Subatomic Particles 1 Subatomic Particles Particle Electrons Symbol

Subatomic Particles
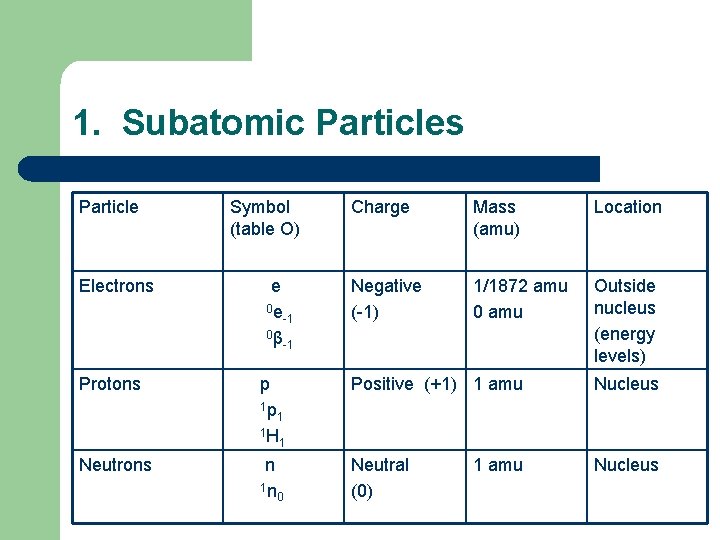
1. Subatomic Particles Particle Electrons Symbol (table O) e 0 e -1 0β -1 Charge Mass (amu) Location Negative (-1) 1/1872 amu 0 amu Outside nucleus (energy levels) Protons p 1 p 1 1 H 1 Positive (+1) 1 amu Nucleus Neutrons n 1 n 0 Neutral (0) Nucleus 1 amu
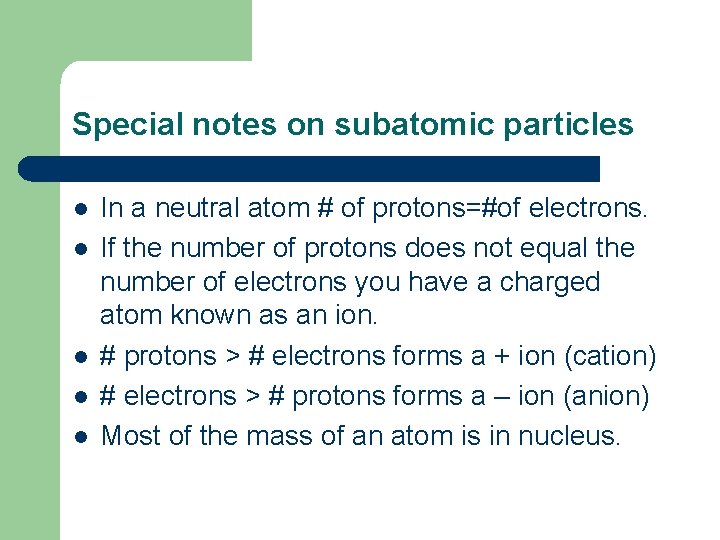
Special notes on subatomic particles l l l In a neutral atom # of protons=#of electrons. If the number of protons does not equal the number of electrons you have a charged atom known as an ion. # protons > # electrons forms a + ion (cation) # electrons > # protons forms a – ion (anion) Most of the mass of an atom is in nucleus.
