Unit 2 History and Structure Particle Charge Location














- Slides: 29

Unit 2: History and Structure


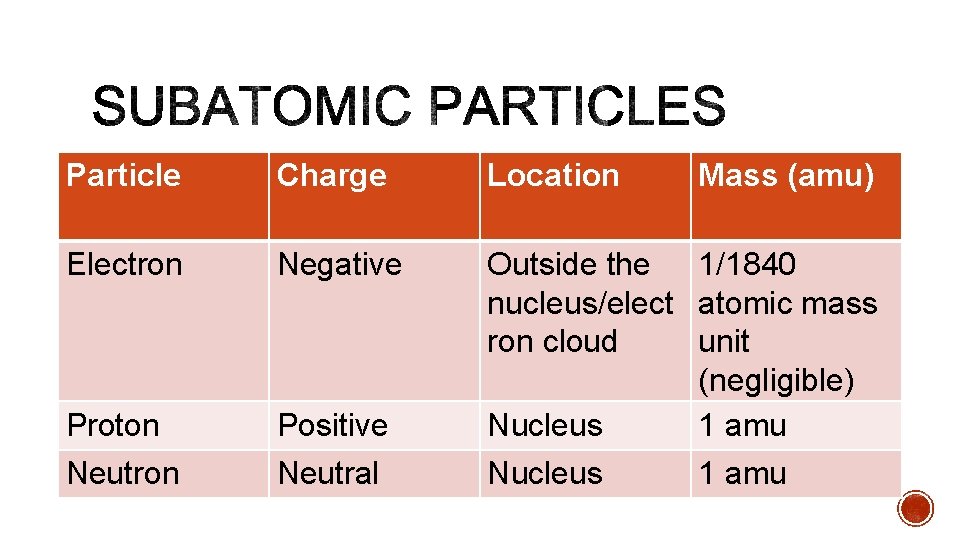
Particle Charge Location Mass (amu) Electron Negative Proton Positive Outside the 1/1840 nucleus/elect atomic mass ron cloud unit (negligible) Nucleus 1 amu Neutron Neutral Nucleus 1 amu

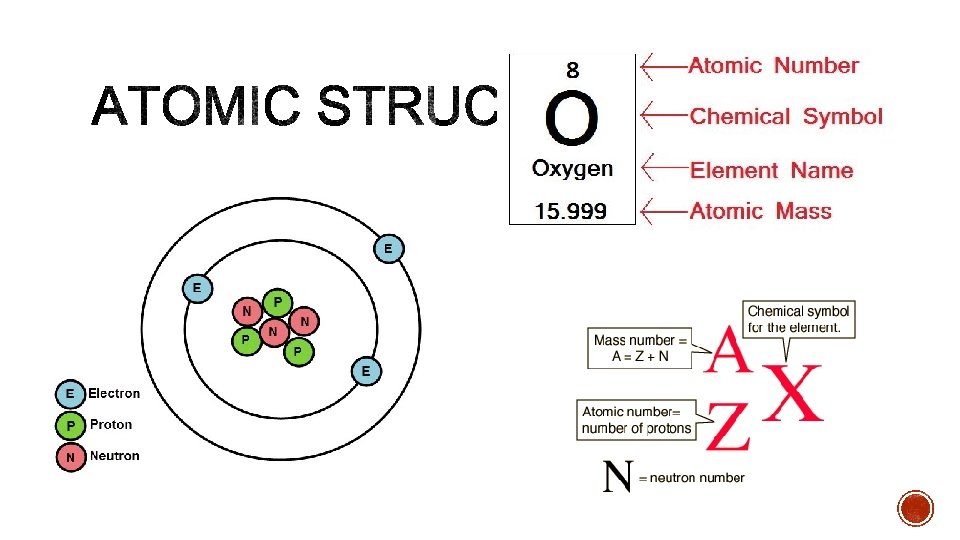
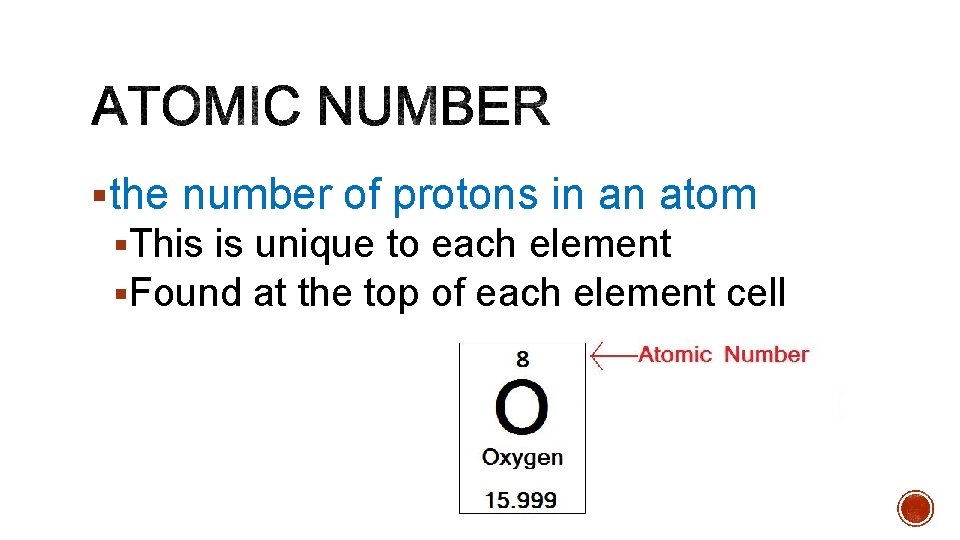
§the number of protons in an atom §This is unique to each element §Found at the top of each element cell

§proton + neutrons §This is NEVER a rounded number §Cannot split neutrons or protons §Is not on the period table §In isotope notation it is the top number (A)

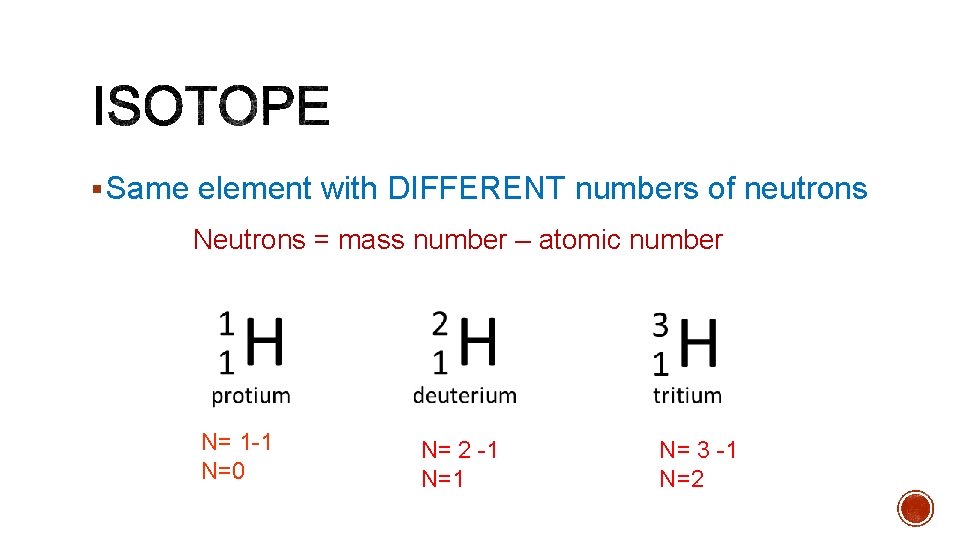
§ Same element with DIFFERENT numbers of neutrons Neutrons = mass number – atomic number N= 1 -1 N=0 N= 2 -1 N= 3 -1 N=2

