1 All matter is made up of 2



























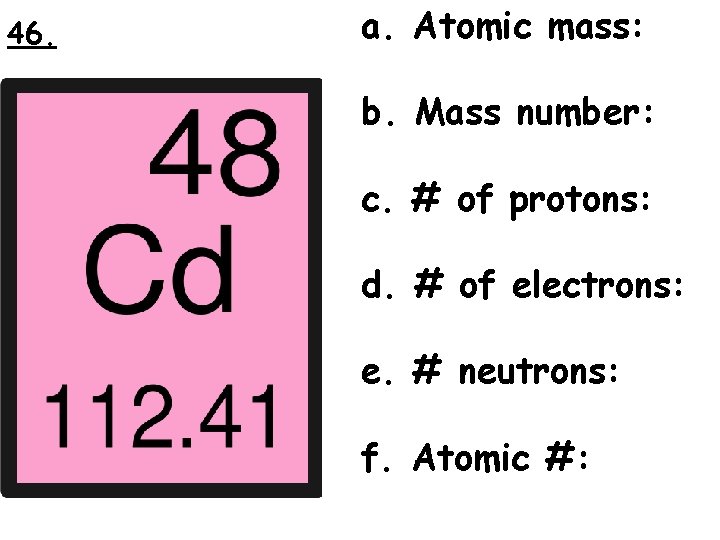





- Slides: 59

1. All matter is made up of ______

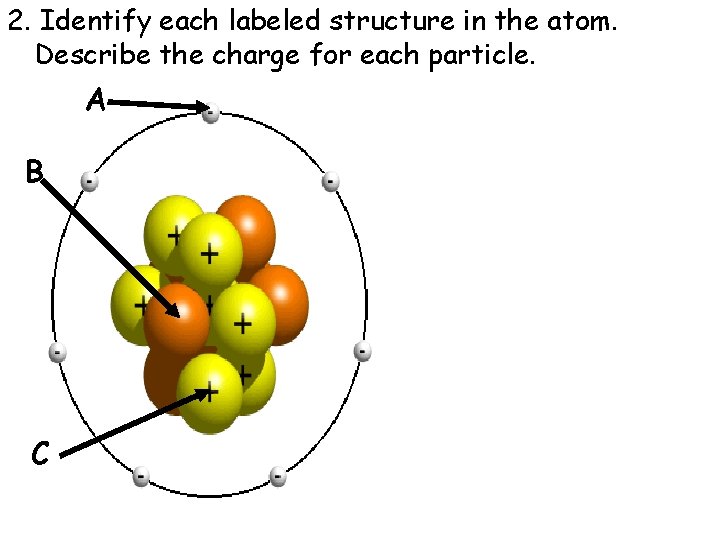
2. Identify each labeled structure in the atom. Describe the charge for each particle. A B C

3. Where are protons and neutrons found in an atom? __________ 4. Where are electrons found in an atom? ____________

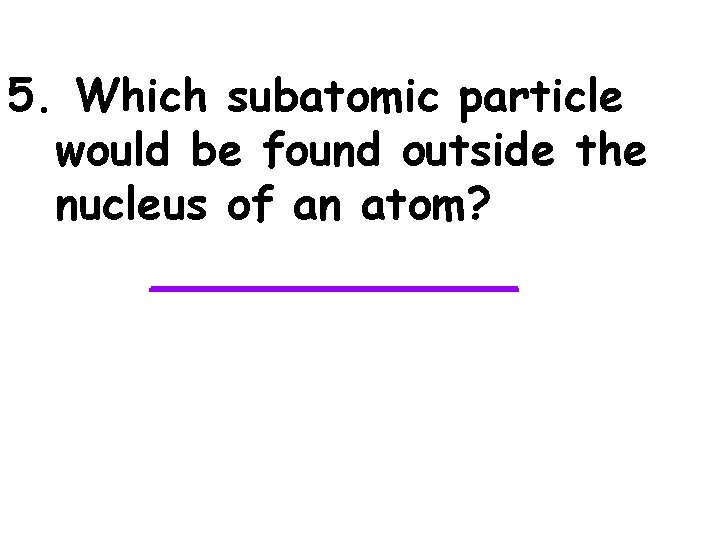
5. Which subatomic particle would be found outside the nucleus of an atom? _______

6. A positively charged particle is a _______

7. Which of the following is not an element? 1. oxygen 3. sodium chloride 2. hydrogen 4. nitrogen

8. A chemical formula like CO 2 represents 1. an element 3. an atom 2. an electron 4. a compound

9. A substance made up of two or more elements that have been chemically combined is a ________

10. A substance made up of two or more elements that have been physcially combined is a ______

11. A substance that cannot be changed into simpler substances by a chemical change is called a (an) 1. element. 3. liquid. 2. solid. 4. mixture.

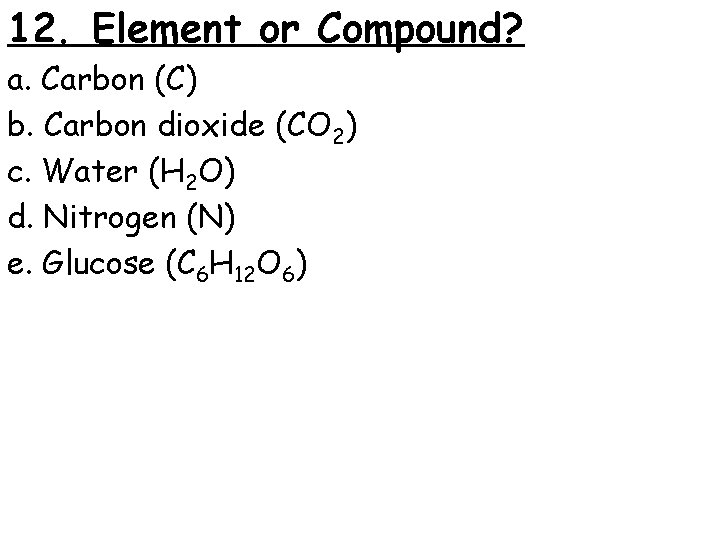
12. Element or Compound? a. Carbon (C) b. Carbon dioxide (CO 2) c. Water (H 2 O) d. Nitrogen (N) e. Glucose (C 6 H 12 O 6)

13.


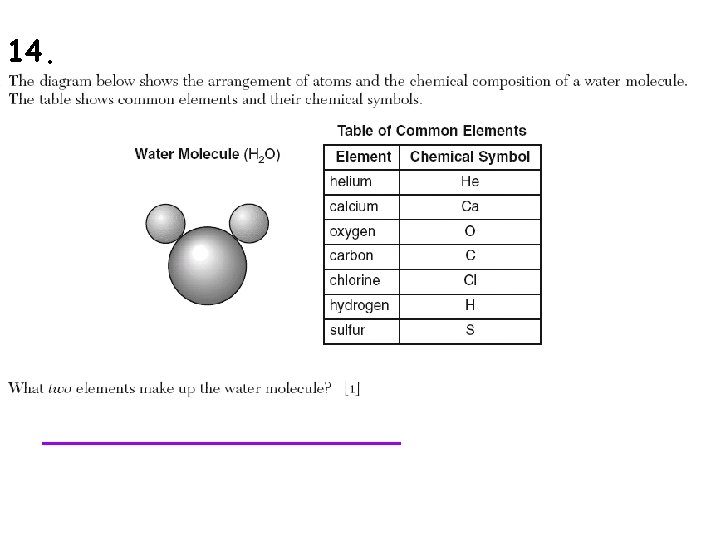
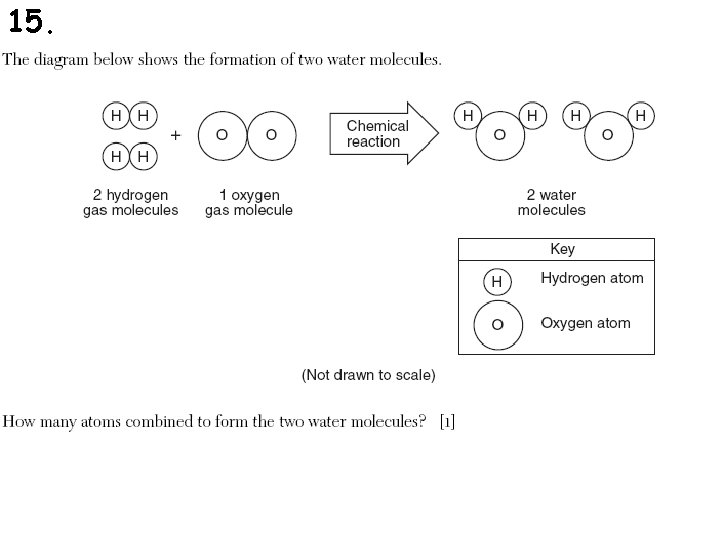
15.

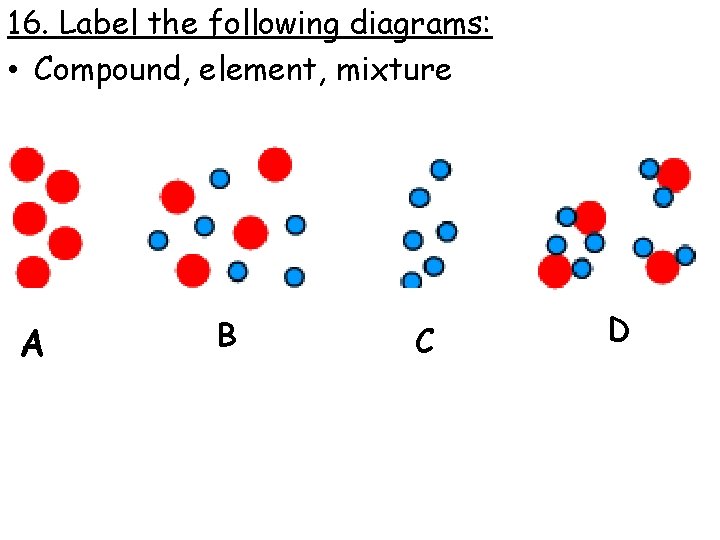
16. Label the following diagrams: • Compound, element, mixture A B C D

17. Sand iron particles that are similar in size and color are mixed together in a beaker. What would be the best method of separating the particles? 1. Use tweezers to separate them. 2. Add water to the mixture. 3. Use a magnet to separate them. 4. Pour the mixture into a filter.

18. The substances in a mixture can be separated by physical means because 1. no chemical change occurs when the substances are combined. 2. the physical and chemical properties of the substances change. 3. none of the properties of the substances change. 4. the chemical, but not the physical, properties of the substances change.

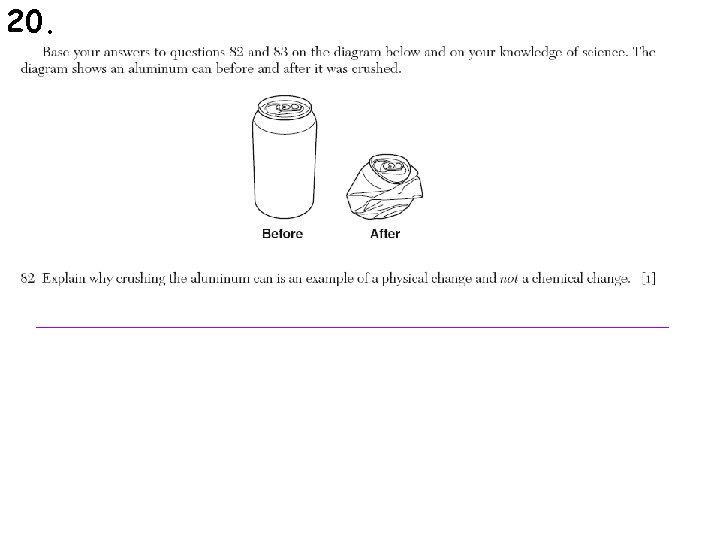
19. For the following pictures, identify whether a physical or chemical change is occurring.








21. Identify the different phases of matter. ___________ 22. The particles of a substance are closest together in a _____. 23. The particles of a substance that does not have a definite volume or shape is ____.

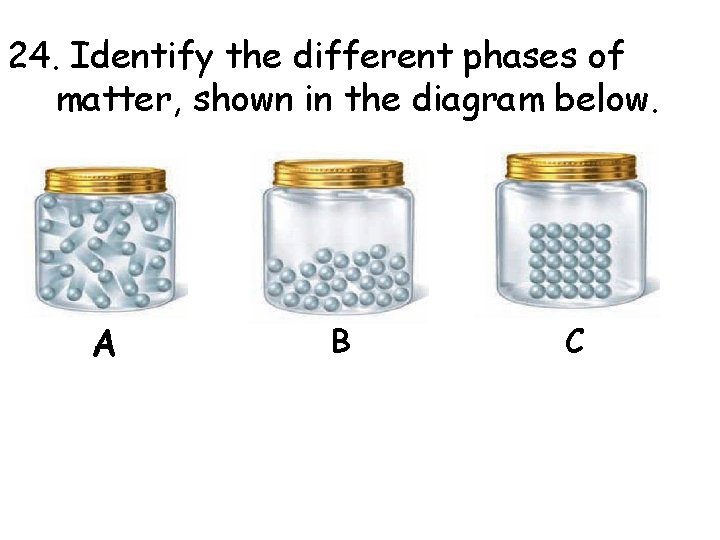
24. Identify the different phases of matter, shown in the diagram below. A B C

25. The particles of a substance are closest together in a ____. 26. The particles of a substance that does not have a definite volume or shape is ______. 27. When a liquid becomes a solid, heat is ____ and the process is called_____. 28. ______ is the process in which liquid changes to gas.

29. Matter is anything that has and _____. 30. Identify the following as a physical or chemical change: a. Bending a Paper Clip. b. Melting ice into water. c. Baking a cake batter into a cake. d. The rusting of a nail

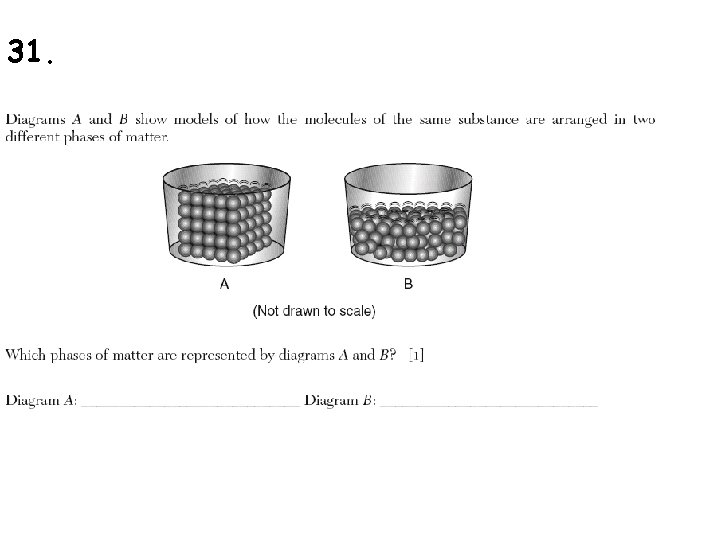
31.

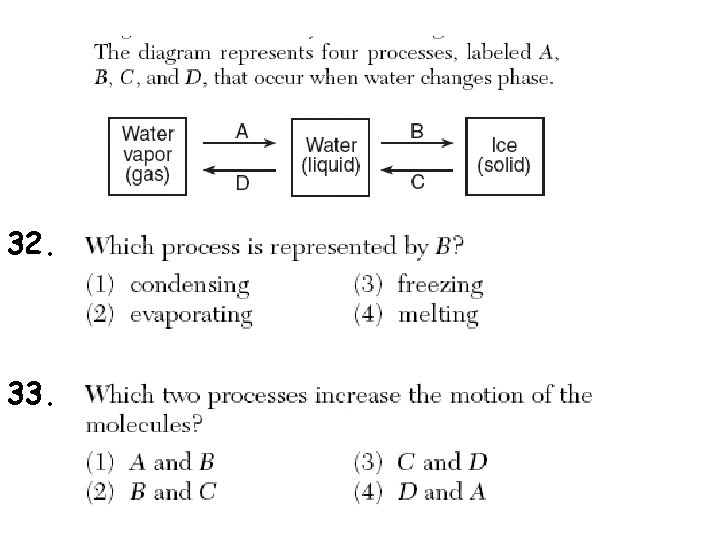
32. 33.

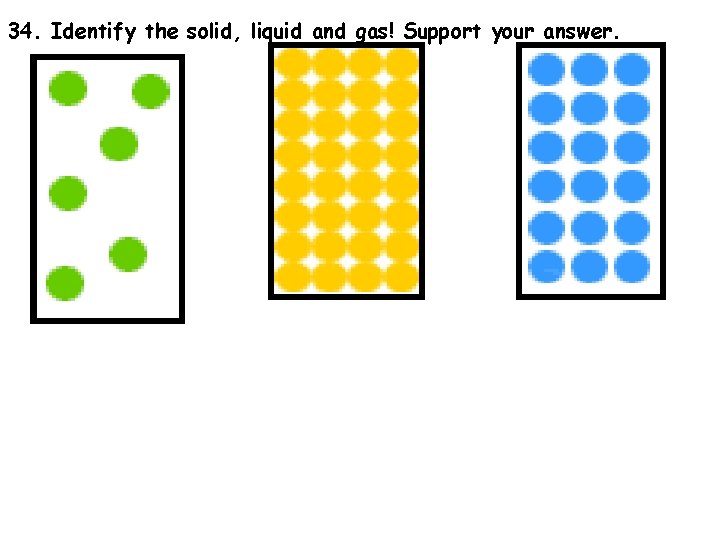
34. Identify the solid, liquid and gas! Support your answer.

35. Identify the phase change described. a. Solid to liquid: b. Gas to liquid: c. Liquid to solid: d. Solid to gas: e. Liquid to gas:

36. For each phase change describe whether energy is RELEASED or ABSORBED. a. Melting: b. Freezing: c. Vaporization: d. Sublimation: e. Condensation:

37. The particles of a substance are closest together in 1. a solid 2. a liquid 3. a gas 4. plasma
38. The particles of a substance move most readily in 1. a solid 2. a liquid 3. a gas
39. The particles of a substance that does not have a definite volume or shape is 1. a solid 2. a liquid 3. a gas

40. When a liquid becomes a solid, energy 1. does not change 2. is released 3. is absorbed 4. is first absorbed, then released

41. _______ is the process in which liquid changes to gas. 1. Sublimation 3. Condensation 2. Evaporation 4. Combustion

42. The change of a liquid to a solid is called 1. freezing 3. melting 2. sublimation 4. vaporization

43. When substances go directly from the solid phase to the gas phase, the phase change is called 1. sublimation 2. condensation 3. evaporation 4. vaporization

44. What happens to the position of water molecules as they lose heat energy? ________________________________

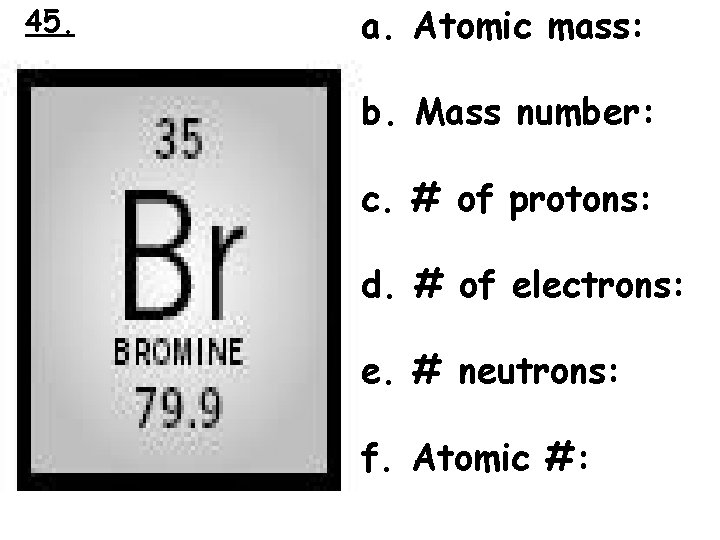
45. a. Atomic mass: b. Mass number: c. # of protons: d. # of electrons: e. # neutrons: f. Atomic #:
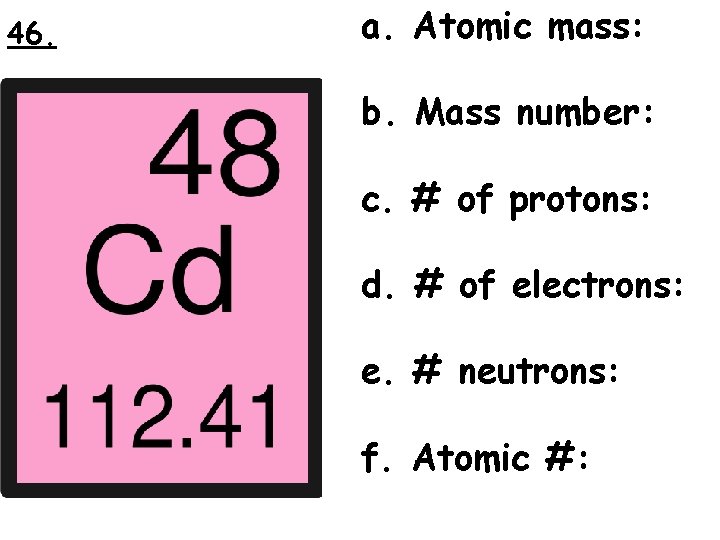
46. a. Atomic mass: b. Mass number: c. # of protons: d. # of electrons: e. # neutrons: f. Atomic #:

47. All samples of an element are composed of atoms with the same 1. atomic mass 2. atomic number 3. number of protons and neutrons 4. number of neutrons

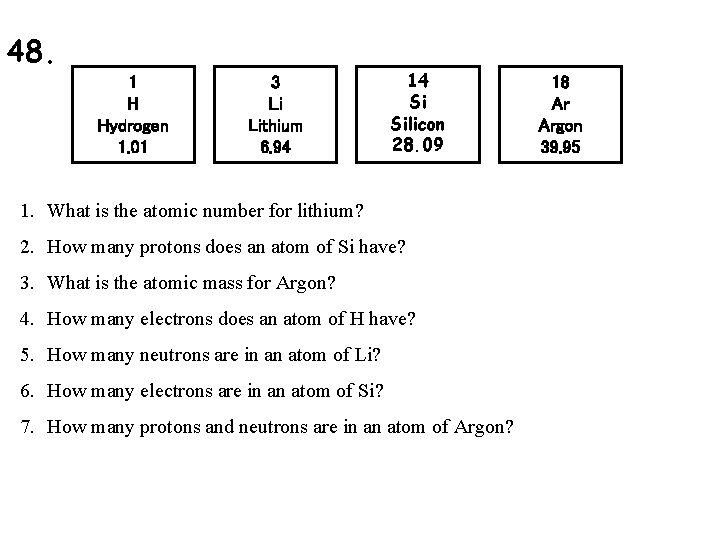
48. 1 H Hydrogen 1. 01 3 Li Lithium 6. 94 14 Si Silicon 28. 09 1. What is the atomic number for lithium? 2. How many protons does an atom of Si have? 3. What is the atomic mass for Argon? 4. How many electrons does an atom of H have? 5. How many neutrons are in an atom of Li? 6. How many electrons are in an atom of Si? 7. How many protons and neutrons are in an atom of Argon? 18 Ar Argon 39. 95

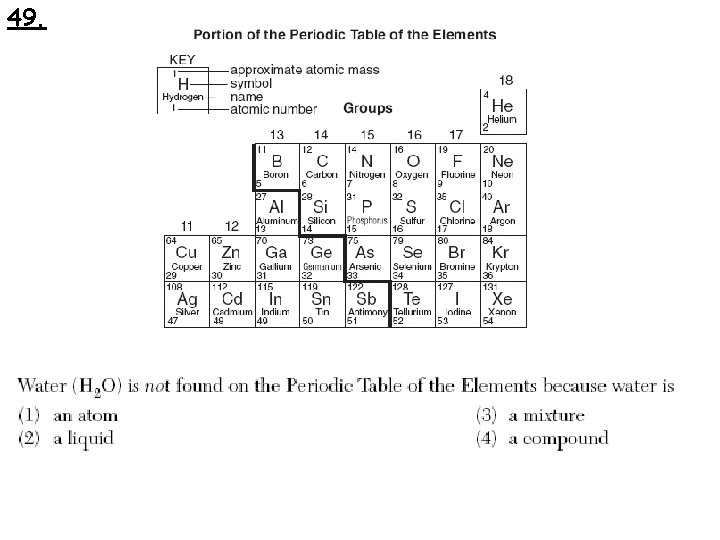
49.

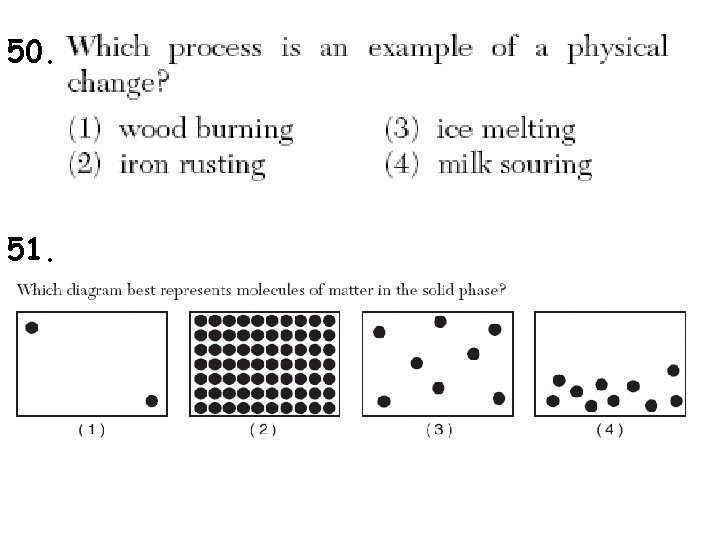
50. 51.

52. Explain how the periodic table of elements is arranged. 53. Elements at the left of the periodic table are known as ______. 54. Elements at the right of the periodic table are known as _______.

