Air Pollution Particulate Matter Particulate air pollution Small


- Slides: 21

Air Pollution Particulate Matter

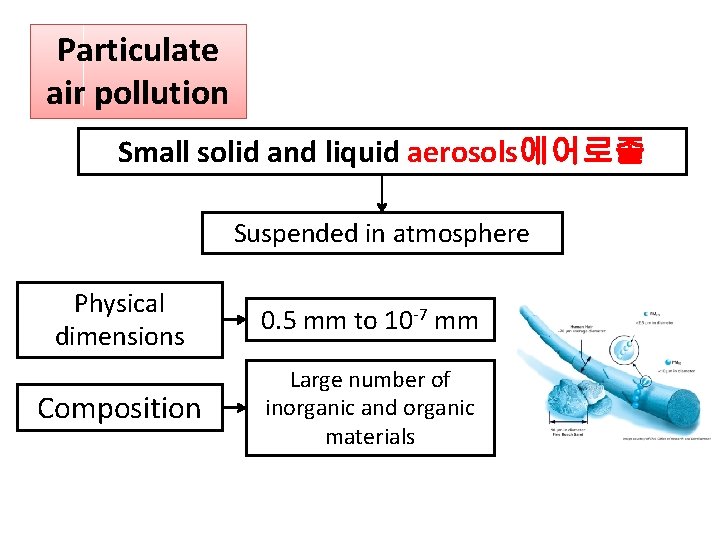
Particulate air pollution Small solid and liquid aerosols에어로졸 Suspended in atmosphere Physical dimensions 0. 5 mm to 10 -7 mm Composition Large number of inorganic and organic materials

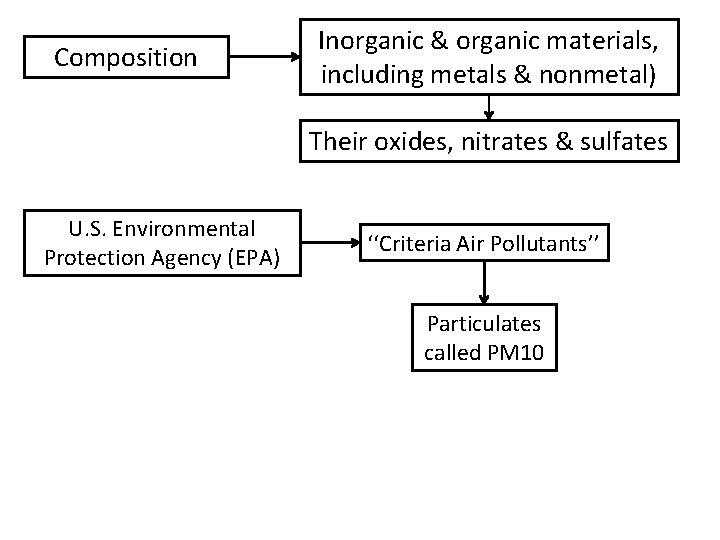
Composition Inorganic & organic materials, including metals & nonmetal) Their oxides, nitrates & sulfates U. S. Environmental Protection Agency (EPA) ‘‘Criteria Air Pollutants’’ Particulates called PM 10

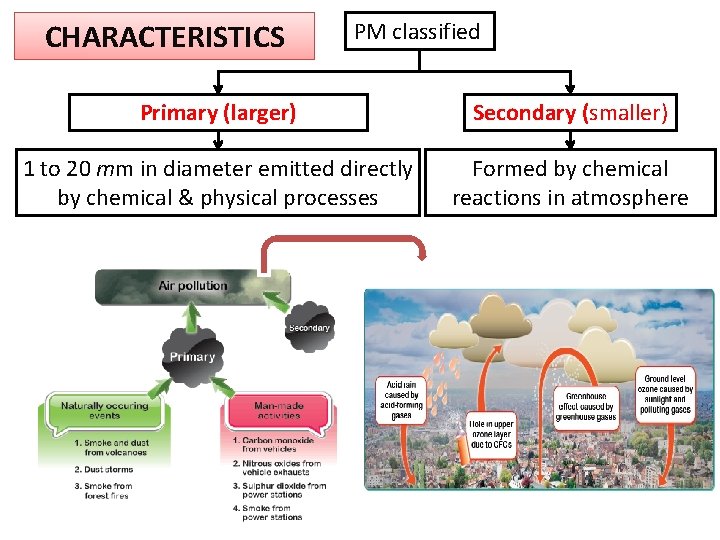
CHARACTERISTICS PM classified Primary (larger) Secondary (smaller) 1 to 20 mm in diameter emitted directly by chemical & physical processes Formed by chemical reactions in atmosphere