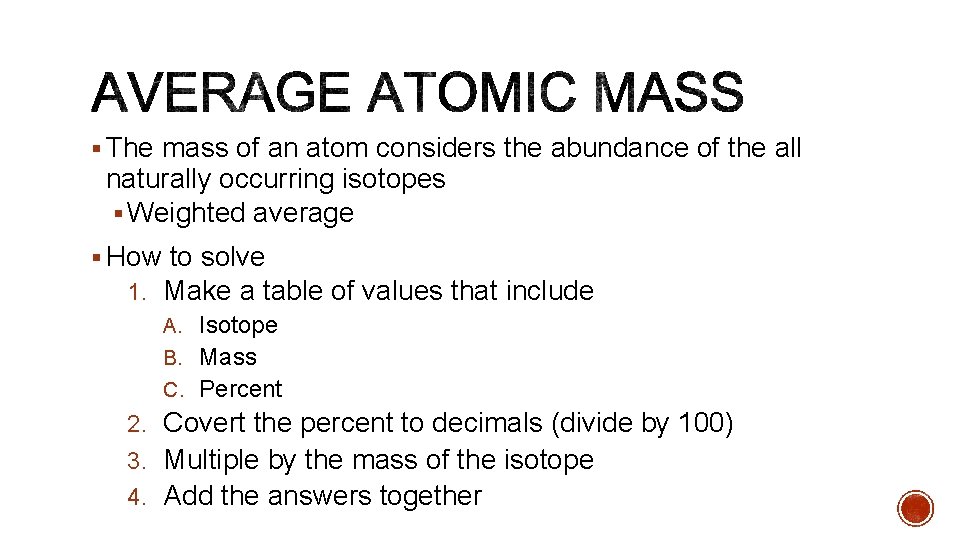
§ The mass of an atom considers the abundance of the all naturally occurring isotopes § Weighted average § How to solve 1. Make a table of values that include A. Isotope B. Mass C. Percent 2. Covert the percent to decimals (divide by 100) 3. Multiple by the mass of the isotope 4. Add the answers together

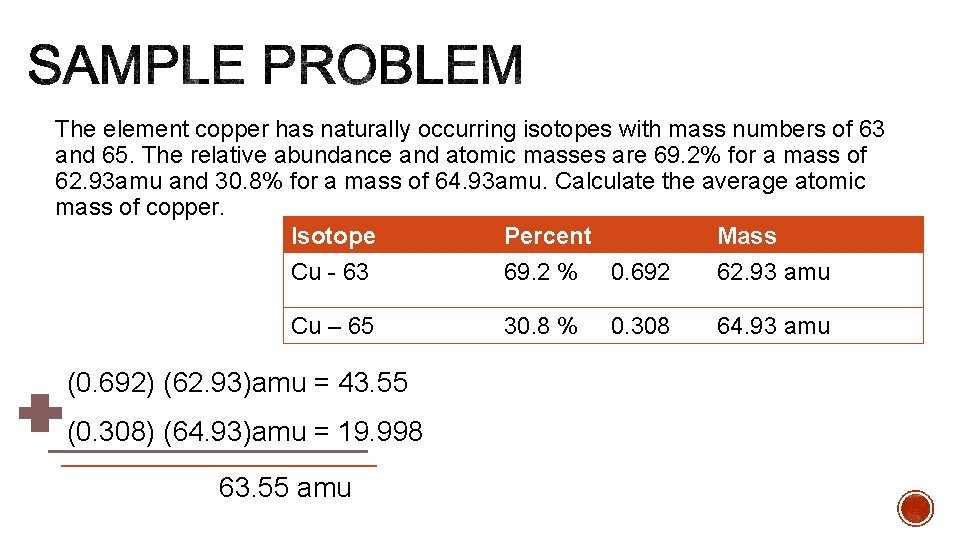
The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are 69. 2% for a mass of 62. 93 amu and 30. 8% for a mass of 64. 93 amu. Calculate the average atomic mass of copper. Isotope Percent Mass Cu - 63 69. 2 % 0. 692 62. 93 amu Cu – 65 (0. 692) (62. 93)amu = 43. 55 (0. 308) (64. 93)amu = 19. 998 63. 55 amu 30. 8 % 0. 308 64. 93 amu

§Same elements with different number of electrons §Cation: positive ion § lower number of electrons than protons §Anion: negative ion § higher number of electrons than protons

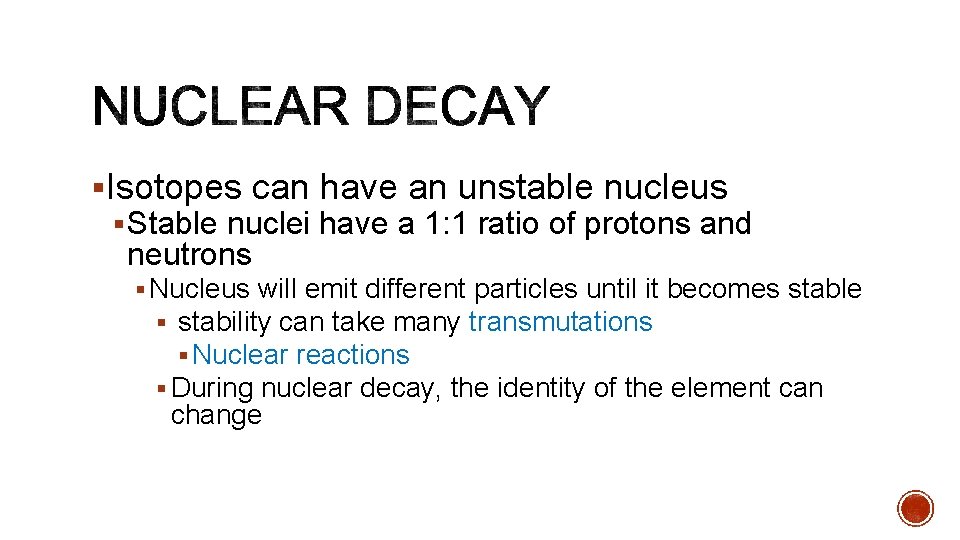
§Isotopes can have an unstable nucleus § Stable nuclei have a 1: 1 ratio of protons and neutrons § Nucleus will emit different particles until it becomes stable § stability can take many transmutations § Nuclear reactions § During nuclear decay, the identity of the element can change


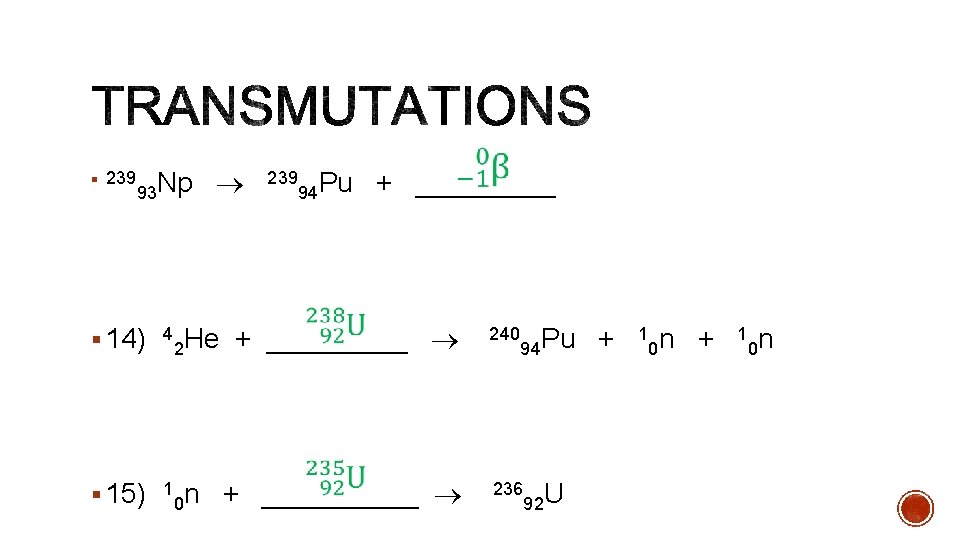
§ 239 93 Np § 14) 4 239 94 Pu + _____ 240 Pu + 1 n He + _____ 2 94 0 0 § 15) 1 236 U n + _____ 0 92
§The amount of time it takes for half the nuclear material within a substance to transmute/ decay §Calculated using a time and mass chart §Always start with time zero

