Subatomic Particles Subatomic Charge Mass amu Particle Location
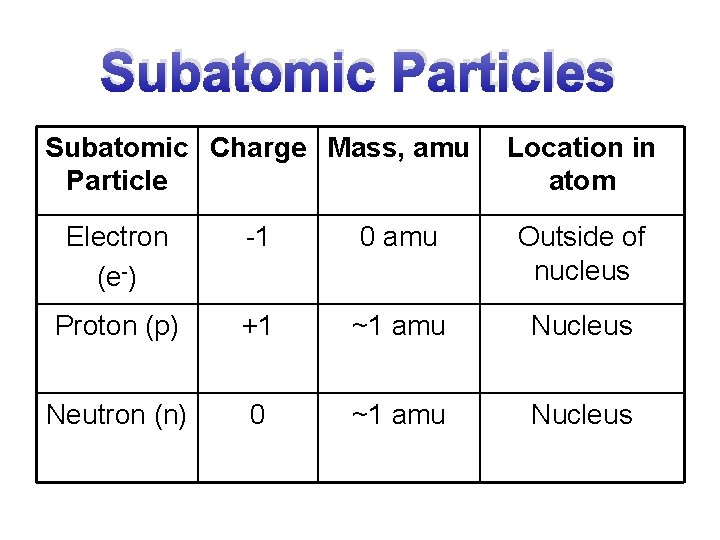
Subatomic Particles Subatomic Charge Mass, amu Particle Location in atom Electron (e-) -1 0 amu Outside of nucleus Proton (p) +1 ~1 amu Nucleus Neutron (n) 0 ~1 amu Nucleus

Mass of Subatomic Particles • The mass of the electron is tiny as compared to that of the proton and neutron. – Therefore, the electron’s mass is considered to be ~0 amu when calculating the mass of an atom.

Subatomic Particles and the Elements • Each element has a unique number of protons. – Number of protons defines the element.

Subatomic Particles and the Elements • Since atoms are neutral, for every proton there is a/n electron. • When atoms interact to form compounds, it is their electrons that “intermingle”/react.

Terms • Atomic number = number of protons in an atom – Also indicates the number of electrons in the atom. – Finding atomic number on the periodic table.

Terms • Mass number = sum of the # of protons and the # neutrons in the nucleus of an atom – FOR MOST ELEMENTS THE MASS NUMBER IF NOT ON THE PERIODIC TABLE. • You will be given enough information to determine mass number or number of neutrons.

Isotopes • Isotopes = atoms of a given element that differ in mass number – Isotopes have the same number of protons. – Isotopes differ in the number of neutrons and their atomic mass.

Isotopes The chemical properties of isotopes does not change because the neutron does not affect the reactivity. The electrons determine the chemical properties and reactivity of an element.

