Particulate View of Matter MATTER All matter is










- Slides: 27

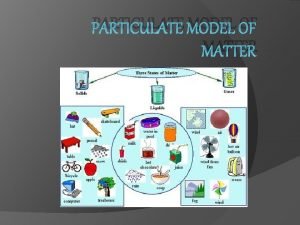
Particulate View of Matter

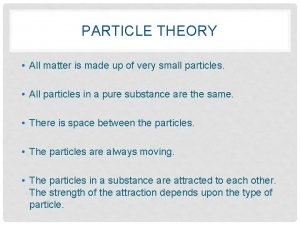
MATTER �All matter is composed of atoms! �Atoms are the building blocks of matter.

Particulate View of Mattter �A Boy and His Atom – IBM �Bill Nye - Atoms

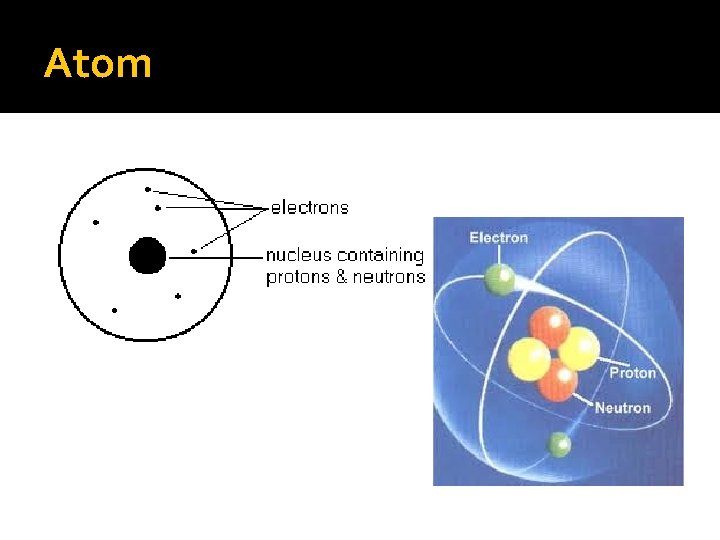
Atom

Parts of Atom �Nucleus – the dense center of the atom that contains the protons and neutrons �Electron cloud – area around the nucleus where the electrons exist. �An atom is mostly empty space.

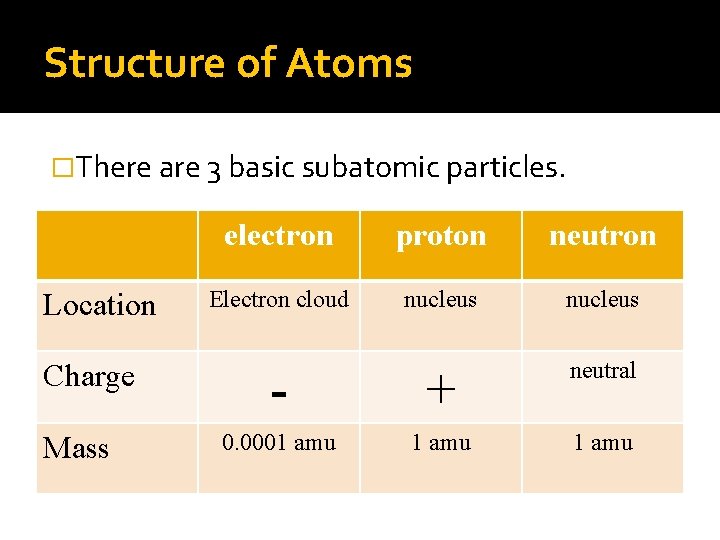
Structure of Atoms �There are 3 basic subatomic particles. Location Charge Mass electron proton neutron Electron cloud nucleus - + neutral 0. 0001 amu

�Matter that is made up of only one kind of atom is known as an element

Elements �Elements: Pure substance – cannot be broken down into other substances Composed of only one kind of atom. ▪ Oxygen is only composed of oxygen atoms. 92 different elements occur naturally Elements of the Periodic Table

Intro to Periodic Table �Mendeleev – discoverer of the periodic law and thus the periodic table. �He arranged the periodic table looking for trends or patterns. �Has been changed over time.

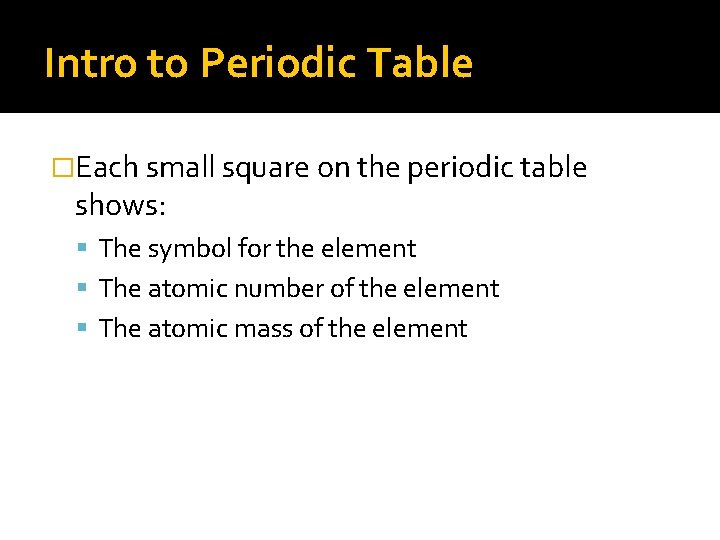
Intro to Periodic Table �Each small square on the periodic table shows: The symbol for the element The atomic number of the element The atomic mass of the element

Intro to Periodic Table �Chemical Symbols Letters that represent elements Each element is assigned a chemical symbol ▪ 1 st letter is capitalized ▪ 2 nd letter is lower case (if there is one) �H = hydrogen �O = oxygen �Ca = calcuim �Pb = lead

Mixture �Two or more physically separable components in ratios varying from sample to sample.

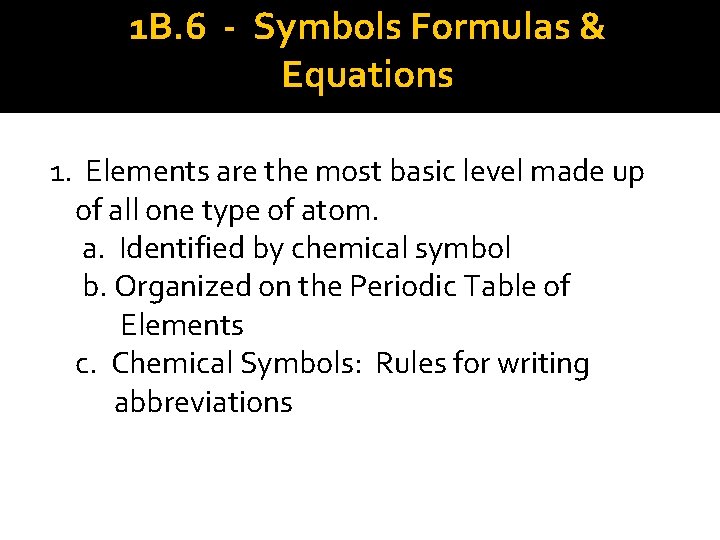
1 B. 6 - Symbols Formulas & Equations 1. Elements are the most basic level made up of all one type of atom. a. Identified by chemical symbol b. Organized on the Periodic Table of Elements c. Chemical Symbols: Rules for writing abbreviations


Rules for writing abbreviations i. iii. First letter always capital Second letter (if present) always lower case Some symbols derived from Latin names: a. b. c. Sodium - Natrium Iron - Ferium (blacksmiths called farriers) Lead - Pb - Plumbum (derived by a plumber)

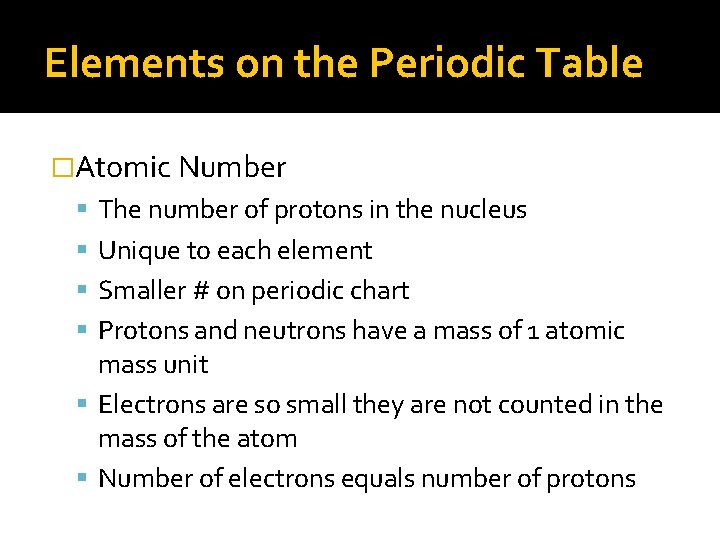
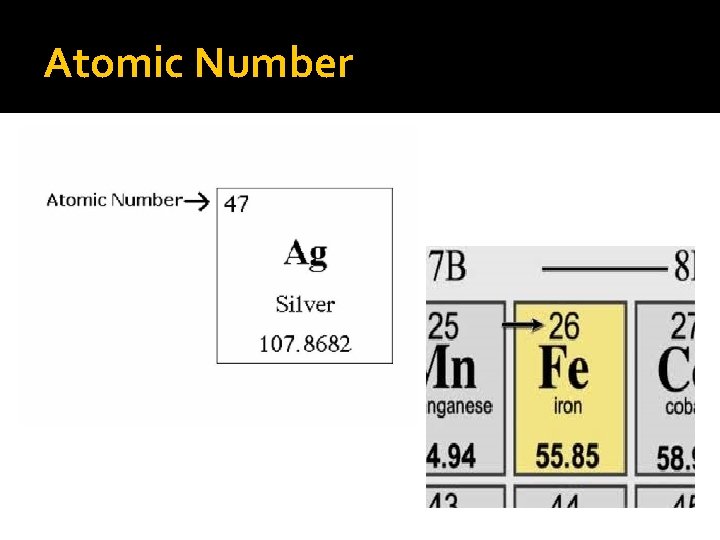
Elements on the Periodic Table �Atomic Number The number of protons in the nucleus Unique to each element Smaller # on periodic chart Protons and neutrons have a mass of 1 atomic mass unit Electrons are so small they are not counted in the mass of the atom Number of electrons equals number of protons

Atomic Number

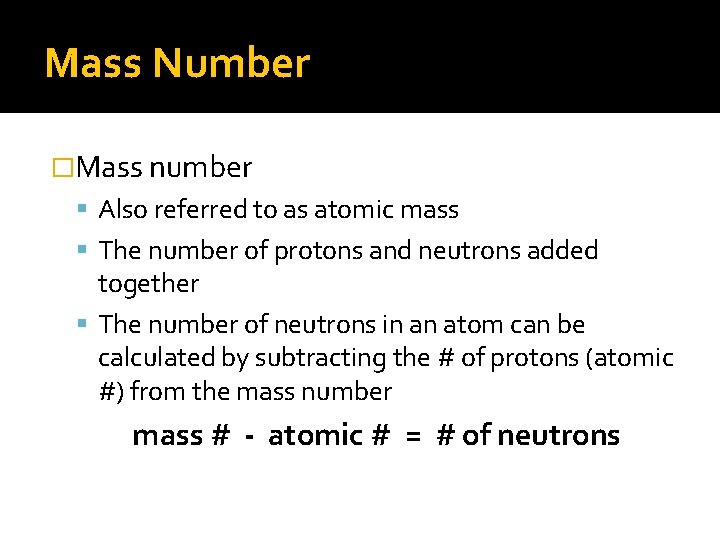
Mass Number �Mass number Also referred to as atomic mass The number of protons and neutrons added together The number of neutrons in an atom can be calculated by subtracting the # of protons (atomic #) from the mass number mass # - atomic # = # of neutrons

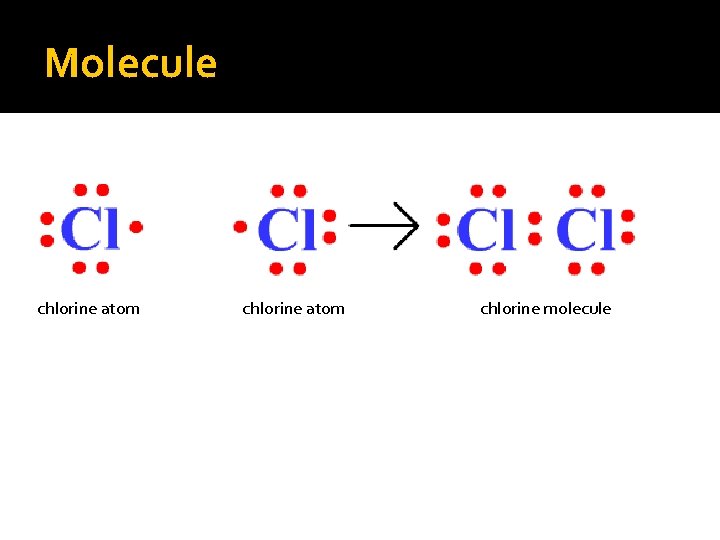
Molecules �Molecules Two or more atoms chemically bonded together by sharing electrons Diatomic molecules are all the same atom ▪ GEN – u – INE Diatomics ▪ Hydrogen, Oxygen, Nitrogen, Fluorine, Chlorine, Bromine and Iodine

Molecule chlorine atom chlorine molecule

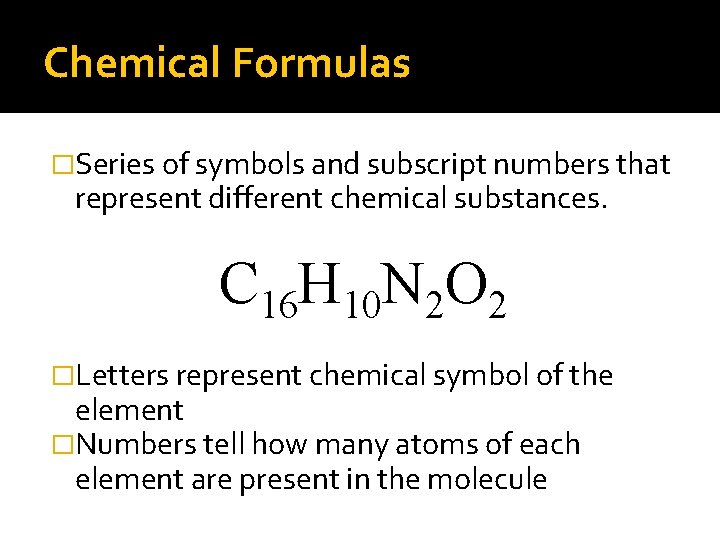
Chemical Formulas �Series of symbols and subscript numbers that represent different chemical substances. C 16 H 10 N 2 O 2 �Letters represent chemical symbol of the element �Numbers tell how many atoms of each element are present in the molecule

Subscripts �Subscripts tell you the ratio of different atoms in a compound H 2 O • tells us there are 2 hydrogen atoms • If there is no subscript, the number is one. (just like coefficients in algebra) ▪ There is just one oxygen in water

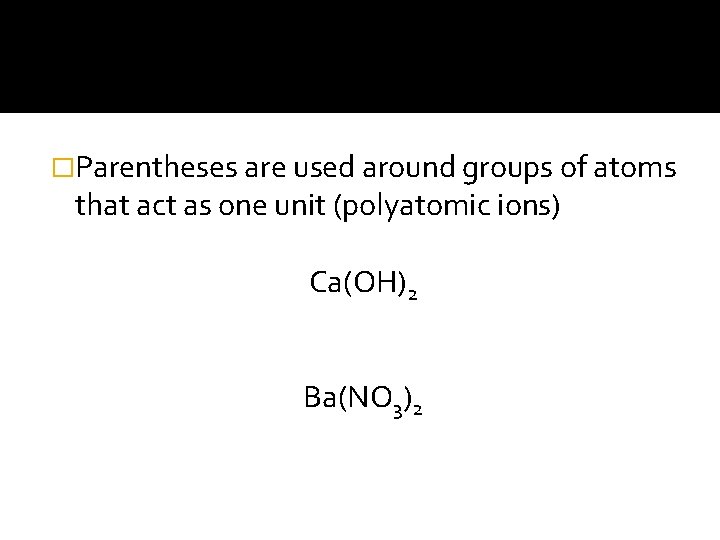
�Parentheses are used around groups of atoms that act as one unit (polyatomic ions) Ca(OH)2 Ba(NO 3)2

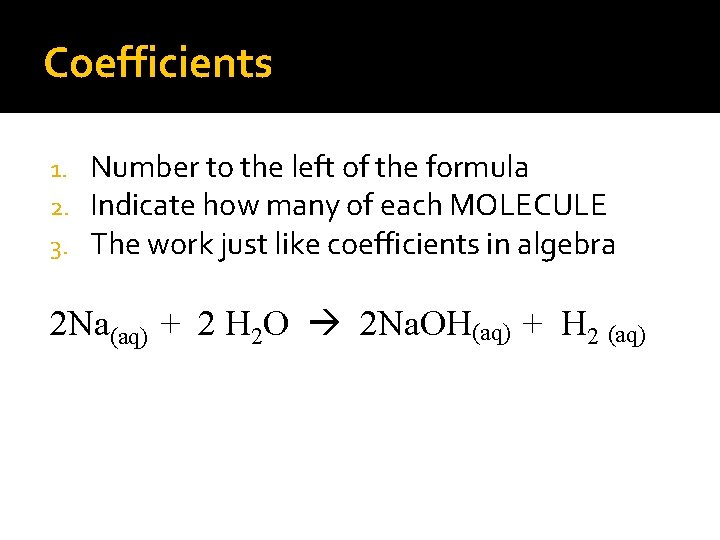
Coefficients 1. 2. 3. Number to the left of the formula Indicate how many of each MOLECULE The work just like coefficients in algebra 2 Na(aq) + 2 H 2 O 2 Na. OH(aq) + H 2 (aq)

Coefficients cont. . �Coefficients are used to balance an equation. �An equation is balanced when the number of each type of atom is the same on both sides of the equation. (Law of Conservation of Mass)

Chemical Reaction �The process of forming new substances from reactants that involves the breaking and forming of new bonds. �Displayed through chemical equations which are “chemical sentences that tell what happens”

Chemical Equations Reactants Products (what you start with) (what you end with)
 The particulate model of matter
The particulate model of matter Wesam al madhoun
Wesam al madhoun Confined space dredging
Confined space dredging Particle theory of matter
Particle theory of matter Particulate processing adalah
Particulate processing adalah Particulate contamination in infusions
Particulate contamination in infusions Particulate forming ceramics
Particulate forming ceramics Particulate radiation
Particulate radiation Is milk macroscopic microscopic or particulate
Is milk macroscopic microscopic or particulate Who was mendal
Who was mendal Name all the lines
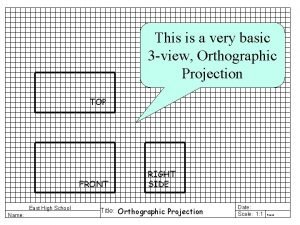
Name all the lines Top view is directly above the front view
Top view is directly above the front view Full sectional view
Full sectional view Sectional view drawing
Sectional view drawing How to draw half sectional view
How to draw half sectional view Birds eye view worms eye view
Birds eye view worms eye view Orthographic elevations
Orthographic elevations Engineering isometric drawing
Engineering isometric drawing For the view create view instructor_info as
For the view create view instructor_info as Simple view and complex view
Simple view and complex view Simple view and complex view
Simple view and complex view Simple view and complex view
Simple view and complex view Partial view in mvc
Partial view in mvc Chest x-ray anatomy
Chest x-ray anatomy Cycle view and push pull view
Cycle view and push pull view Components of os
Components of os Separatist view of business ethics
Separatist view of business ethics Multi-view drawings
Multi-view drawings