PARTICULATE MODEL OF MATTER What is matter made












- Slides: 17

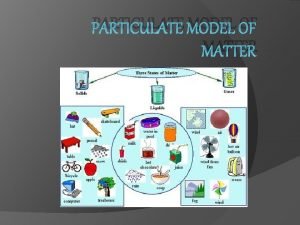
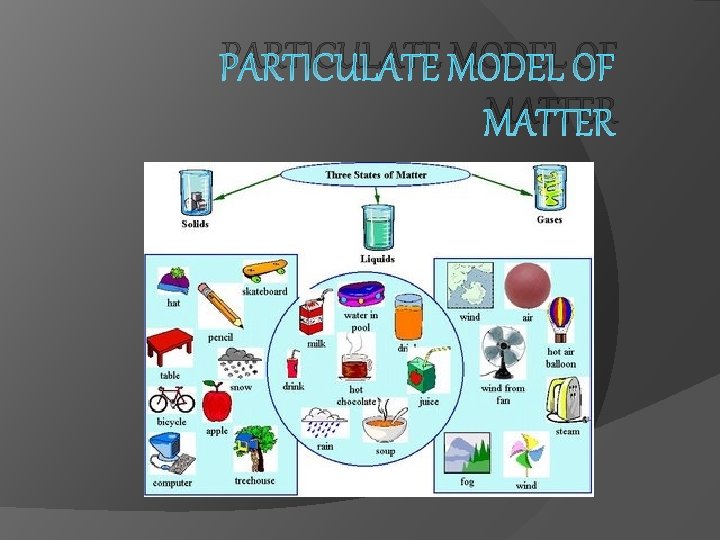
PARTICULATE MODEL OF MATTER

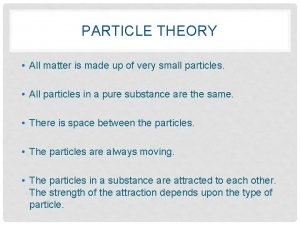
What is matter made up of? � Matter is made of elements � An element is made of tiny particles � All elements is made up of tiny particles � Particles in matter posses kinetic energy and are constantly moving in a random manner.

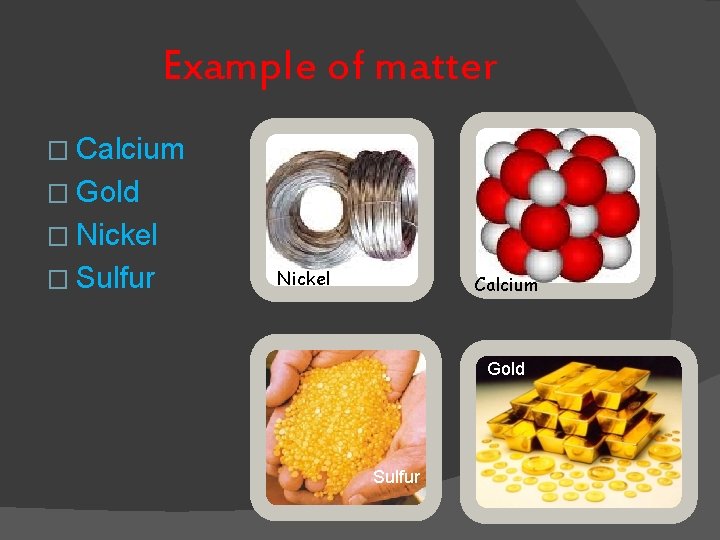
Example of matter � Calcium � Gold � Nickel � Sulfur Nickel Calcium Gold Sulfur

Evidence for moving particles � Diffusion is the process by which particles of matter fill a space because of random motion. � Movement of particles is always from a region of higher concentration to one of lower concentration.

Diffusion in gases � If a bottle of perfume is opened in one corner of a room, it spreads in the whole room by diffusion.

Diffusion in liquid � If a bottle of perfume is opened in one corner of a room, it spreads in the whole room by diffusion. If you drop a little ink in a breaker of water it will spread by itself and the colour spread uniformly.

Diffusion in solid � When a lead plate and a gold plate are placed one over the other if they are kept under pressure for a period of one or two years. It will be observed that the lead particles have diffused into gold and particles have diffused into lead plate. It is believed that fossils of animals like reptiles and leaves lie in contact with stones and rocks for number of years, which leaves an impression on the stones or rocks. This is because of the diffusion of the particles of the organic matter into the stones, which are in contact.

Solid � Regular arrangement � Closely packed � Strong forces of attraction � Vibrate about fixed positions

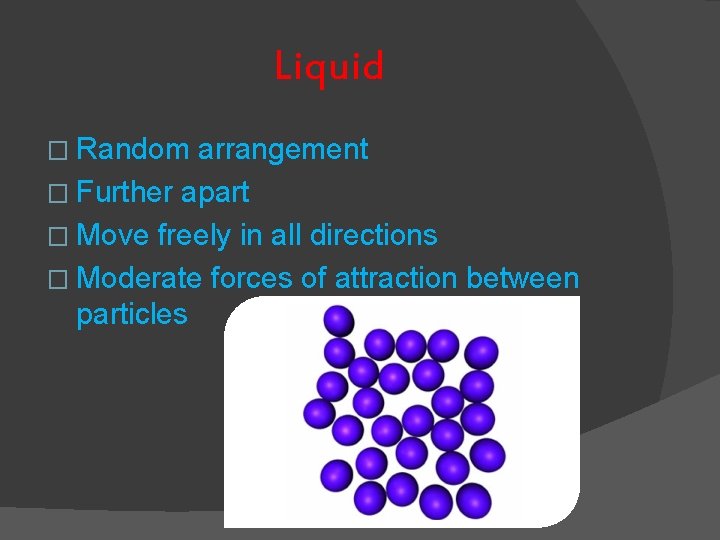
Liquid � Random arrangement � Further apart � Move freely in all directions � Moderate forces of attraction between particles