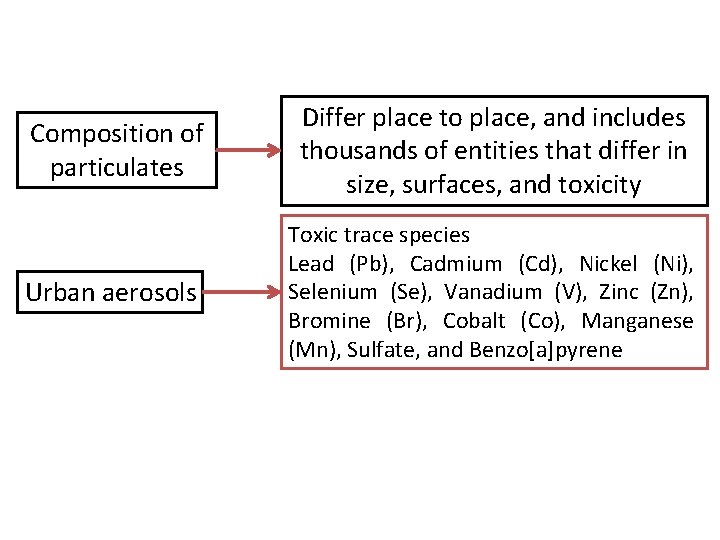
Composition of particulates Urban aerosols Differ place to place, and includes thousands of entities that differ in size, surfaces, and toxicity Toxic trace species Lead (Pb), Cadmium (Cd), Nickel (Ni), Selenium (Se), Vanadium (V), Zinc (Zn), Bromine (Br), Cobalt (Co), Manganese (Mn), Sulfate, and Benzo[a]pyrene

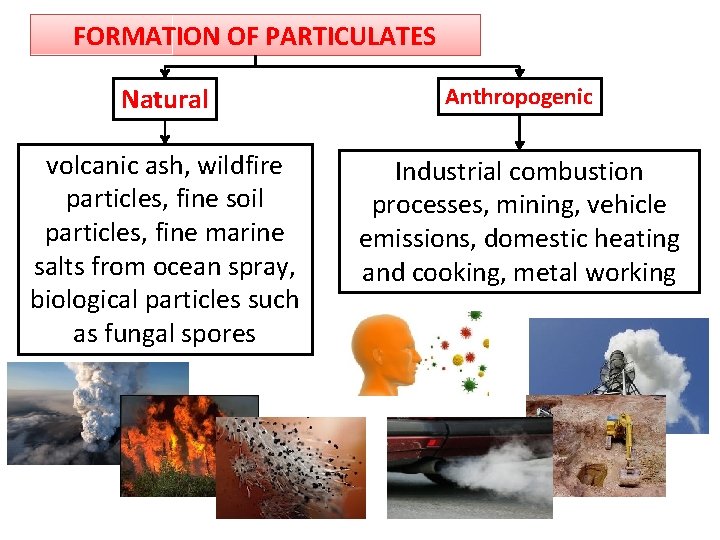
FORMATION OF PARTICULATES Natural Anthropogenic volcanic ash, wildfire particles, fine soil particles, fine marine salts from ocean spray, biological particles such as fungal spores Industrial combustion processes, mining, vehicle emissions, domestic heating and cooking, metal working