Isotopes • Writing atomic symbols for isotopes

Ion Formation • Ions are formed when atoms gain or lose electrons. – Proton and neutron number are unchanged when an ion forms. • Ion – charged atom or group of atoms (an atom with a charge) –Cation = positively charged ion that has lost electrons Metals form cations. Ex: Na+ –Anion = negatively charged ion that has gained electrons. Nonmetals form anions. – Ex: O -2
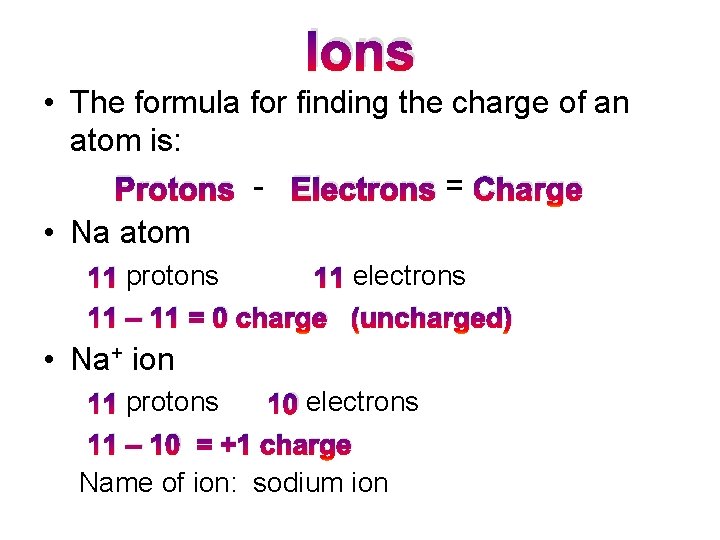
Ions • The formula for finding the charge of an atom is: Protons - Electrons = Charge • Na atom 11 protons 11 electrons 11 – 11 = 0 charge (uncharged) • Na+ ion 11 protons 10 electrons 11 – 10 = +1 charge Name of ion: sodium ion
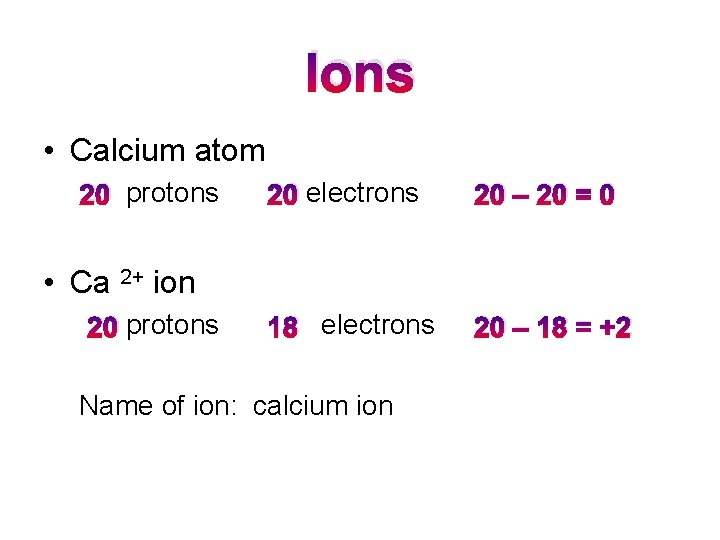
Ions • Calcium atom 20 protons 20 electrons 20 – 20 = 0 18 electrons 20 – 18 = +2 • Ca 2+ ion 20 protons Name of ion: calcium ion

Ions • Sulfur atom 16 protons 16 electrons 16 - 16 = 0 protons 18 electrons 16 - 18 = -2 • S 2 - ion 16 Name of ion: sulfide ion
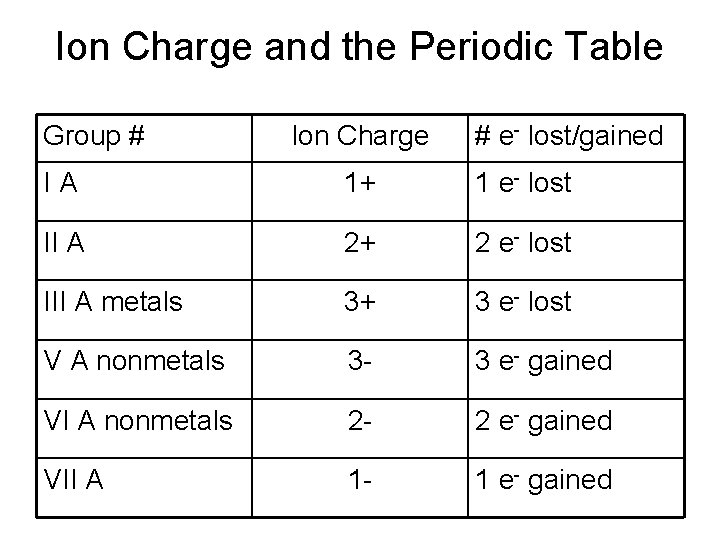
Ion Charge and the Periodic Table Group # Ion Charge # e- lost/gained IA 1+ 1 e- lost II A 2+ 2 e- lost III A metals 3+ 3 e- lost V A nonmetals 3 - 3 e- gained VI A nonmetals 2 - 2 e- gained VII A 1 - 1 e- gained
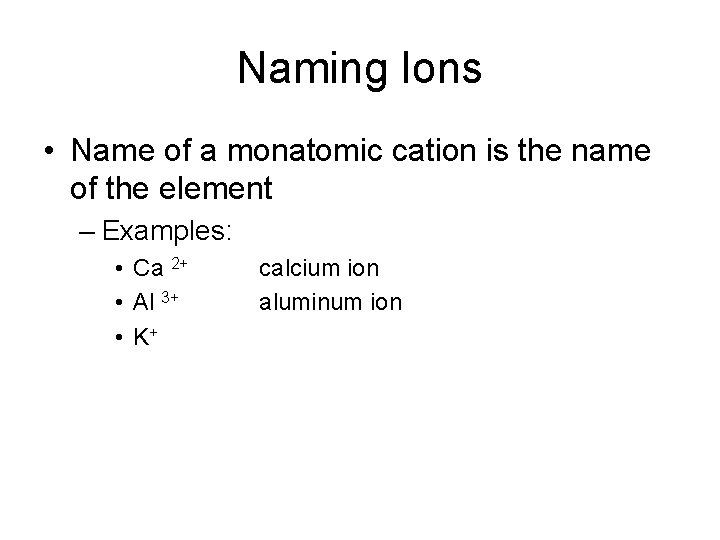
Naming Ions • Name of a monatomic cation is the name of the element – Examples: • Ca 2+ • Al 3+ • K+ calcium ion aluminum ion