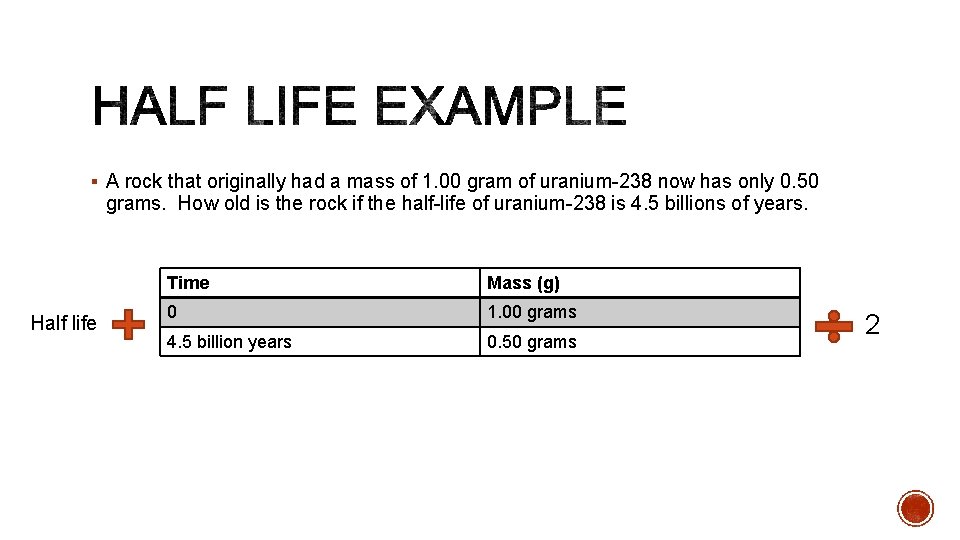
§ A rock that originally had a mass of 1. 00 gram of uranium-238 now has only 0. 50 grams. How old is the rock if the half-life of uranium-238 is 4. 5 billions of years. Half life Time Mass (g) 0 1. 00 grams 4. 5 billion years 0. 50 grams 2

§Democritus § All matter was composed of tiny particles § Atomos § “particles “ were thought to be indivisible §Aristotle §Matter is continuous § Everything in the universe is indefinitely indivisible

§ Had 5 principles to his Atomic theory 1. All matter is composed of particles called atoms 2. Atoms of a given elements have the same properties, atoms of different elements have different properties 3. Atom cannot be subdivided, created, or destroyed 4. Atoms of different elements combined in small whole numbers 5. Chemical reactions are when atoms are combined, separated, and rearranged.

§Not all of Dalton’s principles were correct §Atoms can be subdivide in to subatomic particles § Neutron § Proton § Electron §Elements can combine with themselves to form diatomic molecules

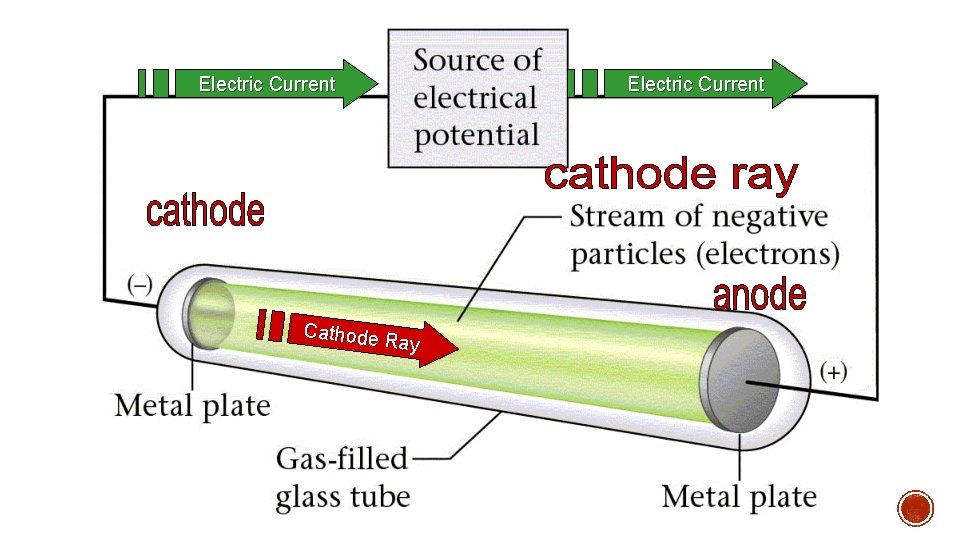
§The discovery of the first subatomic particle took place in the late 1800’s. § A power source was attached to two metal ends of an evacuated glass tube, called a cathode ray tube. § A beam of “light” appears between the two electrodes called a cathode ray.