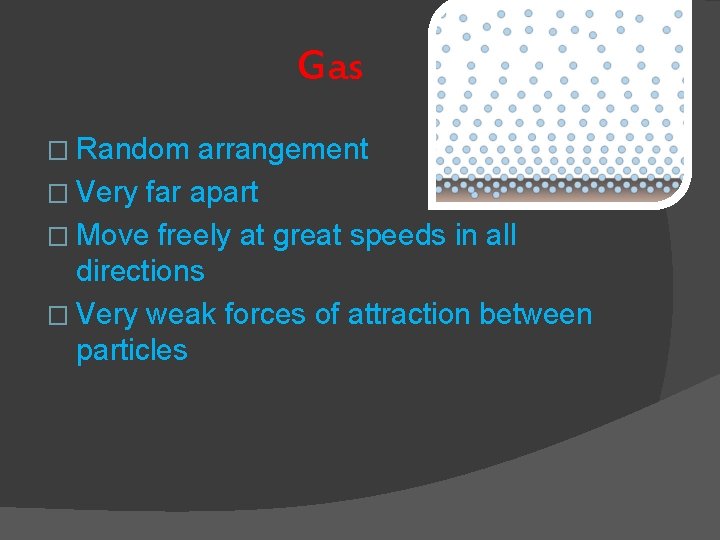
Gas � Random arrangement � Very far apart � Move freely at great speeds in all directions � Very weak forces of attraction between particles

Melting � It is the process of changing a solid into its liquid state. The temperature at which a solid changes to the liquid state is known as melting point. When a solid is heated, the molecules gain more kinetic energy and start vibrating with a greater velocity and greater amplitude. At a particular temperature, the molecules gain kinetic energy sufficient to overcome the force of intermolecular attraction. They then start to slip and slide against each other. This process is called melting.

Evaporation � All the molecules of a liquid are in a state of constant and random motion. They have different velocities. During the motion, molecules collide against each other and some of the molecules gain more kinetic energy than others. The energy rich molecules break through the surface of the liquid. Thus the average kinetic energy of the remaining molecules decreases and this lowers the temperature of the liquid. Thus evaporation results in cooling.

Boiling � When a liquid is heated, the molecules gain more kinetic energy and the temperature starts rising. After it has reached a specific point though heat is being supplied, the temperature becomes steady. At this temperature the energy supplied gets utilized in breaking the inter-molecular force of attraction. Now the molecules start leaving the liquid and escape as vapor. This process is called boiling and the temperature is called boiling point.

Freezing � When a liquid is cooled(heat is removed), the particles lose energy and they move about more slowly.

Condensation � When a gas is cooled(heat is removed), the particles lose energy and they move about less vigorously and at lower speeds.

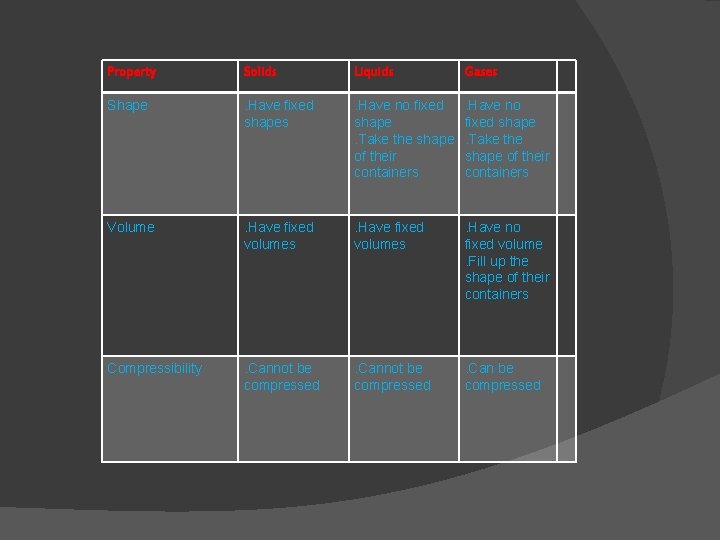
Property Solids Liquids Gases Shape . Have fixed shapes . Have no fixed shape. Take the shape of their containers Volume . Have fixed volumes . Have no fixed volume. Fill up the shape of their containers Compressibility . Cannot be compressed . Can be compressed

Credits � Natalie Woo � Gurpreet Kaur � Anjar Saridiyah Bte Kasman
 The particulate model of matter
The particulate model of matter Wesam al madhoun
Wesam al madhoun Confined space dredging
Confined space dredging Particle theory
Particle theory Shaping process adalah
Shaping process adalah Particulate contamination in infusions
Particulate contamination in infusions Die blanks ceramics
Die blanks ceramics Particulate radiation
Particulate radiation Is milk macroscopic microscopic or particulate
Is milk macroscopic microscopic or particulate Mendel's theory of particulate inheritance __________.
Mendel's theory of particulate inheritance __________. Matter made up of
Matter made up of All matter is made up of
All matter is made up of Gray matter in the brain
Gray matter in the brain All matter is made up of
All matter is made up of Matter is made up of
Matter is made up of Everything around us is made up of matter
Everything around us is made up of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter