Subatomic Particles Subatomic Charge Mass amu Particle Location
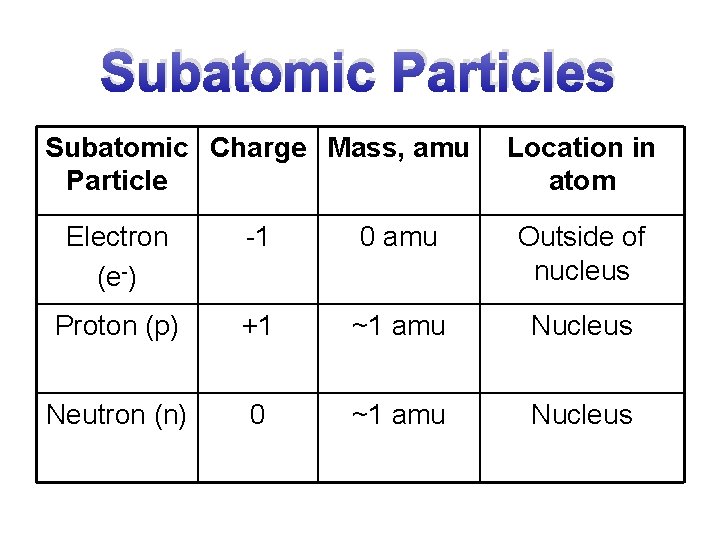
Subatomic Particles Subatomic Charge Mass, amu Particle Location in atom Electron (e-) -1 0 amu Outside of nucleus Proton (p) +1 ~1 amu Nucleus Neutron (n) 0 ~1 amu Nucleus
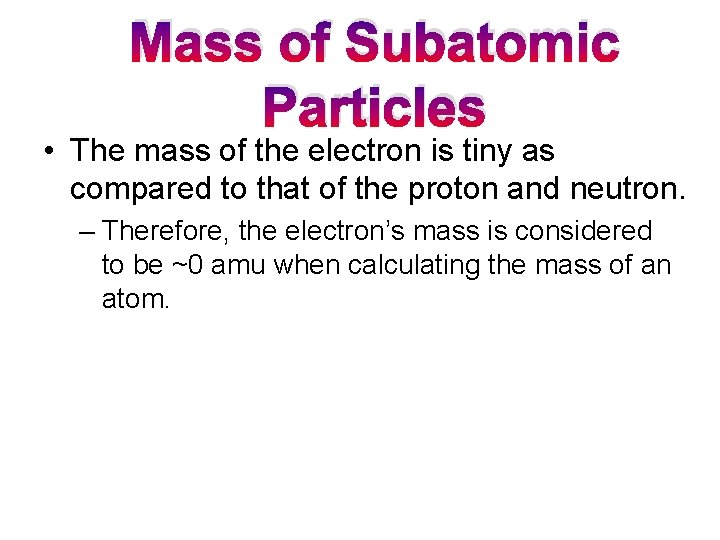
Mass of Subatomic Particles • The mass of the electron is tiny as compared to that of the proton and neutron. – Therefore, the electron’s mass is considered to be ~0 amu when calculating the mass of an atom.
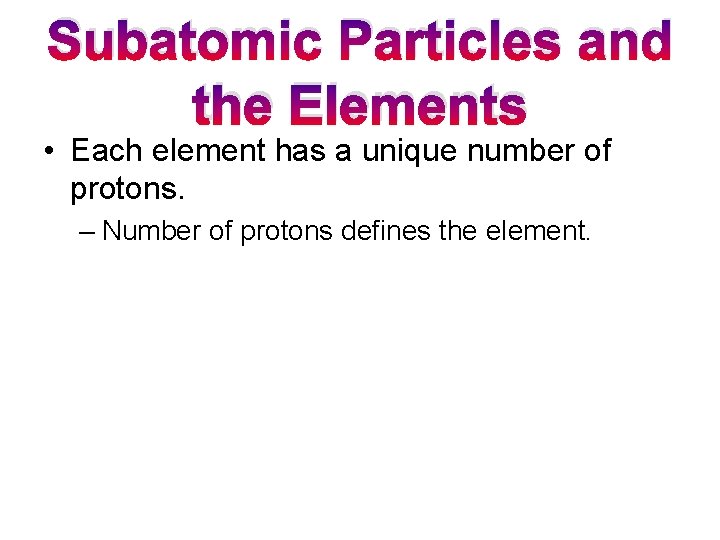
Subatomic Particles and the Elements • Each element has a unique number of protons. – Number of protons defines the element.