Electric Current Cathode Ray Electric Current

1. Cathode rays were deflected by a magnetic field 2. The rays were deflected away from a negatively charged object § Scientists determined the ray was negatively charged. § Electron negatively charged § Must be a positive particle

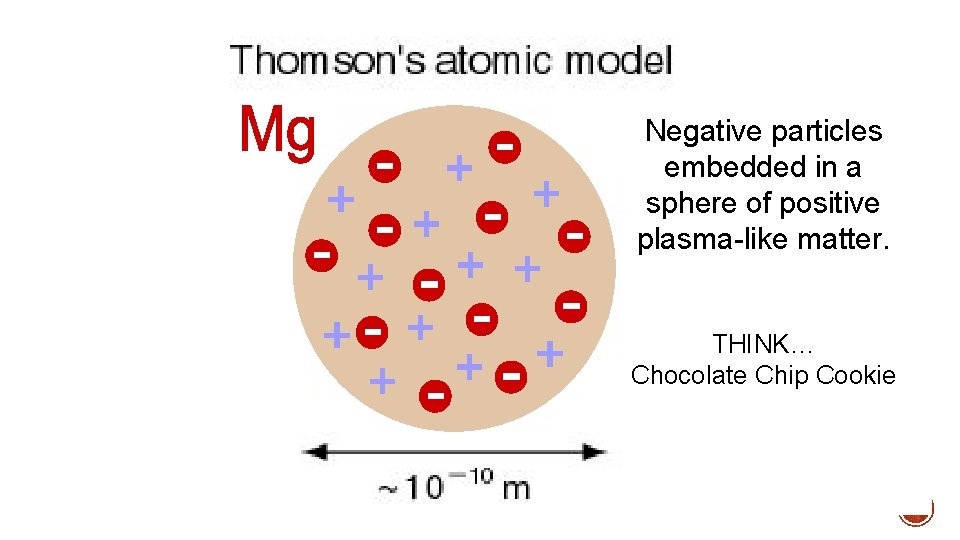
Negative particles embedded in a sphere of positive plasma-like matter. THINK… Chocolate Chip Cookie

§Gold Foil Experiment §directed a narrow beam of alpha particles at a very thin sheet of gold foil. §Alpha particles (a) are He atoms that have been stripped of their electrons


§Alpha particles 1. Went straight through 2. Deflected significantly at an angle 3. Bounced back

§Scientists determined §Atom is made up of mostly empty space §There is a positive particle (like repels like) §Proton positively charged §There is a dense mass in the center of an atom §Nucleus § Must be another particle to account for the mass

§In 1932, the English physicist James Chadwick discovered yet another subatomic particle. § the neutron is electrically neutral § It’s mass is nearly equal to the proton §Therefore the subatomic particles are the electron, proton, and neutron