Naming Ions • Monatomic anions are named by changing end of the name of the element to “ide” Example: S 2 - sulfide ion

Naming Ions • You need to know: N 3 P 3 O 2 S 2 FCl Br. I- nitride ion phosphide ion oxide ion sulfide ion fluoride ion chloride ion bromide ion iodide ion

Ionic Compounds • Structure – In an ionic compound there is a regular arrangement of oppositely charged particles. – Ions are arranged in a 3 -D crystalline structure that maximizes attractive forces and minimizes repulsive forces. • Also called a lattice structure • See page 102

Ionic Compounds • Physical Properties – all are related to the structure of the compounds – Solids at room temperature – Relatively high melting and boiling points – No vapor pressure • Meaning… they don’t evaporate – Electrolytes • Conduct electricity when melted or dissolved in water

Ionic Compounds • The chemical formula for an ionic compound represents the lowest, whole number ratio of the component ions that has a net charge of zero. Total positive charge = total negative charge

Ionic Compounds • Name the compound by naming the ions.

Ionic Compounds • Writing formulas for and naming binary ionic compounds – Magnesium oxide

Ionic Compounds Magnesium oxide – The formula is the simplest ratio of ions that have a net charge of zero. – Ions present: Mg 2+ and O 2– Formula:
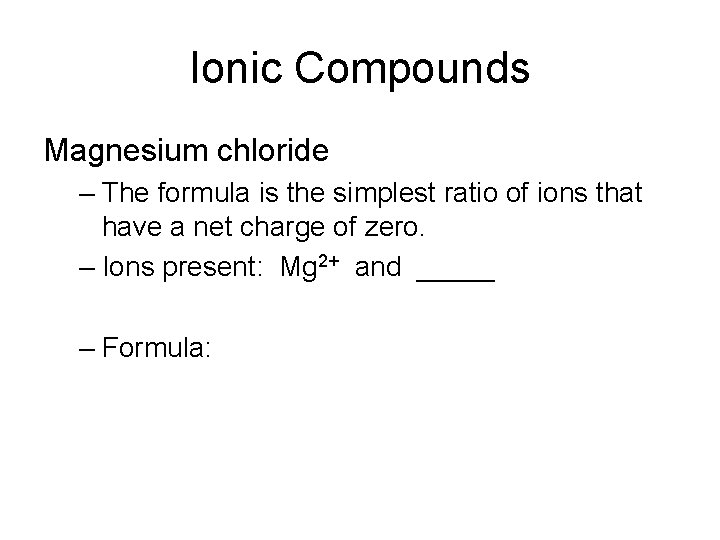
Ionic Compounds Magnesium chloride – The formula is the simplest ratio of ions that have a net charge of zero. – Ions present: Mg 2+ and _____ – Formula:

Ionic Compounds • Practice – Note we are currently applying the content of 4. 11 and 5. 2 (type I binary ionic compounds)
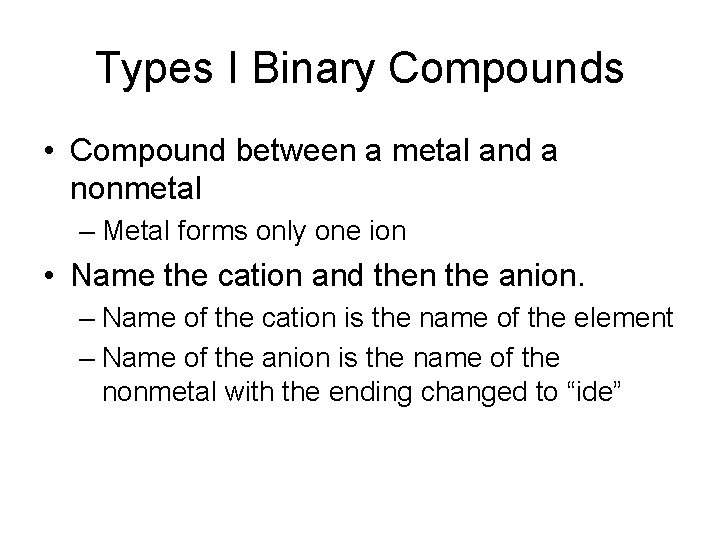
Types I Binary Compounds • Compound between a metal and a nonmetal – Metal forms only one ion • Name the cation and then the anion. – Name of the cation is the name of the element – Name of the anion is the name of the nonmetal with the ending changed to “ide”

Monoatomic cations to know Group # Charge on ion examples IA +1 Na 1+ sodium (ion) K 1+ potassium (ion) IIA +2 Mg 2+ magnesium (ion) IIIA metals +3 Al 3+ aluminum (ion)
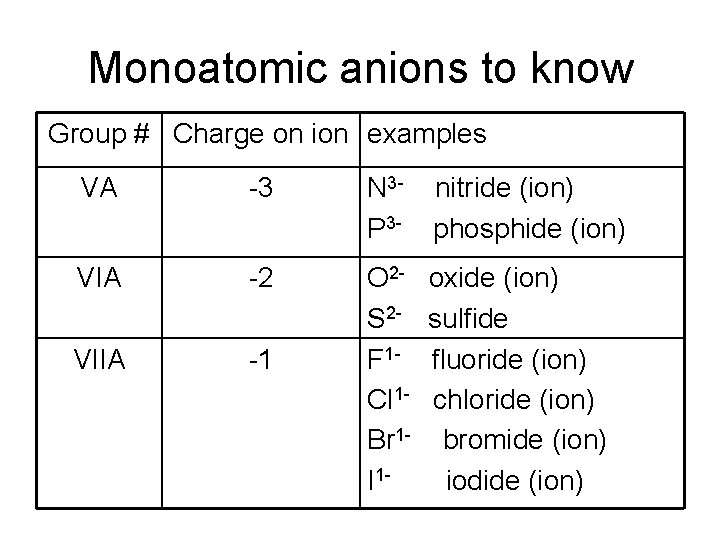
Monoatomic anions to know Group # Charge on ion examples VA -3 N 3 P 3 - nitride (ion) phosphide (ion) VIA -2 VIIA -1 O 2 S 2 F 1 Cl 1 Br 1 I 1 - oxide (ion) sulfide fluoride (ion) chloride (ion) bromide (ion) iodide (ion)

Practice • Name chemical formula • Chemical formula name
- Slides: 29