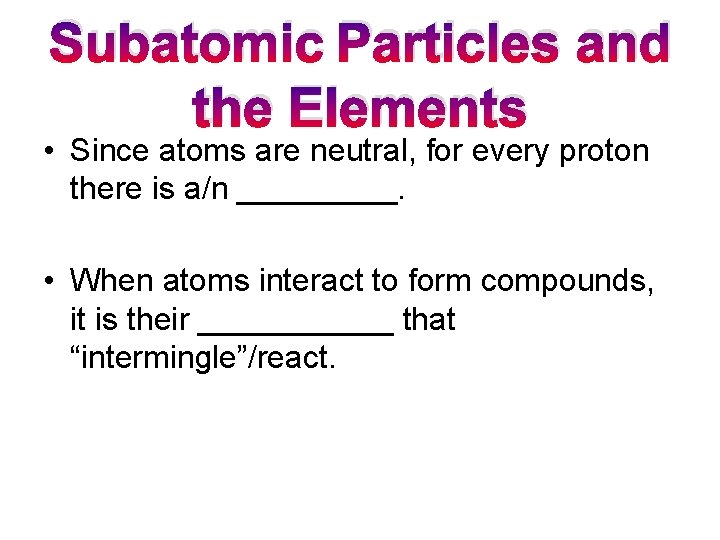
Subatomic Particles and the Elements • Since atoms are neutral, for every proton there is a/n _____. • When atoms interact to form compounds, it is their ______ that “intermingle”/react.
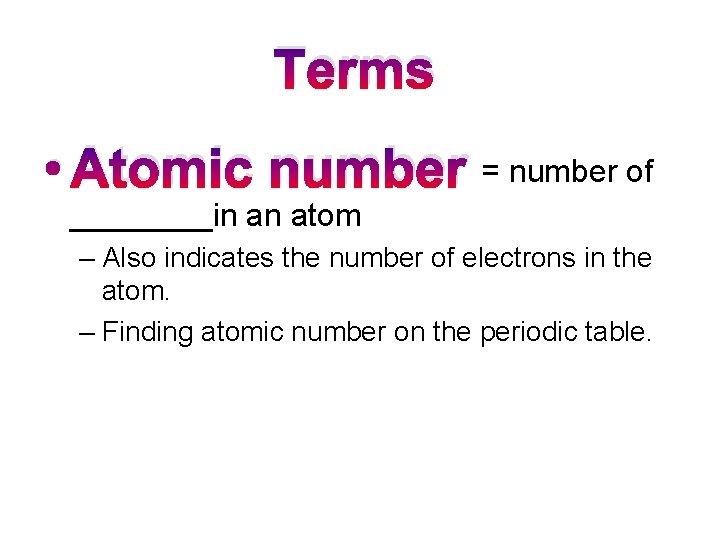
Terms • Atomic number = number of ____in an atom – Also indicates the number of electrons in the atom. – Finding atomic number on the periodic table.
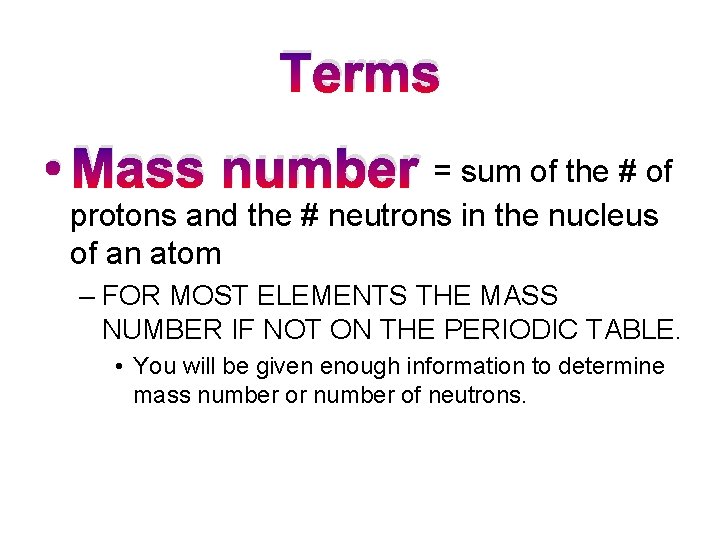
Terms • Mass number = sum of the # of protons and the # neutrons in the nucleus of an atom – FOR MOST ELEMENTS THE MASS NUMBER IF NOT ON THE PERIODIC TABLE. • You will be given enough information to determine mass number or number of neutrons.
