
ERNEST RUTHERFORD • Fired thin alpha particles at a tin gold foil • Thompsons plum pudding model predicted that they would pass thru’ with little deflection

RUTHERFORD’S EXPERIMENT •

RUTHERFORD’S EXPERIMENT

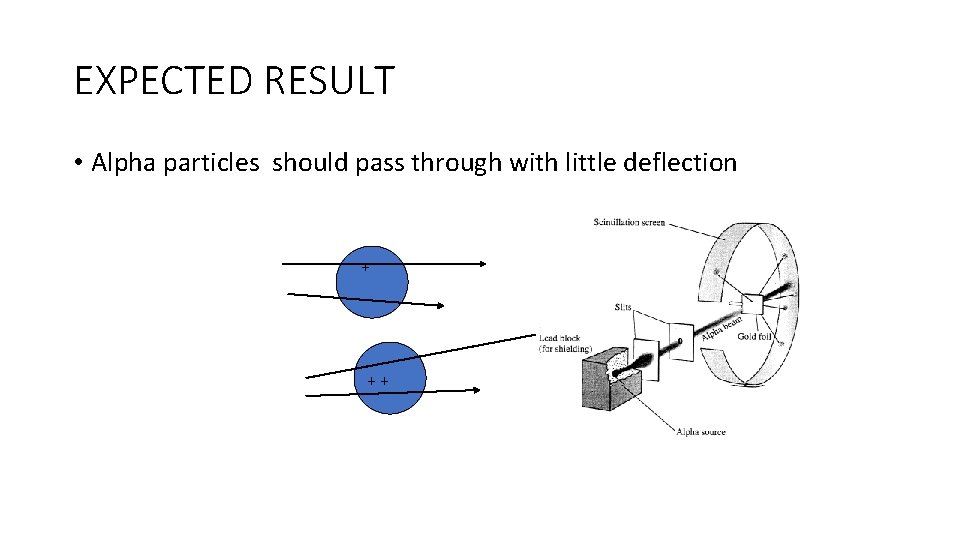
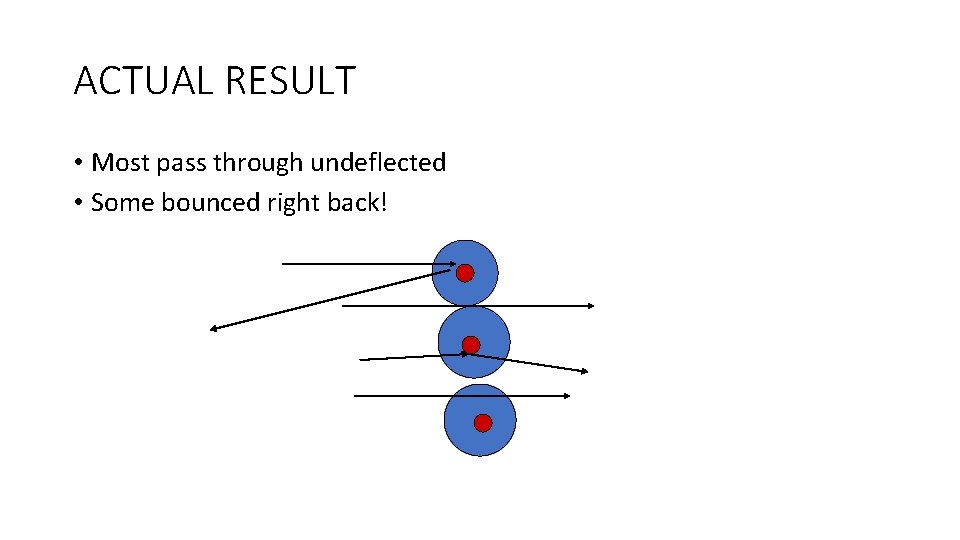
EXPECTED RESULT • Alpha particles should pass through with little deflection + ++

ACTUAL RESULT • Most pass through undeflected • Some bounced right back!