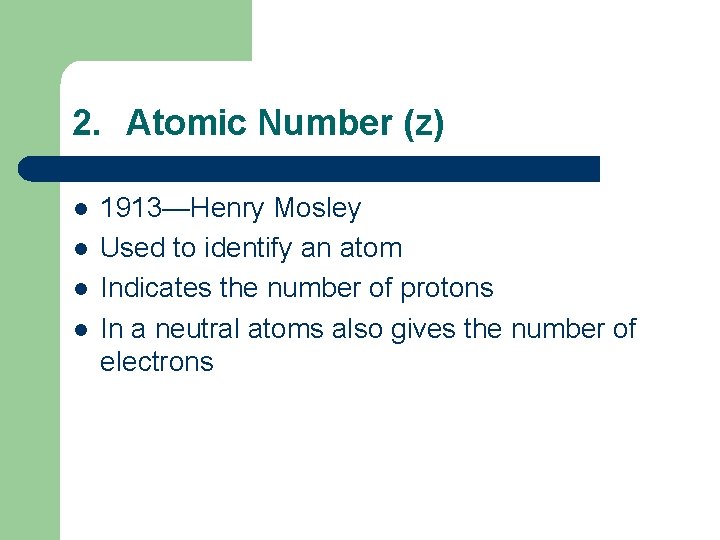
2. Atomic Number (z) l l 1913—Henry Mosley Used to identify an atom Indicates the number of protons In a neutral atoms also gives the number of electrons
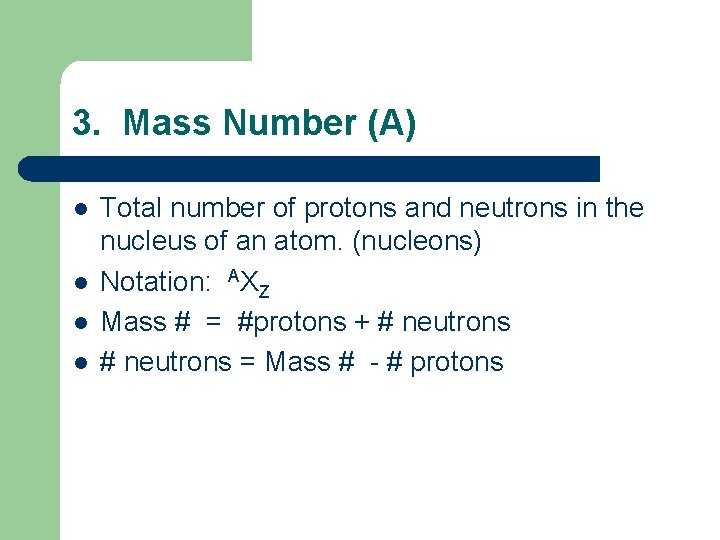
3. Mass Number (A) l l Total number of protons and neutrons in the nucleus of an atom. (nucleons) Notation: AXZ Mass # = #protons + # neutrons = Mass # - # protons
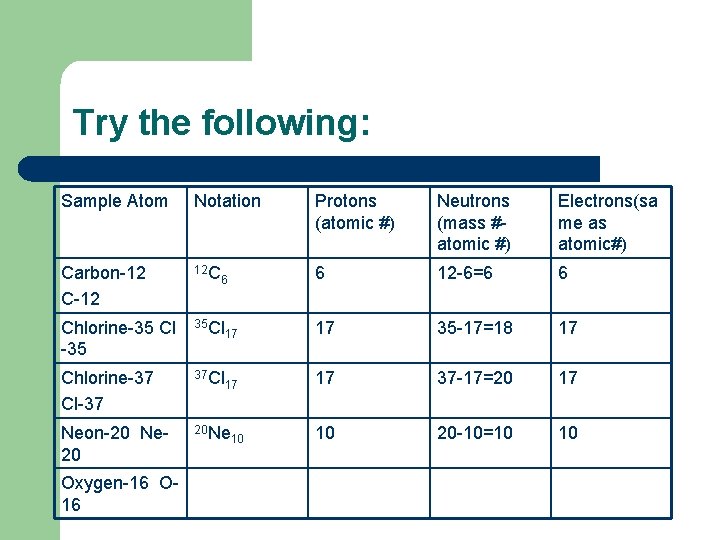
Try the following: Sample Atom Notation Protons (atomic #) Neutrons (mass #atomic #) Electrons(sa me as atomic#) Carbon-12 C-12 12 C 6 12 -6=6 6 Chlorine-35 Cl -35 35 Cl 17 17 35 -17=18 17 Chlorine-37 Cl-37 37 Cl 17 17 37 -17=20 17 Neon-20 Ne 20 20 Ne 10 20 -10=10 10 Oxygen-16 O 16 6 10
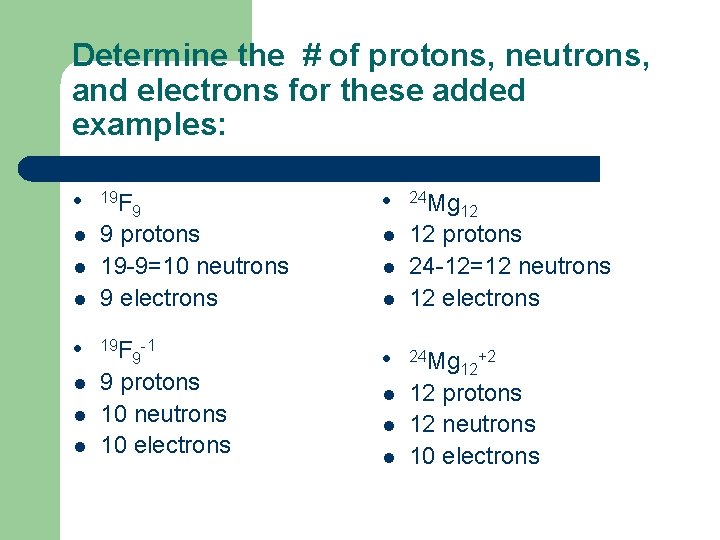
Determine the # of protons, neutrons, and electrons for these added examples: l 19 F l 9 protons 19 -9=10 neutrons 9 electrons l l l 9 19 F -1 9 9 protons 10 neutrons 10 electrons l 24 Mg l 12 protons 24 -12=12 neutrons 12 electrons l l 12 l 24 Mg l 12 protons 12 neutrons 10 electrons l l 12 +2