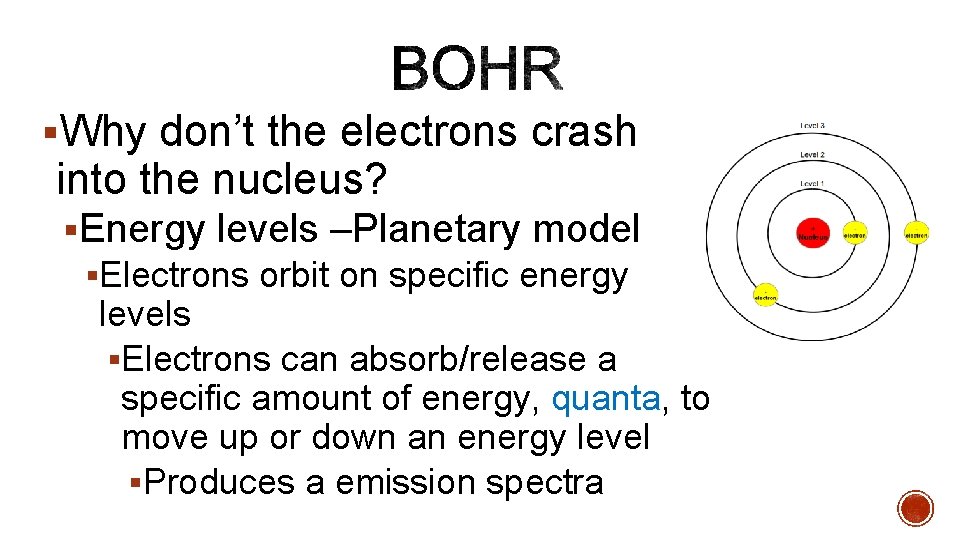
§Why don’t the electrons crash into the nucleus? §Energy levels –Planetary model §Electrons orbit on specific energy levels §Electrons can absorb/release a specific amount of energy, quanta, to move up or down an energy level §Produces a emission spectra

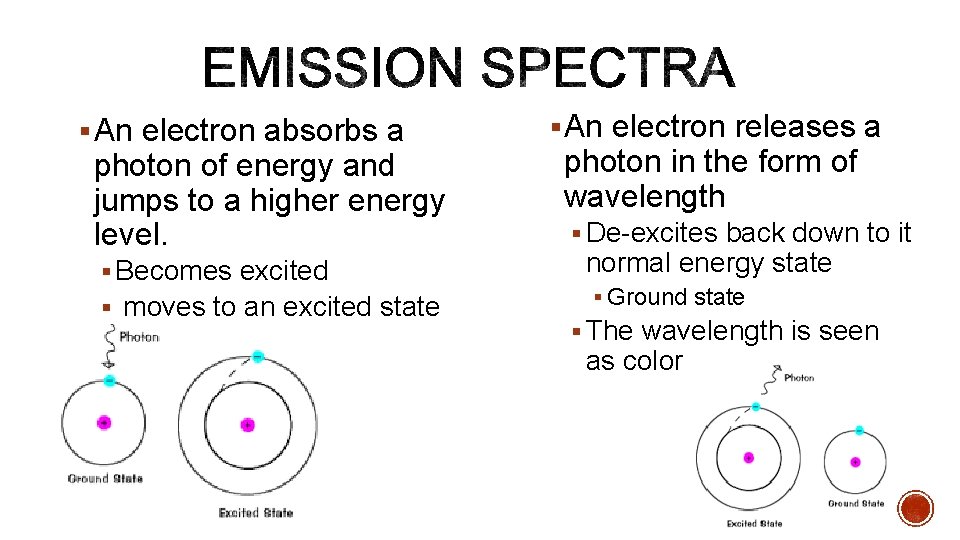
§ An electron absorbs a photon of energy and jumps to a higher energy level. § Becomes excited § moves to an excited state § An electron releases a photon in the form of wavelength § De-excites back down to it normal energy state § Ground state § The wavelength is seen as color

§ Strong: works over small distances, so can bring about interactions between particles colliding at high kinetic energy § Weak: involved in certain decays and interactions, and its involvement is signaled by interactions over 10 -12 seconds or longer § Electromagnetic: acts between all charged particles and is signaled by photon emissions or absorption. It may be involved in the internal re-arrangement of electron within an atom.