EXPLANATION • Hard dense core of positive matter in the center of each atom-nucleus • Atoms are mostly empty space.

THE PROTON • Rutherford continued to bombard different elements such as nitrogen and oxygen • Small positive particles were given off--- protons

THE NEUTRON • James Chadwick bombarded beryllium with alpha particles. • Small particles were given off which were neutral and had the same mass as the proton—the neutron.


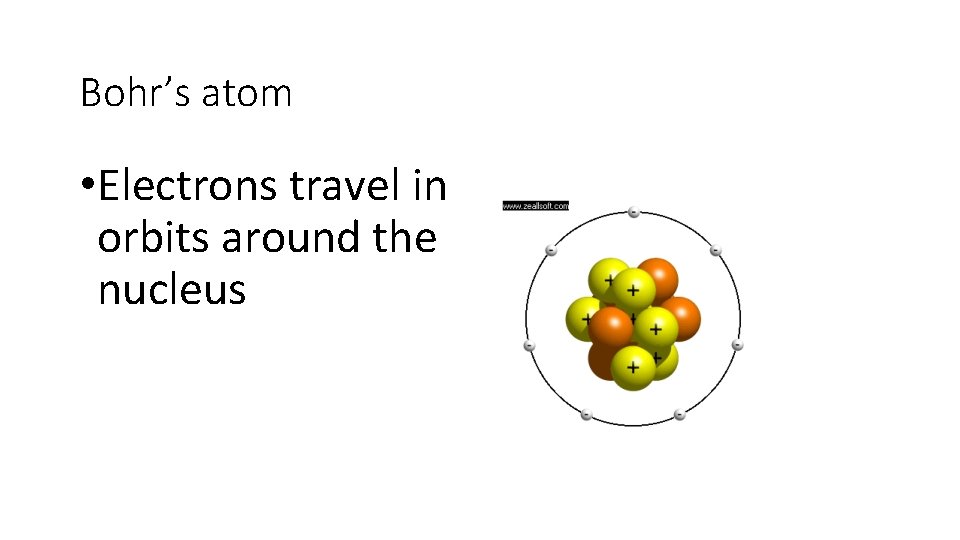
Bohr’s atom • Electrons travel in orbits around the nucleus


Learning Outcomes • Properties of electrons, protons and neutrons • (relative mass, relative charge, location within atom).

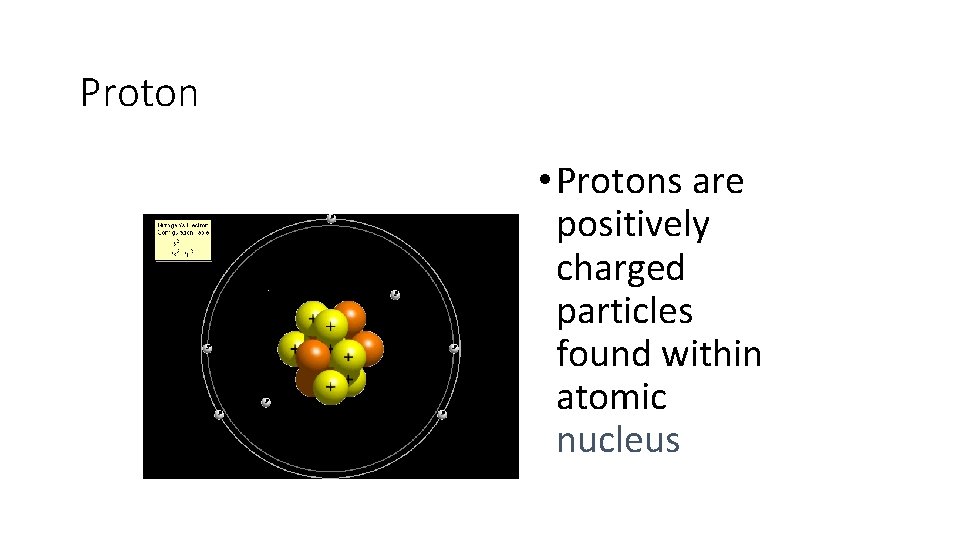
Proton • Protons are positively charged particles found within atomic nucleus



Learning Outcomes Atomic number (Z ), mass number (A), isotopes; hydrogen and carbon as examples of isotopes. Relative atomic mass (A r). The 12 C scale for relative atomic masses.