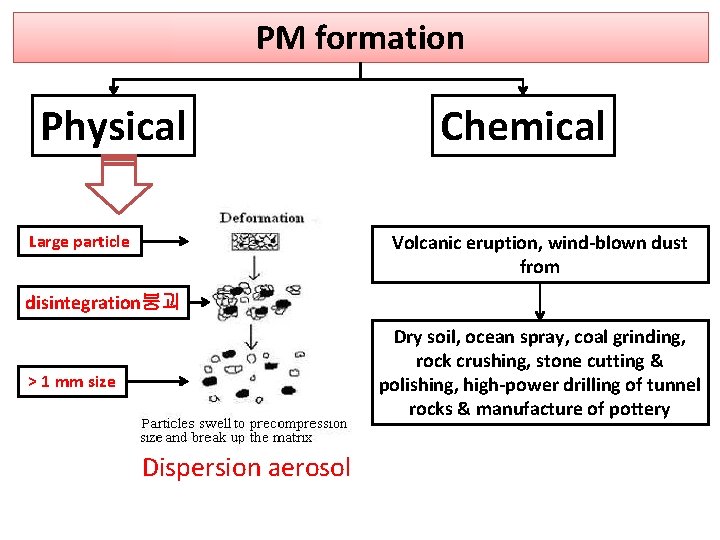
PM formation Physical Chemical Volcanic eruption, wind-blown dust from Large particle disintegration붕괴 Dry soil, ocean spray, coal grinding, rock crushing, stone cutting & polishing, high-power drilling of tunnel rocks & manufacture of pottery > 1 mm size Dispersion aerosol

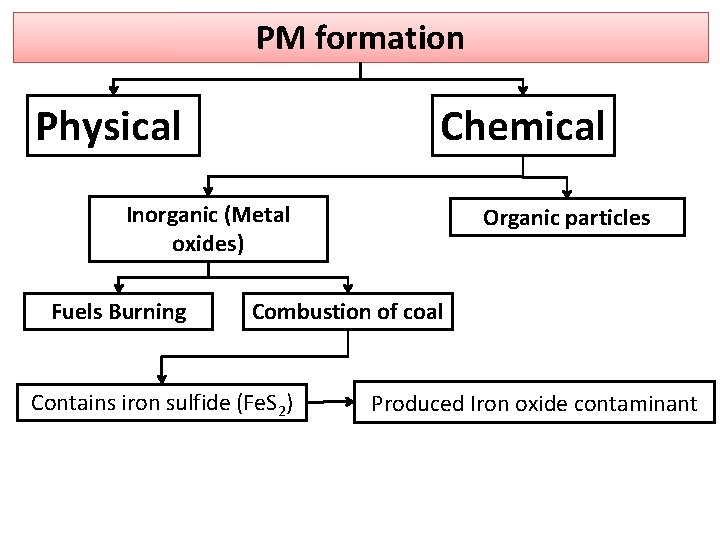
PM formation Chemical Physical Inorganic (Metal oxides) Fuels Burning Organic particles Combustion of coal Contains iron sulfide (Fe. S 2) Produced Iron oxide contaminant

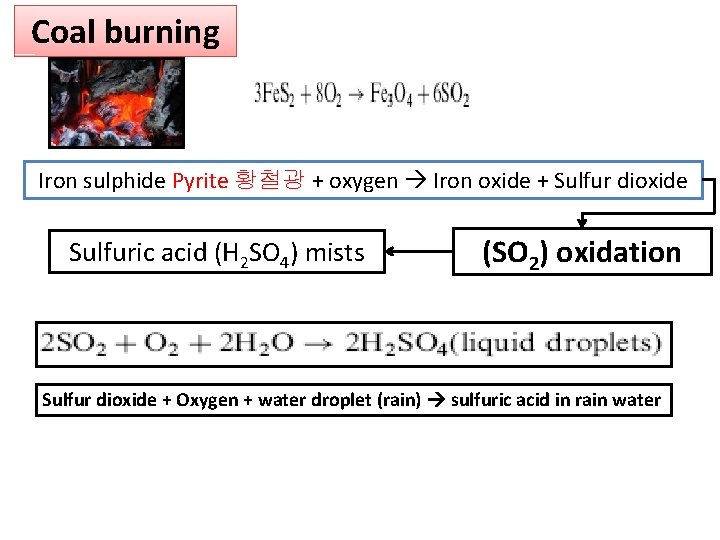
Coal burning Iron sulphide Pyrite 황철광 + oxygen Iron oxide + Sulfur dioxide Sulfuric acid (H 2 SO 4) mists (SO 2) oxidation Sulfur dioxide + Oxygen + water droplet (rain) sulfuric acid in rain water

