How Atoms Differ a Properties of Subatomic Particles

How Atoms Differ

a. Properties of Subatomic Particles Particle Symbol Location e- outside the nucleus Proton p+ in the nucleus Neutron n 0 in the nucleus Electron Relative Charge Relative mass Actual mass (g) 1 1840 9. 10 x 10 -28 g +1 1 1. 673 x 10 -24 g 0 1 1. 675 x 10 -24 g -1

Elements on the Periodic Table

b. Atomic Number • the number of protons in an atom • Identifies element c. Mass Number • represents the total number of protons and neutrons in the nucleus Mass number atomic number A Z X

d. Isotopes • Atoms that have the same number of protons but have a different masses • Ex: 3 isotopes of carbon: 12 6 C 13 6 C 14 6 C
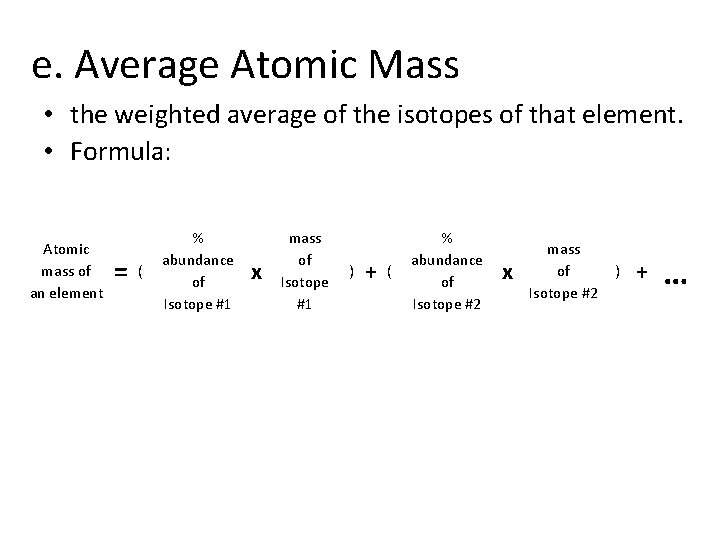
e. Average Atomic Mass • the weighted average of the isotopes of that element. • Formula: Atomic mass of an element = ( % abundance of Isotope #1 x mass of Isotope #1 ) + ( % abundance of Isotope #2 x mass of Isotope #2 ) + …

Average Atomic Mass • The mass of an atom is so small it is difficult to work with, so chemists have developed an atomic standard to compare all the masses • The standard is the atomic mass unit (amu) • If the mass of an element is not close to a whole number, it is because the atom has several isotopes • The atomic mass is the weighted average of the isotopes of that element

Example 1 Silver has two naturally occurring isotopes. Ag-107 has an abundance of 51. 82% and mass of 106. 9 amu. Ag-109 has a relative abundance of 48. 18% and a mass of 108. 9 amu. Calculate the average atomic mass of silver.

Example 1 • Silver has two naturally occurring isotopes. Ag -107 has an abundance of 51. 82% and mass of 106. 9 amu. Ag-109 has a relative abundance of 48. 18% and a mass of 108. 9 amu. Calculate the atomic mass of silver. . 5182(106. 9 amu) +. 4818(108. 9 amu) (remember to round at the end with more than one operation) = 107. 87 amu **Round to the hundredths for amu values

Example 2 Rubidium is a soft, silvery-white metal that has two 87 common isotopes, 85 Rb and Rb. 37 37 If the abundance of 85 Rb is 72. 2% with 84. 911794 amu and the abundance of 87 Rb is 27. 8% with 86. 909187 amu, what is the average atomic mass of rubidium?
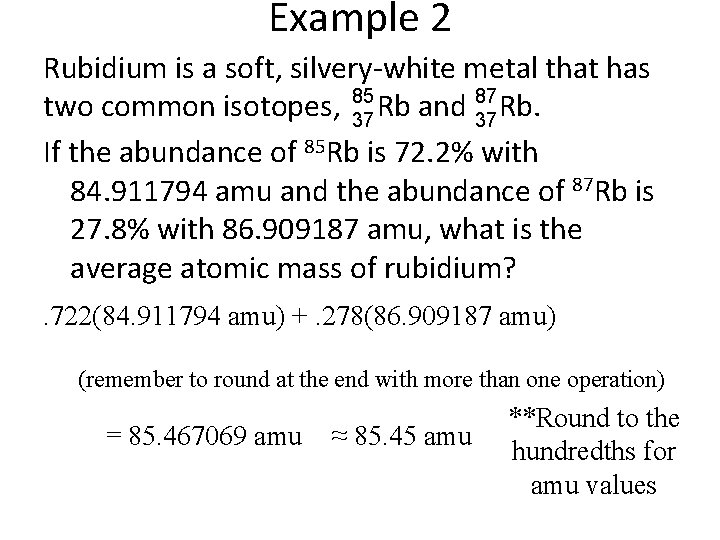
Example 2 Rubidium is a soft, silvery-white metal that has 85 87 two common isotopes, 37 Rb and 37 Rb. If the abundance of 85 Rb is 72. 2% with 84. 911794 amu and the abundance of 87 Rb is 27. 8% with 86. 909187 amu, what is the average atomic mass of rubidium? . 722(84. 911794 amu) +. 278(86. 909187 amu) (remember to round at the end with more than one operation) = 85. 467069 amu ≈ 85. 45 amu **Round to the hundredths for amu values

Honors Example 3 Boron has two naturally occurring isotopes. If the abundance of 11 B is 80. 10% with an amu of 11. 0093, find the abundance of 10 B.
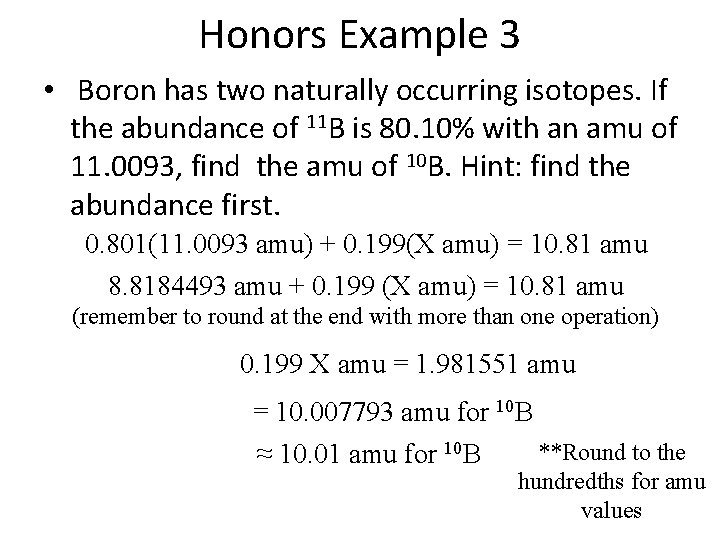
Honors Example 3 • Boron has two naturally occurring isotopes. If the abundance of 11 B is 80. 10% with an amu of 11. 0093, find the amu of 10 B. Hint: find the abundance first. 0. 801(11. 0093 amu) + 0. 199(X amu) = 10. 81 amu 8. 8184493 amu + 0. 199 (X amu) = 10. 81 amu (remember to round at the end with more than one operation) 0. 199 X amu = 1. 981551 amu = 10. 007793 amu for 10 B **Round to the ≈ 10. 01 amu for 10 B hundredths for amu values
- Slides: 13