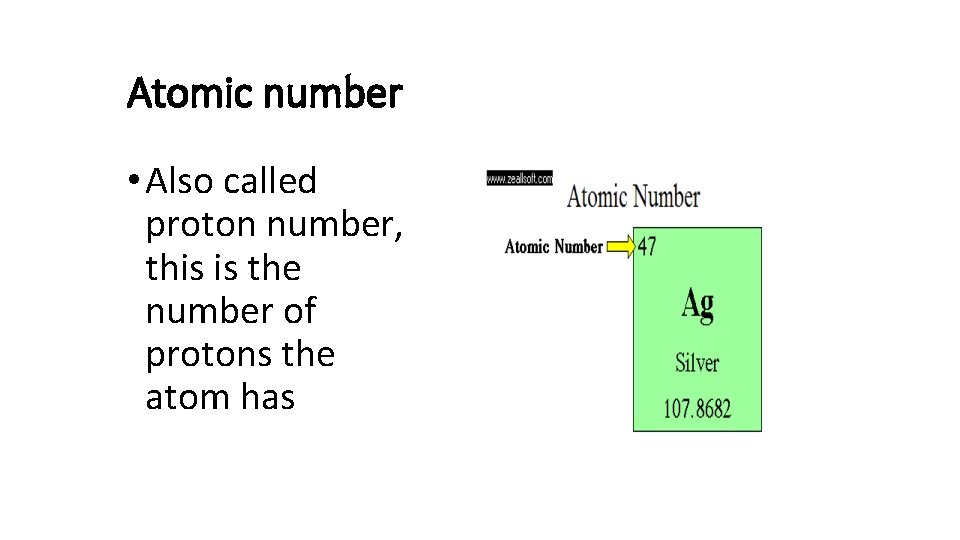
Atomic number • Also called proton number, this is the number of protons the atom has

The Number of Electrons • Atoms must have equal numbers of protons and electrons. • An atom of krypton must contain 36 electrons since it contains 36 protons.

Mass number • Mass Number = • (Number of Protons) + (Number of Neutrons)

Isotope • Atoms that have the same atomic number of protons but different numbers of neutrons are called isotopes


Hydrogen isotopes • The element hydrogen for example, has three commonly known isotopes: protium, deuterium and tritium

Protium • an atom of protium consists of one proton, no neutron and one electron

Deuterium • an atom of deuterium consists of one proton one neutron and one electron

Tritium • An atom of tritium consists of one proton two neutrons and one electron


Relative Atomic Mass • The relative atomic mass is the average mass of the atoms of an element as they occur in nature with the mass of each atom taken relative to one twelfth of the mass of a Carbon 12 atom

Learning Outcomes • Calculation of approximate relative atomic masses from abundance of isotopes of given mass number (e. g. Calculation of approximate relative atomic mass of chlorine).

Chlorine • Chlorine-35 and Chlorine-37 are both isotopes of chlorine

Relative mass of chlorine • Chlorine consists of roughly 75% Chlorine-35 and roughly 25% Chlorine-37. We take an average of the two figures The relative atomic mass of chlorine is usually quoted as 35. 5.


Learning outcomes • Use of the mass spectrometer in determining relative atomic mass. • Fundamental processes that occur in a mass spectrometer: • vaporisation of substance, • production of positive ions, • acceleration, separation, • detection (mathematical • treatment excluded).

THE MASS SPECTROMETER • The Mass Spectrometer is used in determining relative atomic mass • It is based on: • Atoms can be deflected by magnetic fields provided the atom is first turned into an ion.