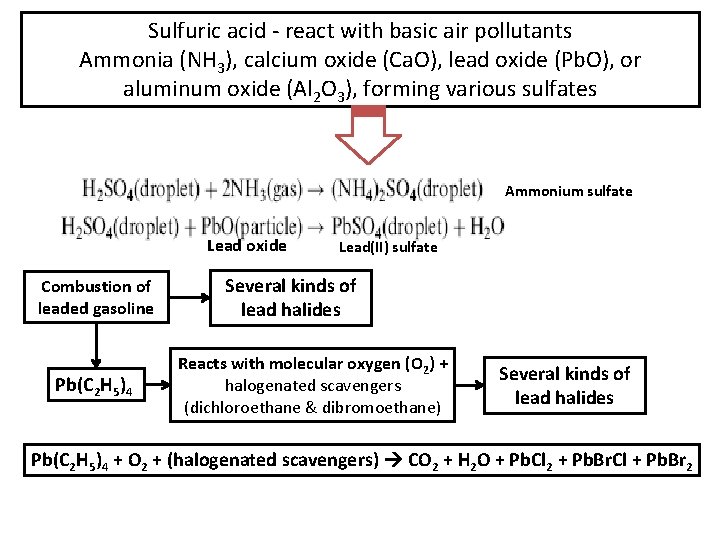
Sulfuric acid - react with basic air pollutants Ammonia (NH 3), calcium oxide (Ca. O), lead oxide (Pb. O), or aluminum oxide (Al 2 O 3), forming various sulfates Ammonium sulfate Lead oxide Combustion of leaded gasoline Pb(C 2 H 5)4 Lead(II) sulfate Several kinds of lead halides Reacts with molecular oxygen (O 2) + halogenated scavengers (dichloroethane & dibromoethane) Several kinds of lead halides Pb(C 2 H 5)4 + O 2 + (halogenated scavengers) CO 2 + H 2 O + Pb. Cl 2 + Pb. Br. Cl + Pb. Br 2

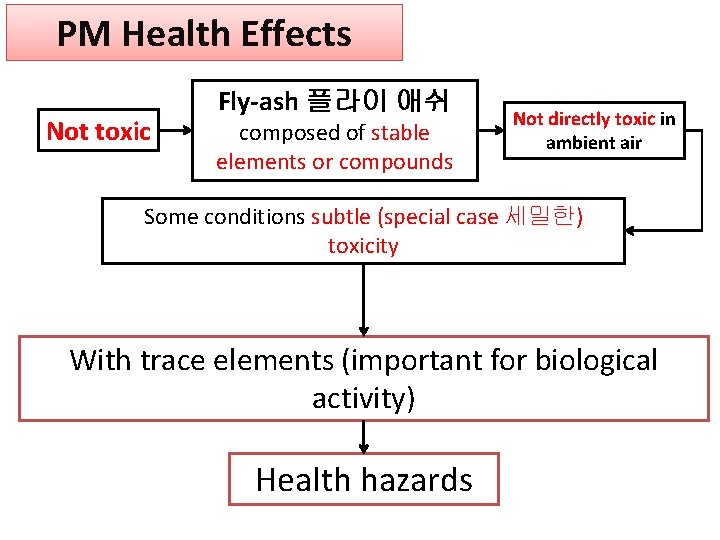
PM Health Effects Not toxic Fly-ash 플라이 애쉬 composed of stable elements or compounds Not directly toxic in ambient air Some conditions subtle (special case 세밀한) toxicity With trace elements (important for biological activity) Health hazards