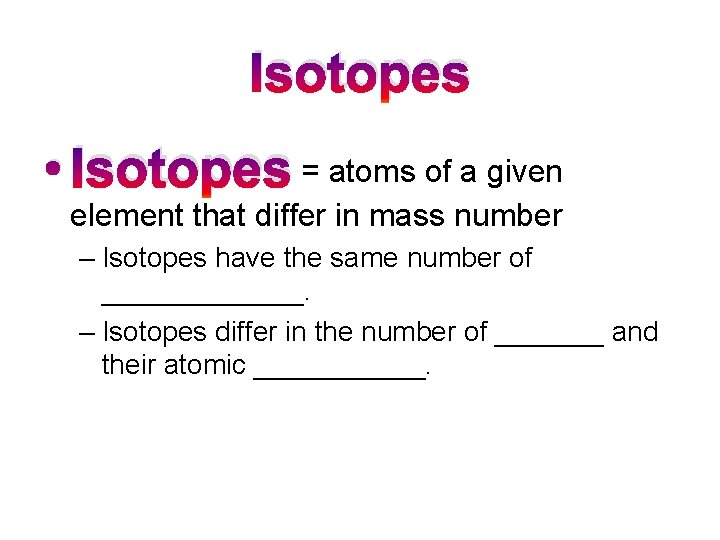
Isotopes • Isotopes = atoms of a given element that differ in mass number – Isotopes have the same number of _______. – Isotopes differ in the number of _______ and their atomic ______.
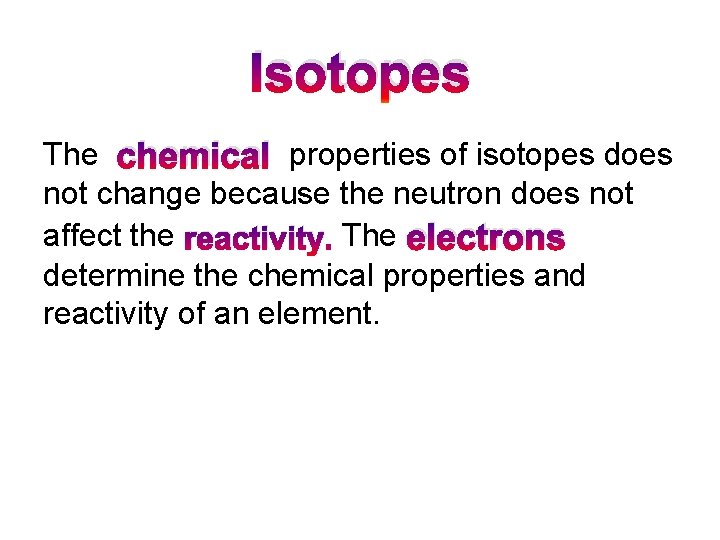
Isotopes The chemical properties of isotopes does not change because the neutron does not affect the reactivity. The electrons determine the chemical properties and reactivity of an element.
