What are subatomic particles Subatomic Particles Protons Electrons
What are subatomic particles? Subatomic Particles Protons Electrons Neutrons
� Protons = positively charged, in the nucleus, each is assigned a charge 1+ � Electron = negatively charged, outside the nucleus, each has a charge 1 - � Neutron = neutral charge, in the nucleus, each has no charge (0) �Symbols = p+, e-, n �Mass = 1, 1/1836, 1

atoms of any given element always have the same # of protons Hydrogen’s atomic number is 1 which means it has only 1 proton. Atomic # = number of protons Atomic #’s are unique to that element. Each positive (protons) charge is balanced by an equal # of negative charge. So, the atomic # of an element also equals the # of electrons in an atom. How do we find the # of neutron’s in an atom’s nucleus?
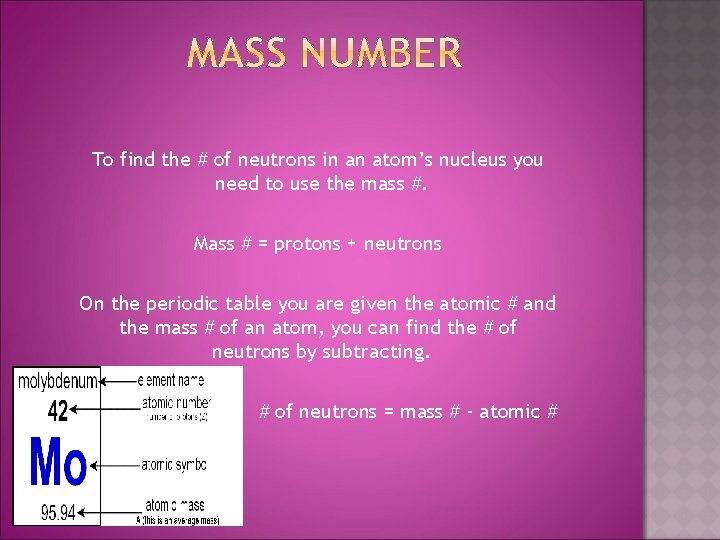
To find the # of neutrons in an atom’s nucleus you need to use the mass #. Mass # = protons + neutrons On the periodic table you are given the atomic # and the mass # of an atom, you can find the # of neutrons by subtracting. # of neutrons = mass # - atomic #
� Every atom of a given element has the same # of protons and electrons � Every atom of a given element does not have the same # of neutrons � Isotopes: atoms of the same element that have different #’s of neutrons and different mass #’s �Isotopes of an element have the same atomic # but different mass #’s because they have different #’s of neutrons.
� Oxygen has an Atomic # of 8 and a Mass # of 15. 999 (therefore it has 8 neutrons) � An isotope of an Oxygen atom will still have an atomic # of 8, however the mass number will be different. �For example: an isotope of oxygen could have a mass # of 19. 999 and still has an atomic # of 8 (therefore it has 12 neutrons) � Because it has a different # of neutrons, it’s mass # will change
- Slides: 6