Stage 1: Ionisation • The atom is ionised by knocking one or more electrons off to give a positive ion.

Stage 2: Acceleration • The ions are accelerated so that they all have the same kinetic energy.

Stage 3: Deflection • The ions are then deflected by a magnetic field according to their masses. The lighter they are, the more they are deflected.

Stage 4: Detection • The beam of ions passing through the machine is detected electrically.


 All life is composed of matter
All life is composed of matter An explanation of how particles in matter behave
An explanation of how particles in matter behave Building block of matter which contains subatomic particles
Building block of matter which contains subatomic particles The mutual force of attraction between particles of matter
The mutual force of attraction between particles of matter Expected outcome example
Expected outcome example Learning outcomes of water cycle
Learning outcomes of water cycle Notice writing learning outcomes
Notice writing learning outcomes Objective of swot
Objective of swot Rhymes learning outcomes
Rhymes learning outcomes Planning goals and learning outcomes
Planning goals and learning outcomes What is photolysis in photosynthesis
What is photolysis in photosynthesis Learning objectives of nutrition in plants class 7
Learning objectives of nutrition in plants class 7 What is ncbts?
What is ncbts? Objectives of linear equations in one variable
Objectives of linear equations in one variable A machine that converts mechanical energy into electricity
A machine that converts mechanical energy into electricity Learning outcomes of reported speech
Learning outcomes of reported speech Purpose of learning outcomes
Purpose of learning outcomes Objective of input output devices
Objective of input output devices Mri ib psychology
Mri ib psychology How to write reflective essay
How to write reflective essay Learning outcomes of holy week
Learning outcomes of holy week Holy week objectives
Holy week objectives Headstart early learning outcomes framework
Headstart early learning outcomes framework Learning outcomes of vegetables
Learning outcomes of vegetables Gagne learning outcomes
Gagne learning outcomes Arithmetic progression lesson plan
Arithmetic progression lesson plan What i learnt from a gift of chappals
What i learnt from a gift of chappals Learning outcomes of work and energy
Learning outcomes of work and energy Learning objectives of profit and loss
Learning objectives of profit and loss Cooking learning outcomes
Cooking learning outcomes Professional standard strand
Professional standard strand Humalog dosage scale
Humalog dosage scale Learning outcomes of work and energy
Learning outcomes of work and energy Cas learning outcomes
Cas learning outcomes Conclusion of learning outcomes
Conclusion of learning outcomes Pythagoras theorem
Pythagoras theorem Learning outcomes should be
Learning outcomes should be Chapter 8 digestive system
Chapter 8 digestive system Learning outcome
Learning outcome Pathophysiology of angina pectoris
Pathophysiology of angina pectoris Learning outcomes of empathy
Learning outcomes of empathy Mqf learning outcomes domains
Mqf learning outcomes domains Hindu writing
Hindu writing Learning outcomes of myself
Learning outcomes of myself Human resources learning outcomes
Human resources learning outcomes Business and communication systems gcse
Business and communication systems gcse Objectives of email writing
Objectives of email writing Define training program
Define training program Examples of learning outcomes
Examples of learning outcomes Learning objectives for mental health
Learning objectives for mental health Affective domain
Affective domain Learning outcomes definition
Learning outcomes definition Junior cycle learning outcomes
Junior cycle learning outcomes Bloom's taxonomy of learning objectives
Bloom's taxonomy of learning objectives Learning outcomes examples english
Learning outcomes examples english Learning outcomes framework
Learning outcomes framework Institutional learning outcomes examples
Institutional learning outcomes examples Cas learning outcomes
Cas learning outcomes Cuadro comparativo entre e-learning b-learning y m-learning
Cuadro comparativo entre e-learning b-learning y m-learning Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Grey matter in nervous system
Grey matter in nervous system Section 1 composition of matter
Section 1 composition of matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Primary taste cortex
Primary taste cortex Composition of matter section 1
Composition of matter section 1 Gray matter and white matter
Gray matter and white matter