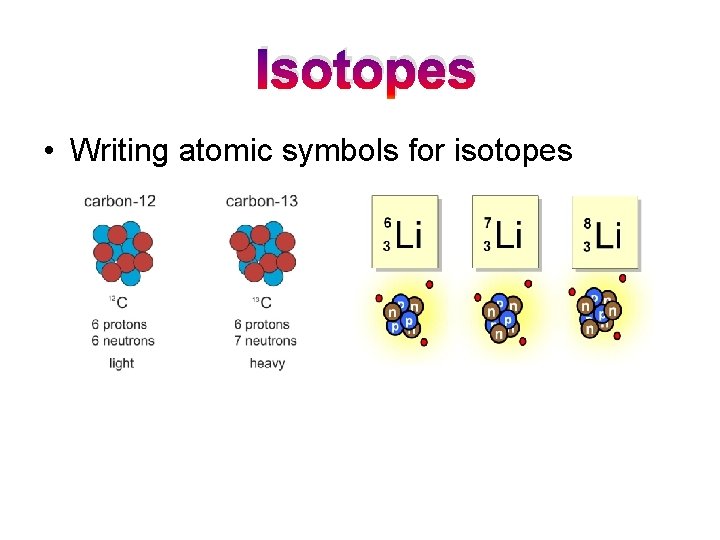
Isotopes • Writing atomic symbols for isotopes
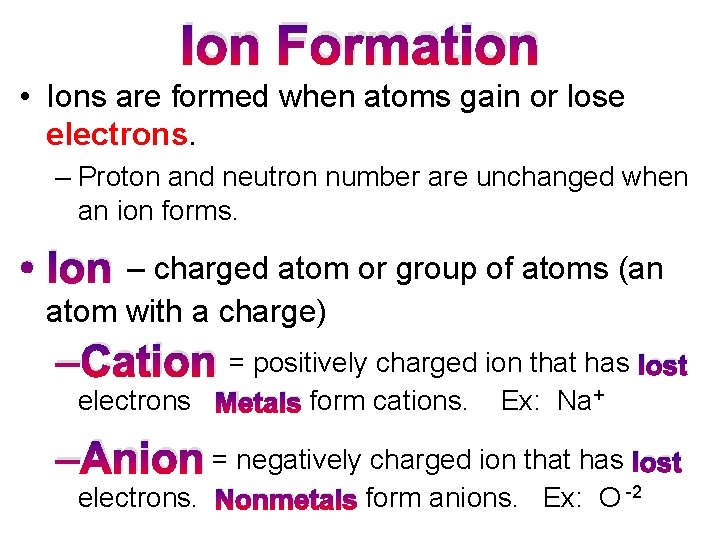
Ion Formation • Ions are formed when atoms gain or lose electrons. – Proton and neutron number are unchanged when an ion forms. • Ion – charged atom or group of atoms (an atom with a charge) –Cation = positively charged ion that has lost electrons Metals form cations. Ex: Na+ –Anion = negatively charged ion that has lost electrons. Nonmetals form anions. Ex: O -2
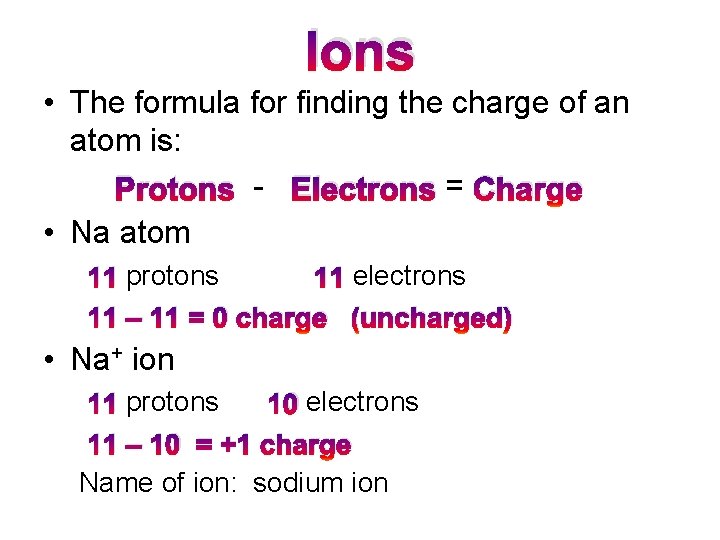
Ions • The formula for finding the charge of an atom is: Protons - Electrons = Charge • Na atom 11 protons 11 electrons 11 – 11 = 0 charge (uncharged) • Na+ ion 11 protons 10 electrons 11 – 10 = +1 charge Name of ion: sodium ion
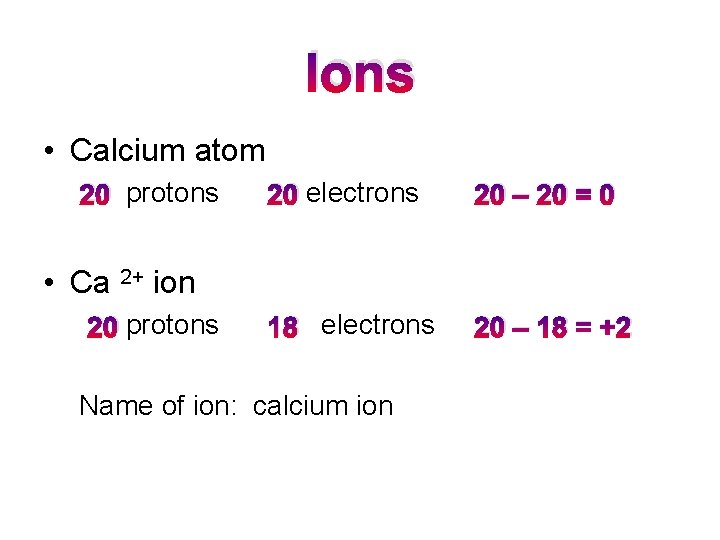
Ions • Calcium atom 20 protons 20 electrons 20 – 20 = 0 18 electrons 20 – 18 = +2 • Ca 2+ ion 20 protons Name of ion: calcium ion
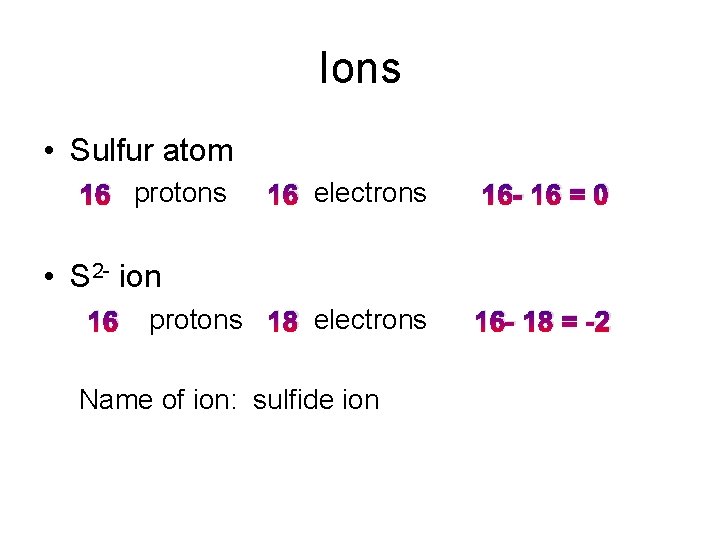