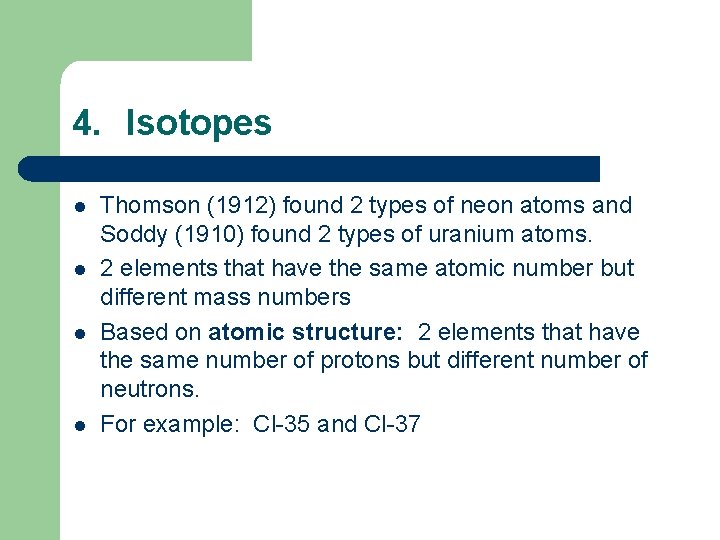
4. Isotopes l l Thomson (1912) found 2 types of neon atoms and Soddy (1910) found 2 types of uranium atoms. 2 elements that have the same atomic number but different mass numbers Based on atomic structure: 2 elements that have the same number of protons but different number of neutrons. For example: Cl-35 and Cl-37
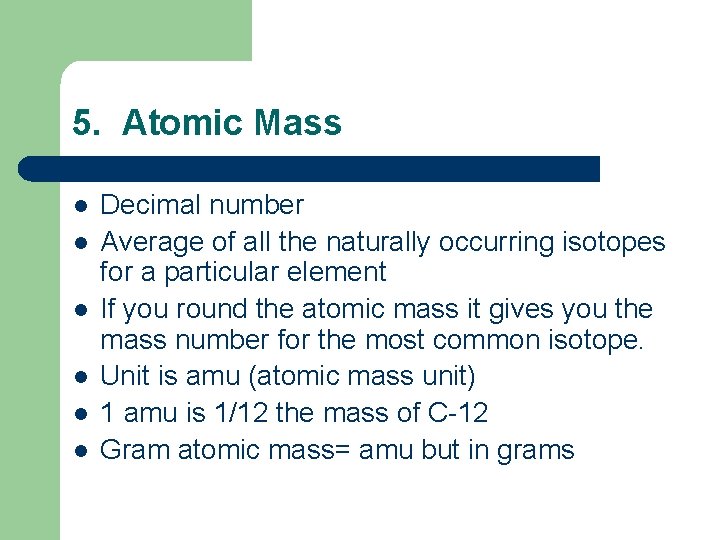
5. Atomic Mass l l l Decimal number Average of all the naturally occurring isotopes for a particular element If you round the atomic mass it gives you the mass number for the most common isotope. Unit is amu (atomic mass unit) 1 amu is 1/12 the mass of C-12 Gram atomic mass= amu but in grams
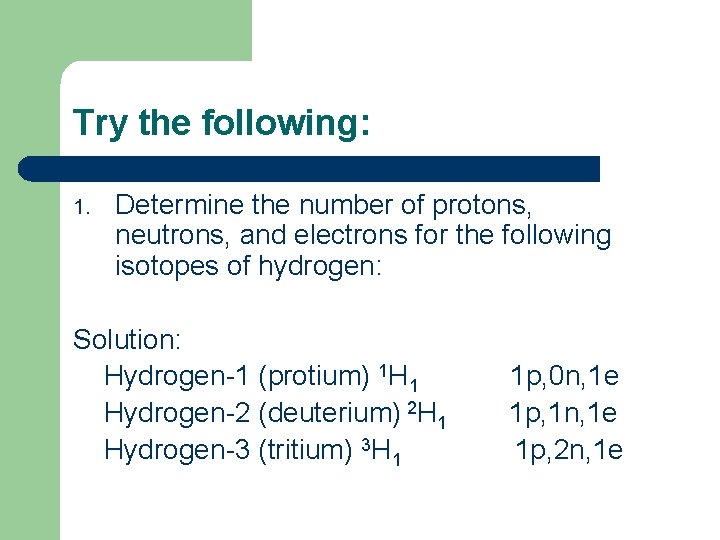
Try the following: 1. Determine the number of protons, neutrons, and electrons for the following isotopes of hydrogen: Solution: Hydrogen-1 (protium) 1 H 1 Hydrogen-2 (deuterium) 2 H 1 Hydrogen-3 (tritium) 3 H 1 1 p, 0 n, 1 e 1 p, 1 n, 1 e 1 p, 2 n, 1 e

