Particles All matter is made up of very




- Slides: 7

Particles • All matter is made up of very tiny particles which are far too small to see with the naked eye. • The structure of each type of matter can be explained in terms of particles.

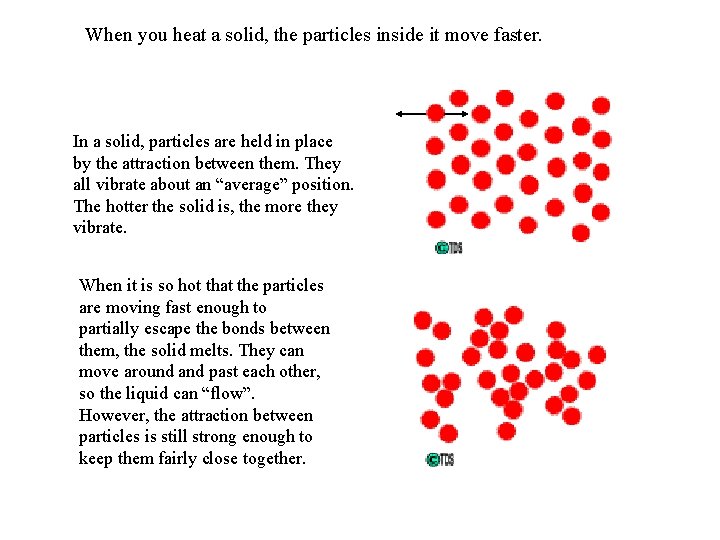
When you heat a solid, the particles inside it move faster. In a solid, particles are held in place by the attraction between them. They all vibrate about an “average” position. The hotter the solid is, the more they vibrate. When it is so hot that the particles are moving fast enough to partially escape the bonds between them, the solid melts. They can move around and past each other, so the liquid can “flow”. However, the attraction between particles is still strong enough to keep them fairly close together.

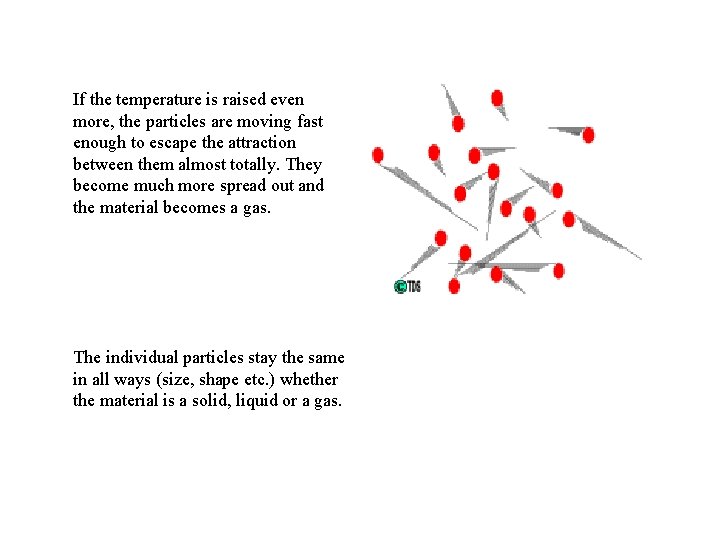
If the temperature is raised even more, the particles are moving fast enough to escape the attraction between them almost totally. They become much more spread out and the material becomes a gas. The individual particles stay the same in all ways (size, shape etc. ) whether the material is a solid, liquid or a gas.

Task Explain how the particles are arranged in a solid, a liquid and a gas. Include a diagram in your answer and use your text book to help you. For each type of matter, explain how the arrangement of the particles leads to the properties of the matter.

Instructions 1. Heat water bath to boiling pt (100 degrees) and ensure acid is all melted. Then switch off Bunsen. 2. Remove clamped acid tube from water bath and allow it to start cooling. 3. Take readings of the temperature every minute and record in a neat table in your book. 4. At the same time, record any observations about the appearance of the acid. 5. Plot a graph of temperature against time.

1. Draw a sketch of your cooling curve graph and label the state of matter during each section. 2. At 60 degrees C or so, the Acid stopped cooling altogether for some time. What is the explanation for this (in terms of particles)? 3. What temperature would the acid eventually reach? Why? 4. Why did the acid cool off at a faster rate to begin with, and then slower later on? 5. Before an injection, a nurse can wipe the relevant area with alcohol to numb it. This feels very cool, why is this? 6. Draw a sketch showing a temperature against time graph of a sample of ice being heated so it melts, and then boils. Label the relevant temperatures.

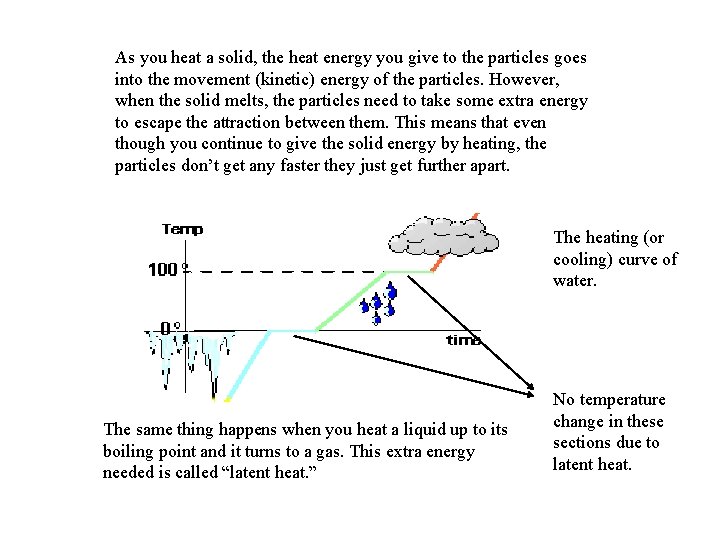
As you heat a solid, the heat energy you give to the particles goes into the movement (kinetic) energy of the particles. However, when the solid melts, the particles need to take some extra energy to escape the attraction between them. This means that even though you continue to give the solid energy by heating, the particles don’t get any faster they just get further apart. The heating (or cooling) curve of water. The same thing happens when you heat a liquid up to its boiling point and it turns to a gas. This extra energy needed is called “latent heat. ” No temperature change in these sections due to latent heat.