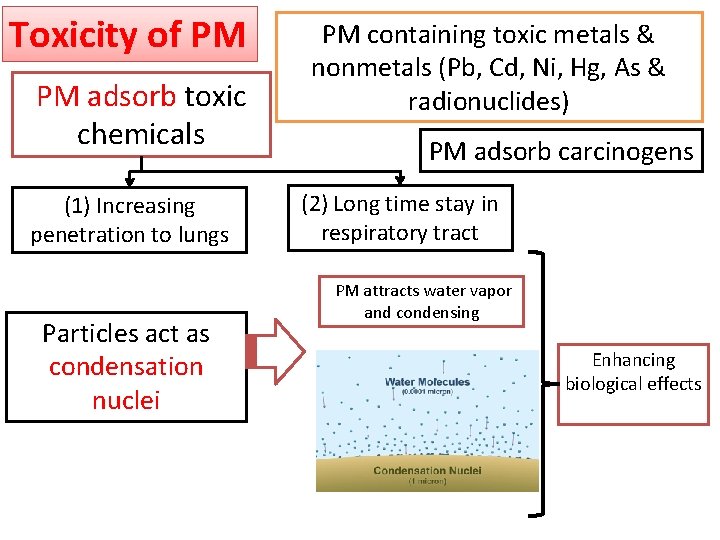
Toxicity of PM PM adsorb toxic chemicals (1) Increasing penetration to lungs Particles act as condensation nuclei PM containing toxic metals & nonmetals (Pb, Cd, Ni, Hg, As & radionuclides) PM adsorb carcinogens (2) Long time stay in respiratory tract PM attracts water vapor and condensing Enhancing biological effects

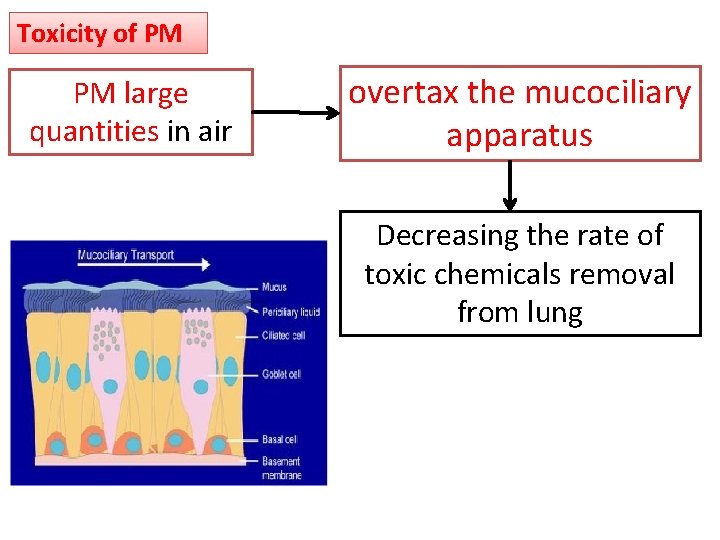
Toxicity of PM PM large quantities in air overtax the mucociliary apparatus Decreasing the rate of toxic chemicals removal from lung

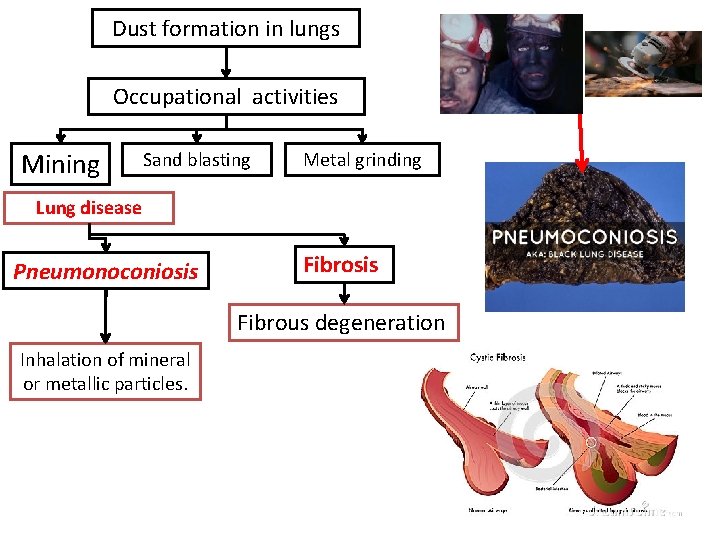
Dust formation in lungs Occupational activities Mining Sand blasting Metal grinding Lung disease Pneumonoconiosis Fibrous degeneration Inhalation of mineral or metallic particles.

