How Atoms Differ Properties of Subatomic Particles Particle

How Atoms Differ
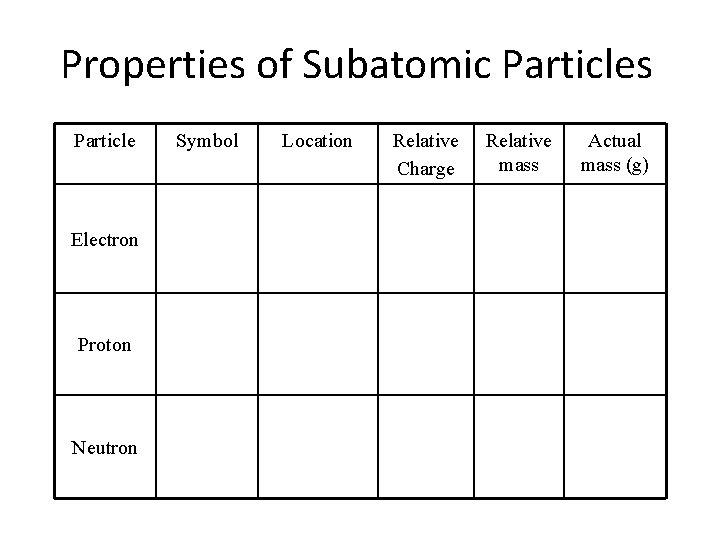
Properties of Subatomic Particles Particle Electron Proton Neutron Symbol Location Relative Charge Relative mass Actual mass (g)
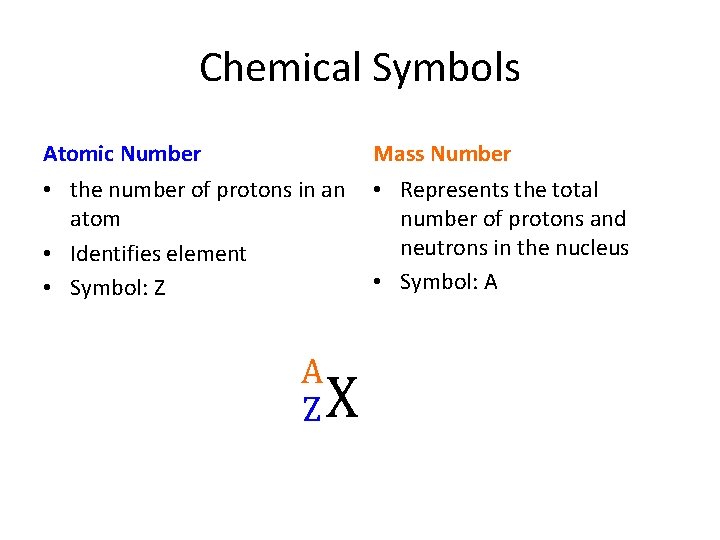
Chemical Symbols Atomic Number Mass Number • the number of protons in an atom • Identifies element • Symbol: Z • Represents the total number of protons and neutrons in the nucleus • Symbol: A A Z X
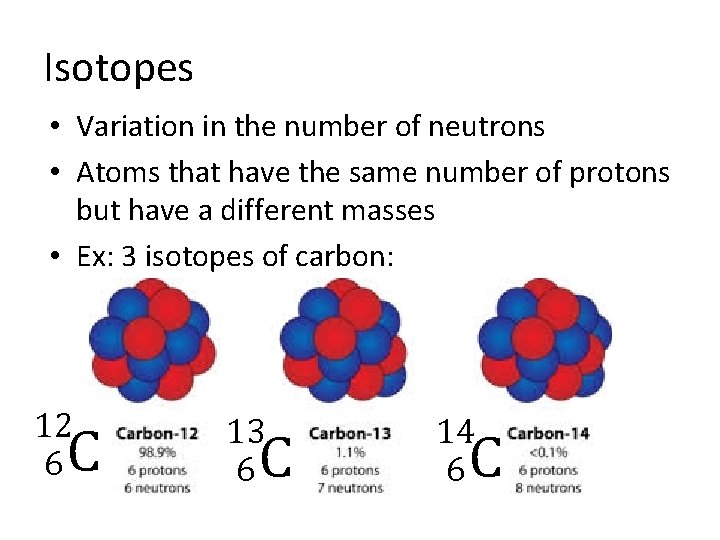
Isotopes • Variation in the number of neutrons • Atoms that have the same number of protons but have a different masses • Ex: 3 isotopes of carbon: 12 6 C 13 6 C 14 6 C
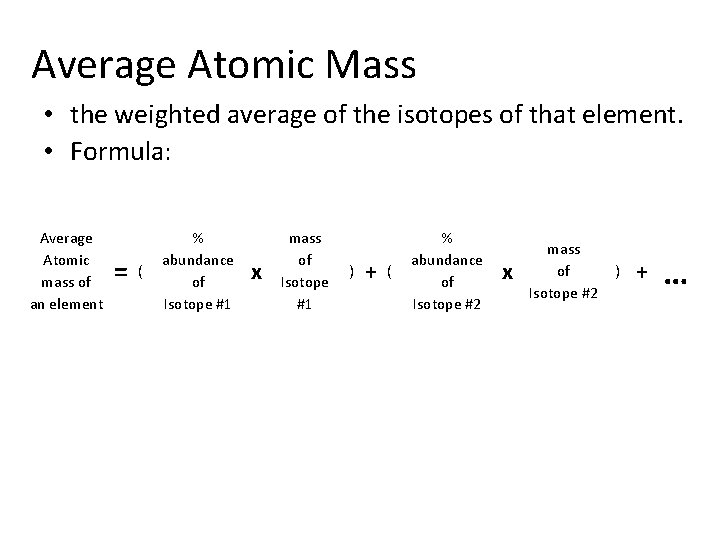
Average Atomic Mass • the weighted average of the isotopes of that element. • Formula: Average Atomic mass of an element = ( % abundance of Isotope #1 x mass of Isotope #1 ) + ( % abundance of Isotope #2 x mass of Isotope #2 ) + …