Ions • Sulfur atom 16 protons 16 electrons 16 - 16 = 0 protons 18 electrons 16 - 18 = -2 • S 2 - ion 16 Name of ion: sulfide ion
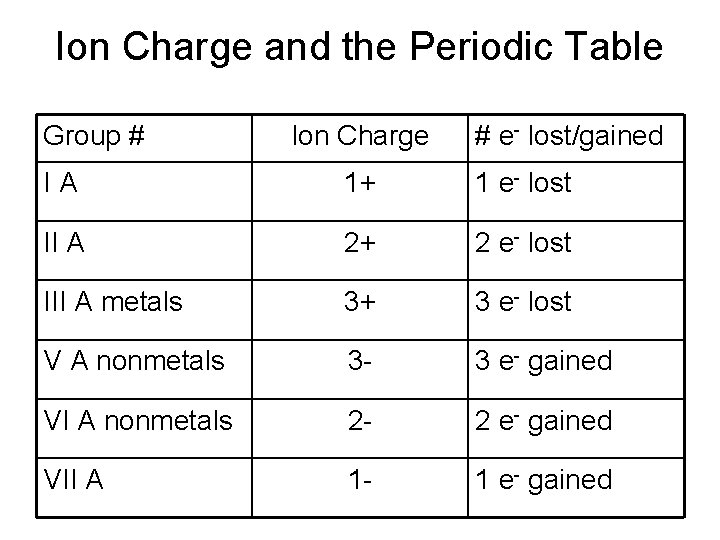
Ion Charge and the Periodic Table Group # Ion Charge # e- lost/gained IA 1+ 1 e- lost II A 2+ 2 e- lost III A metals 3+ 3 e- lost V A nonmetals 3 - 3 e- gained VI A nonmetals 2 - 2 e- gained VII A 1 - 1 e- gained
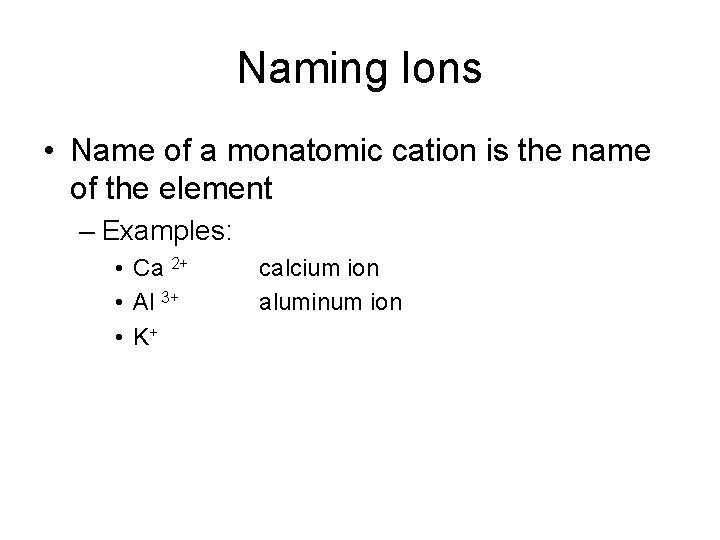
Naming Ions • Name of a monatomic cation is the name of the element – Examples: • Ca 2+ • Al 3+ • K+ calcium ion aluminum ion

Naming Ions • Monatomic anions are named by changing end of the name of the element to “ide” Example: S 2 - sulfide ion

Naming Ions • You need to know: N 3 P 3 O 2 S 2 FCl Br. I- nitride ion phosphide ion oxide ion sulfide ion fluoride ion chloride ion bromide ion iodide ion

Ionic Compounds • Structure – In an ionic compound there is a regular arrangement of oppositely charged particles. – Ions are arranged in a 3 -D crystalline structure that maximizes attractive forces and minimizes repulsive forces. • Also called a lattice structure • See page 102