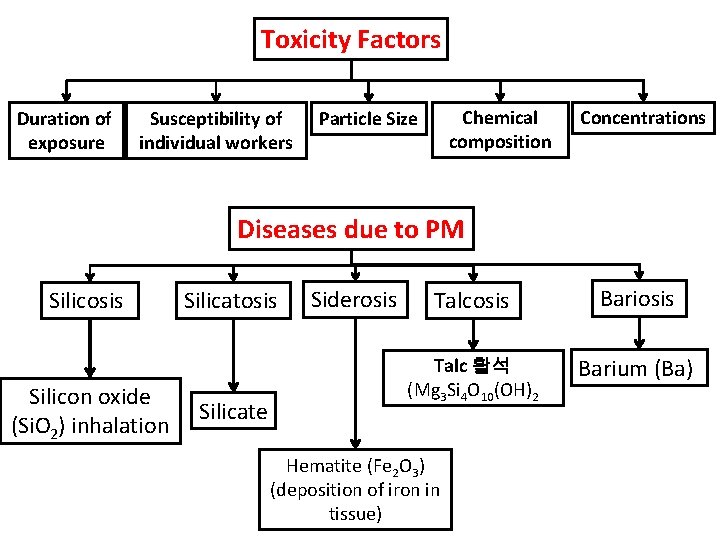
Toxicity Factors Duration of exposure Susceptibility of individual workers Chemical composition Particle Size Concentrations Diseases due to PM Silicosis Silicon oxide (Si. O 2) inhalation Silicatosis Silicate Siderosis Talcosis Bariosis Talc 활석 (Mg 3 Si 4 O 10(OH)2 Barium (Ba) Hematite (Fe 2 O 3) (deposition of iron in tissue)

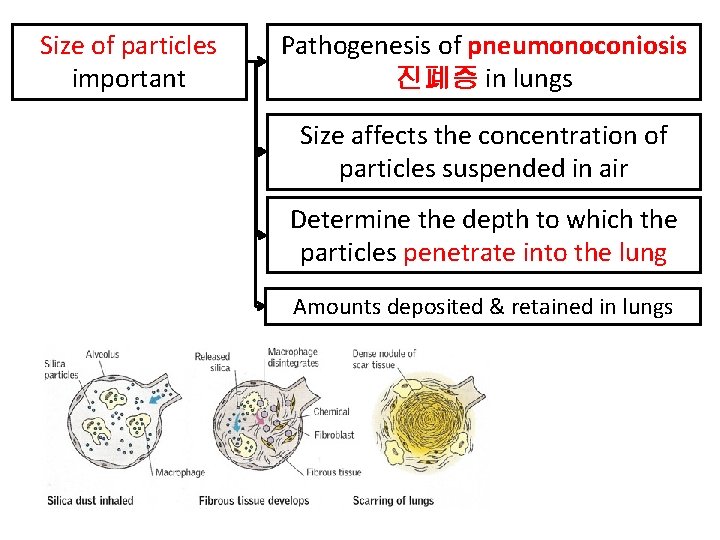
Size of particles important Pathogenesis of pneumonoconiosis 진폐증 in lungs Size affects the concentration of particles suspended in air Determine the depth to which the particles penetrate into the lung Amounts deposited & retained in lungs

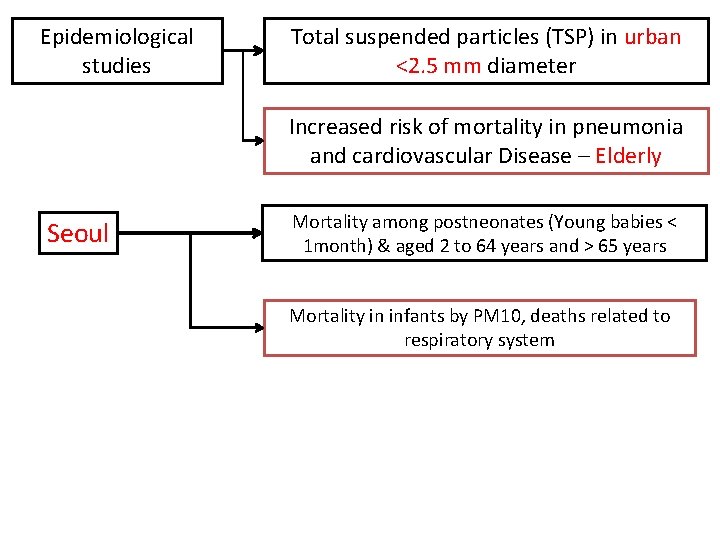
Epidemiological studies Total suspended particles (TSP) in urban <2. 5 mm diameter Increased risk of mortality in pneumonia and cardiovascular Disease – Elderly Seoul Mortality among postneonates (Young babies < 1 month) & aged 2 to 64 years and > 65 years Mortality in infants by PM 10, deaths related to respiratory system