55. Explain some of the properties of metals. • They are malleable, ductile, have luster, and are good conductors of heat and electricity 56. Explain some of the properties of nonmetals. • They are brittle, have no luster and they are not good conductors of heat and electricity.

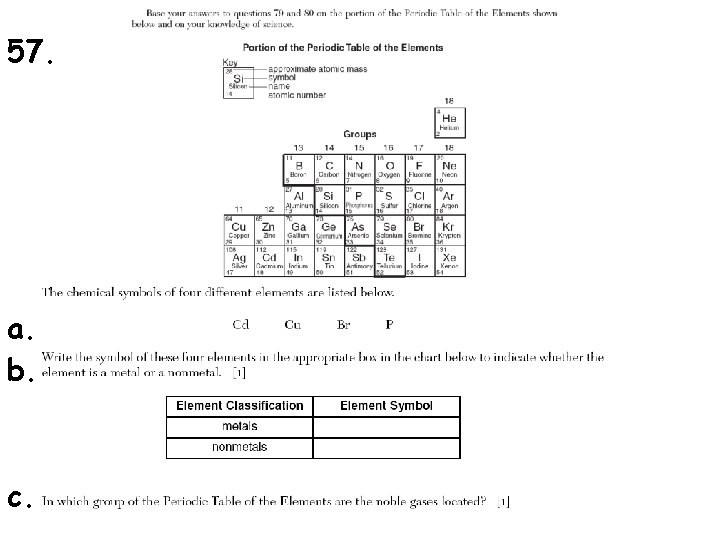
57. a. b. c.

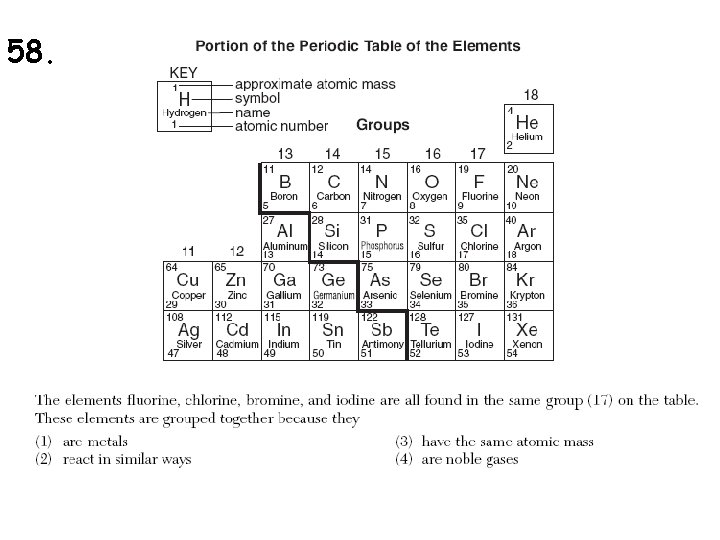
58.

59. Use a periodic table to help you identify each element. Give its symbol for the answer. a. I am a member of the Boron family with 49 protons. b. I have a total of 74 electrons in an atom. c. I have an atomic mass of 55. 847. d. I have 8 neutrons in an atom.

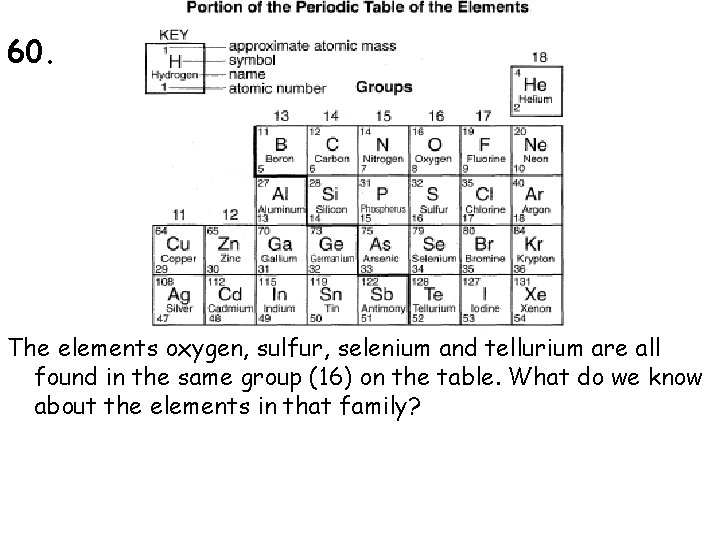
60. The elements oxygen, sulfur, selenium and tellurium are all found in the same group (16) on the table. What do we know about the elements in that family?