Ionic Compounds • Physical Properties – all are related to the structure of the compounds – Solids at room temperature – Relatively high melting and boiling points – No vapor pressure • Meaning… they don’t evaporate – Electrolytes • Conduct electricity when melted or dissolved in water

Ionic Compounds • The chemical formula for an ionic compound represents the lowest, whole number ratio of the component ions that has a net charge of zero. Total positive charge = total negative charge

Ionic Compounds • Name the compound by naming the ions.

Ionic Compounds • Writing formulas for and naming binary ionic compounds – Magnesium oxide

Ionic Compounds Magnesium oxide – The formula is the simplest ratio of ions that have a net charge of zero. – Ions present: Mg 2+ and O 2– Formula:
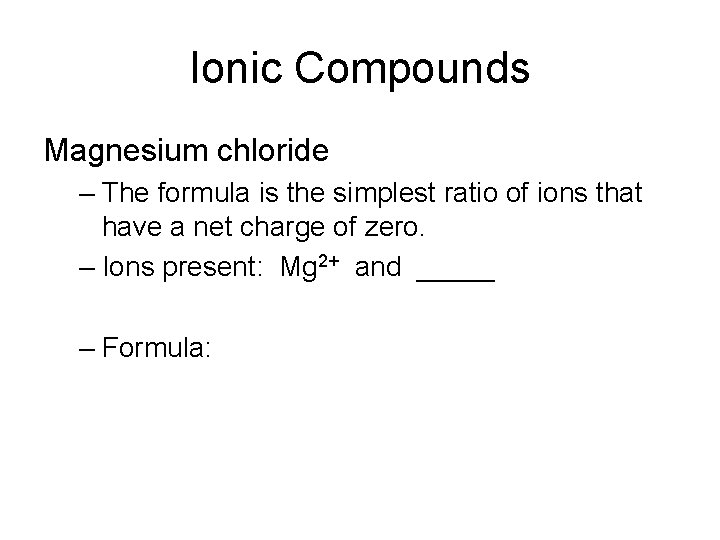
Ionic Compounds Magnesium chloride – The formula is the simplest ratio of ions that have a net charge of zero. – Ions present: Mg 2+ and _____ – Formula:

Ionic Compounds • Practice – Note we are currently applying the content of 4. 11 and 5. 2 (type I binary ionic compounds)
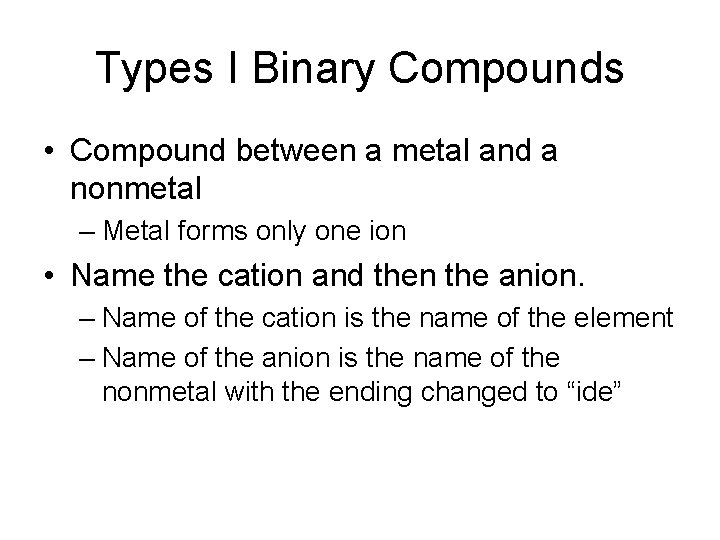
Types I Binary Compounds • Compound between a metal and a nonmetal – Metal forms only one ion • Name the cation and then the anion. – Name of the cation is the name of the element – Name of the anion is the name of the nonmetal with the ending changed to “ide”
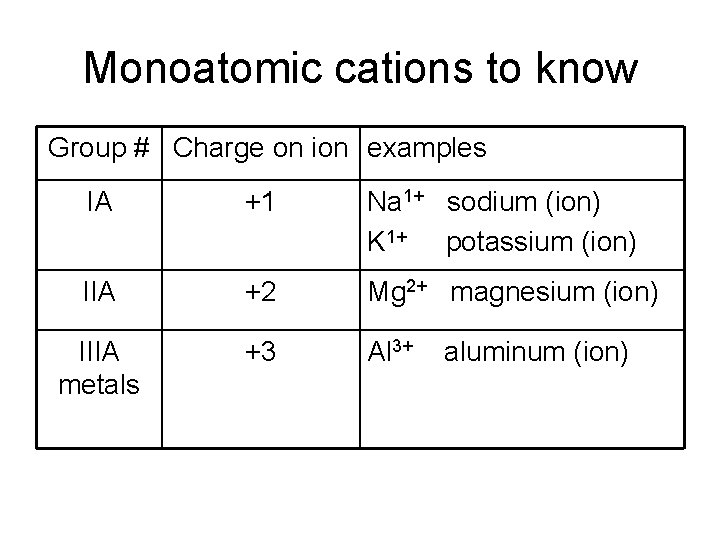
Monoatomic cations to know Group # Charge on ion examples IA +1 Na 1+ sodium (ion) K 1+ potassium (ion) IIA +2 Mg 2+ magnesium (ion) IIIA metals +3 Al 3+ aluminum (ion)
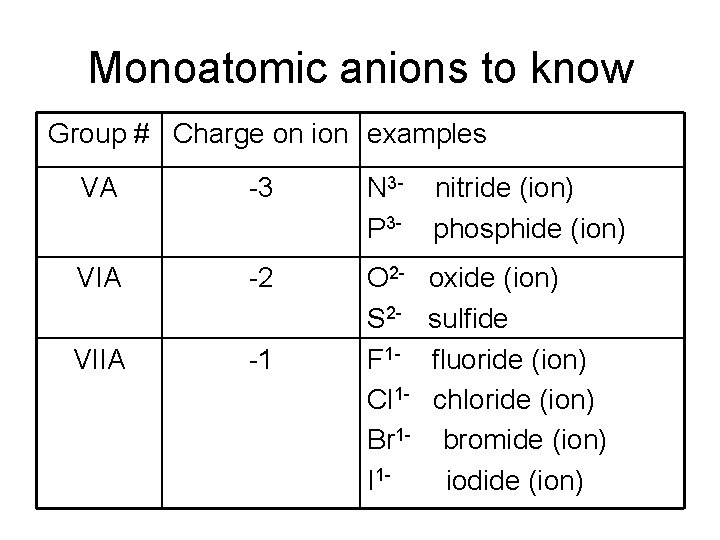
Monoatomic anions to know Group # Charge on ion examples VA -3 N 3 P 3 - nitride (ion) phosphide (ion) VIA -2 VIIA -1 O 2 S 2 F 1 Cl 1 Br 1 I 1 - oxide (ion) sulfide fluoride (ion) chloride (ion) bromide (ion) iodide (ion)

Practice • Name chemical formula • Chemical formula name
- Slides: 29