Example 1 Silver has two naturally occurring isotopes. Ag-107 has an abundance of 51. 82% and mass of 106. 9 amu. Ag-109 has a relative abundance of 48. 18% and a mass of 108. 9 amu. Calculate the average atomic mass of silver.

Example 2 Rubidium is a soft, silvery-white metal that has two 87 common isotopes, 85 Rb and Rb. 37 37 If the abundance of 85 Rb is 72. 2% and the abundance of 87 Rb is 27. 8%, what is the average atomic mass of rubidium?

Example 3 Boron has two naturally occurring isotopes. If the abundance of 11 B is 80. 10% with an amu of 11. 0093, find the abundance of 10 B.
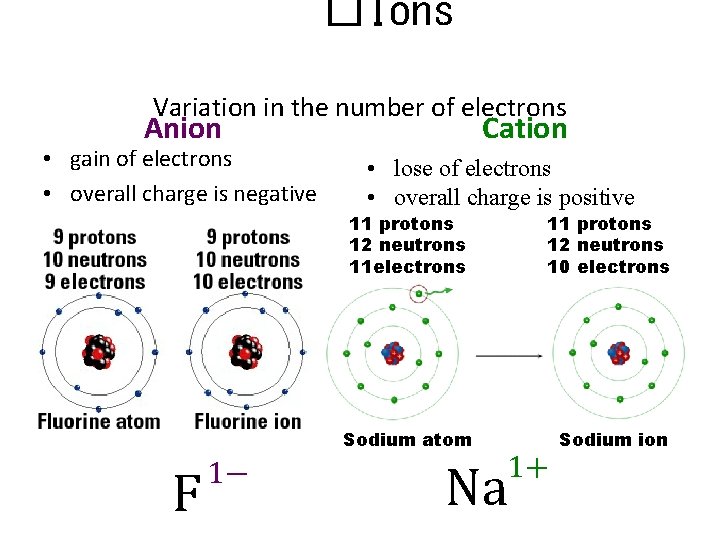
�Ions Variation in the number of electrons Anion • gain of electrons • overall charge is negative F 1− Cation • lose of electrons • overall charge is positive 11 protons 12 neutrons 11 electrons 11 protons 12 neutrons 10 electrons Sodium atom Sodium ion 1+ Na
- Slides: 9