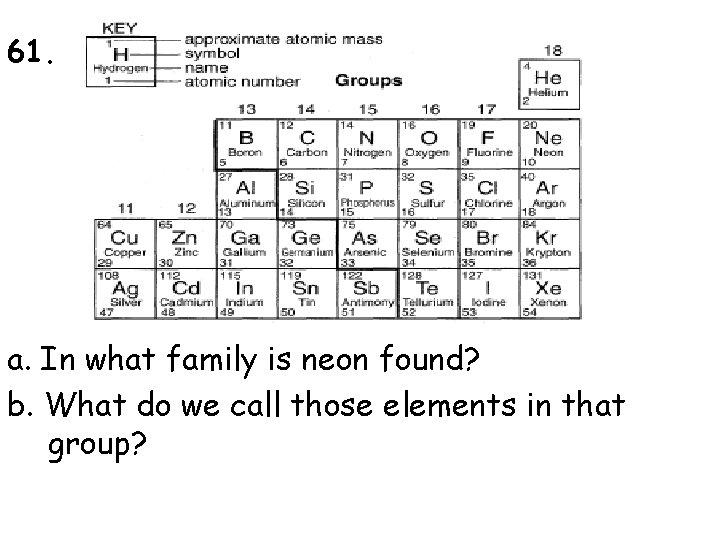
61. a. In what family is neon found? b. What do we call those elements in that group?

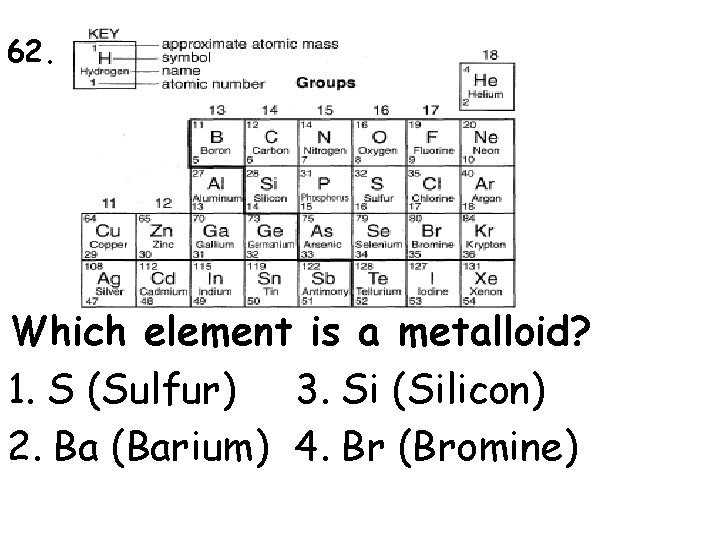
62. Which element is a metalloid? 1. S (Sulfur) 3. Si (Silicon) 2. Ba (Barium) 4. Br (Bromine)

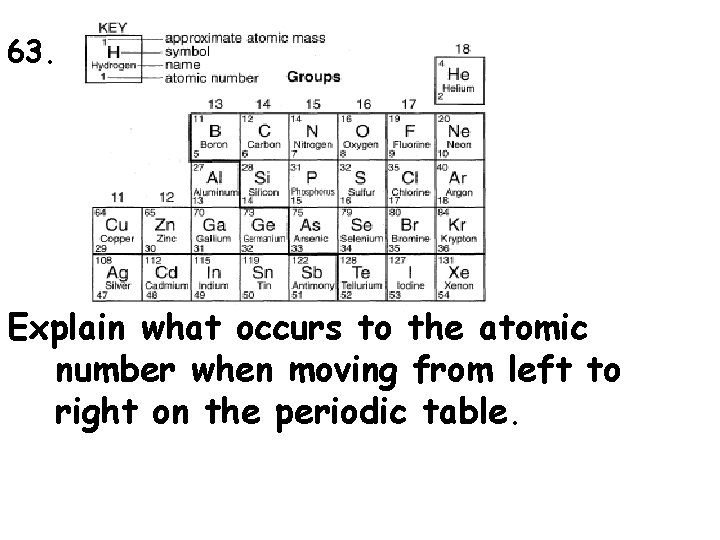
63. Explain what occurs to the atomic number when moving from left to right on the periodic table.

64. Which three elements have the most similar chemical properties? 1. Ar, Kr, Br 2. K, Rb, Cs 3. B, C, N 4. O, N, Si

65. Which element is a noble gas? 1. hydrogen 2. oxygen 3. neon 4. nitrogen