2. Naturally occurring chlorine consists of 75% Cl-35 and 25% Cl-37. Find the average atomic mass. l . 75(35) +. 25(37)= 35. 50 amu or l 75(35) + 25(37) =35. 50 amu 100
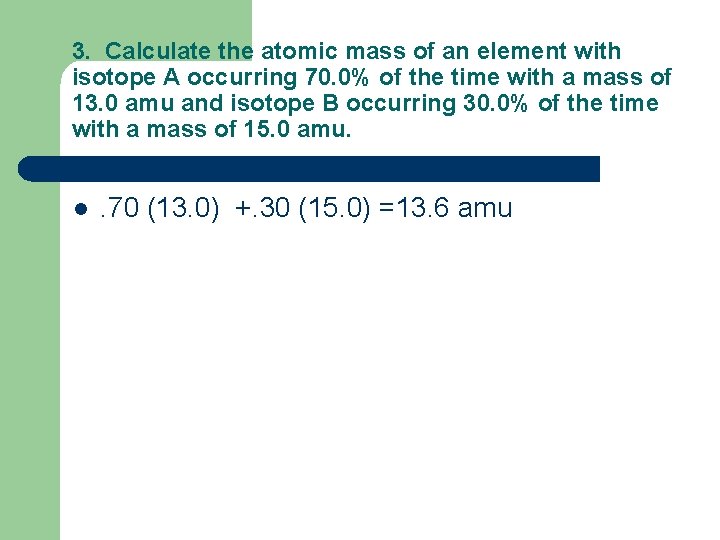
3. Calculate the atomic mass of an element with isotope A occurring 70. 0% of the time with a mass of 13. 0 amu and isotope B occurring 30. 0% of the time with a mass of 15. 0 amu. l . 70 (13. 0) +. 30 (15. 0) =13. 6 amu

4. An element X has three isotopes X-30 has a 50. 0% abundance, X-28 has a 30. 0% abundance and X-31 has a 20. 0% abundance. l . 500(30) +. 300(28) +. 200 (31) = 29. 6 amu
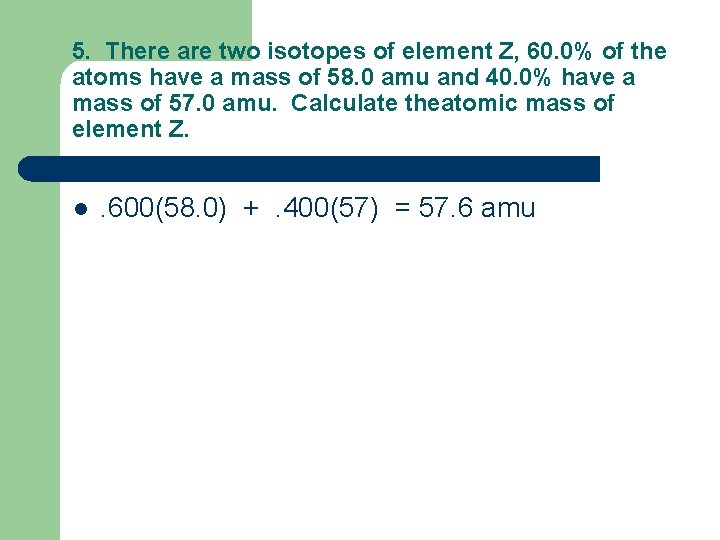
5. There are two isotopes of element Z, 60. 0% of the atoms have a mass of 58. 0 amu and 40. 0% have a mass of 57. 0 amu. Calculate theatomic mass of element Z. l . 600(58. 0) +. 400(57) = 57. 6 amu
- Slides: 14