Silica Silicon dioxide, Si. O 2 and silicates – rocks (soils, sands & clays) Second most abundant element (after oxygen) in the earth. Si. O 2 occurs free form or combined state (Silicate) Free silica in crystalline form (quartz 석영, granite 화강암, flint, and diatomite 규조토 or noncrystalline form)

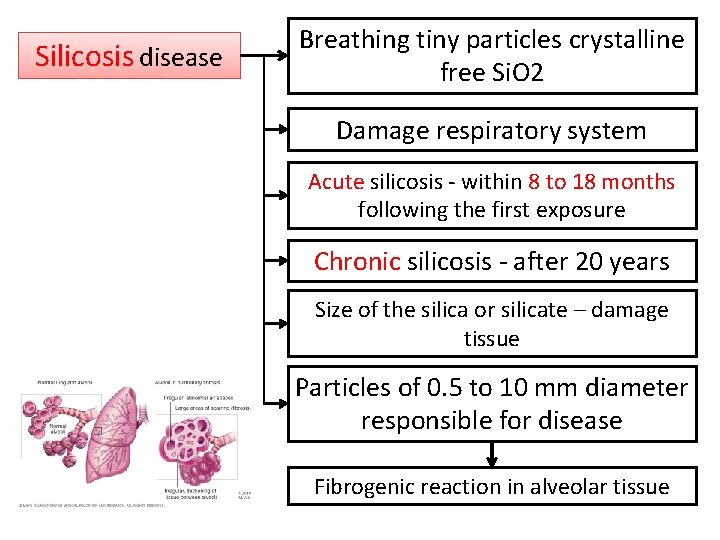
Silicosis disease Breathing tiny particles crystalline free Si. O 2 Damage respiratory system Acute silicosis - within 8 to 18 months following the first exposure Chronic silicosis - after 20 years Size of the silica or silicate – damage tissue Particles of 0. 5 to 10 mm diameter responsible for disease Fibrogenic reaction in alveolar tissue

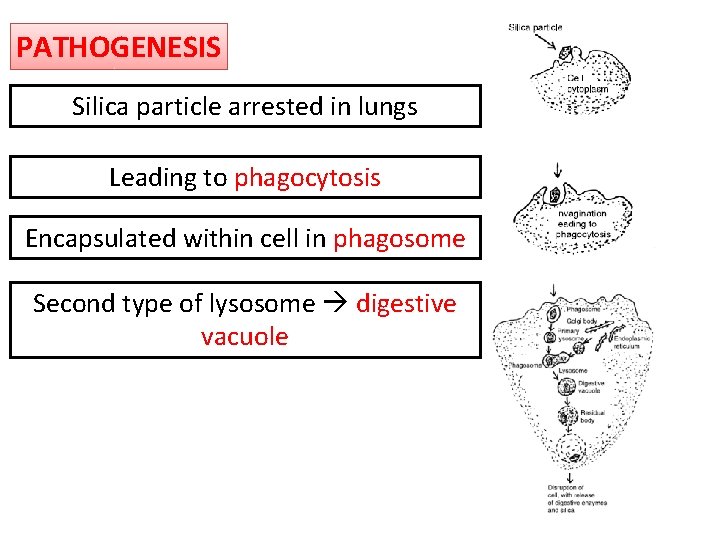
PATHOGENESIS Silica particle arrested in lungs Leading to phagocytosis Encapsulated within cell in phagosome Second type of lysosome digestive vacuole

Beryllium (Be) & Asbestos 석면 toxic elements