Subatomic Particles And Isotopes Subatomic Particle Symbol Location
Subatomic Particles And Isotopes
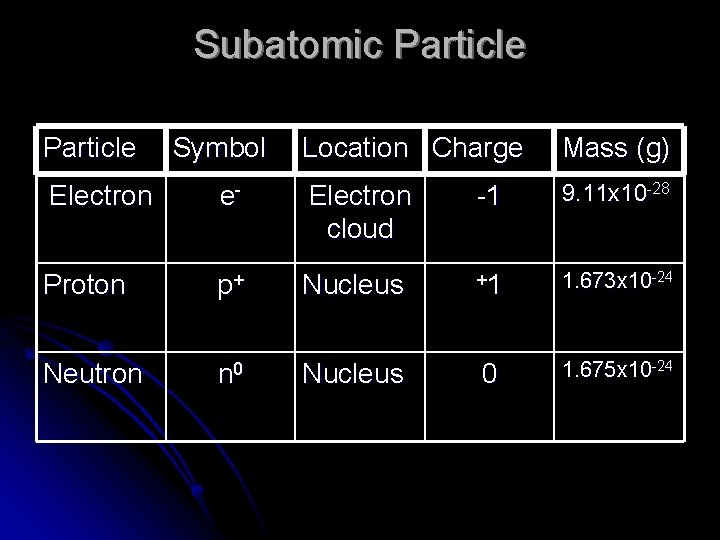
Subatomic Particle Symbol Location Charge Mass (g) -1 9. 11 x 10 -28 Electron e- Electron cloud Proton p+ Nucleus +1 1. 673 x 10 -24 Neutron n 0 Nucleus 0 1. 675 x 10 -24
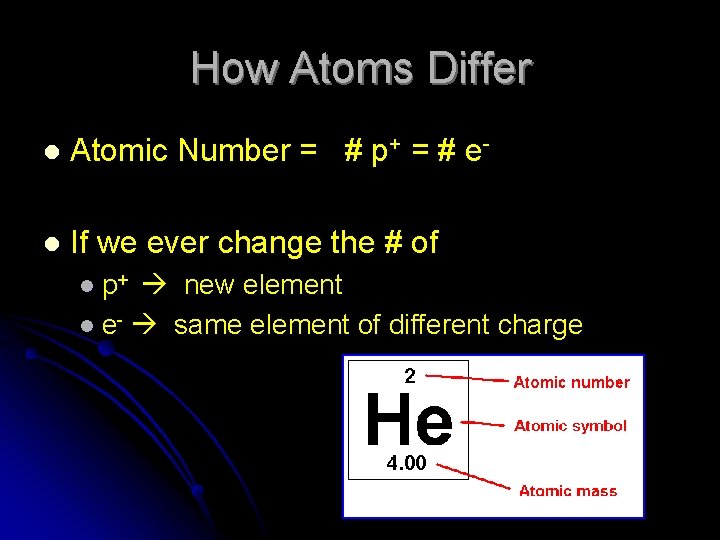
How Atoms Differ l Atomic Number = # p+ = # e- l If we ever change the # of l p+ new element l e- same element of different charge
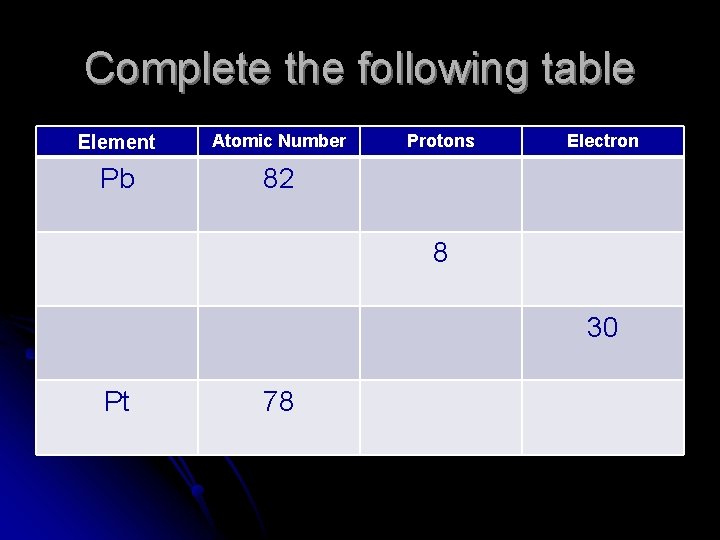
Complete the following table Element Atomic Number Pb 82 Protons Electron 8 30 Pt 78
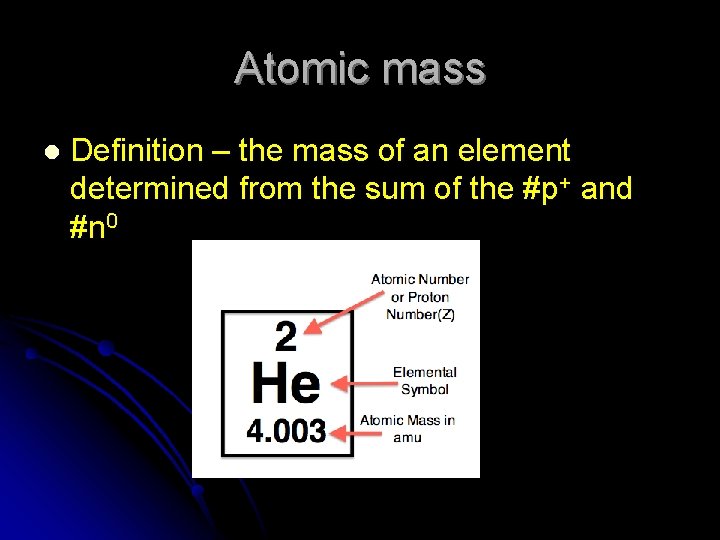
Atomic mass l Definition – the mass of an element determined from the sum of the #p+ and #n 0

Can Atomic mass Differ? l YES!!! l Isotope – same element of different mass l How? ? ? l Remember – if change # p+ then change element l What if change # n 0? different mass!!
Isotopes l 3 types of potassium l All have 19 protons cannot change these l 1 has 20 neutrons l 1 has 21 neutrons and l 1 has 22 neutrons l Atoms like these with the same number of protons but different number of neutrons are isotopes!
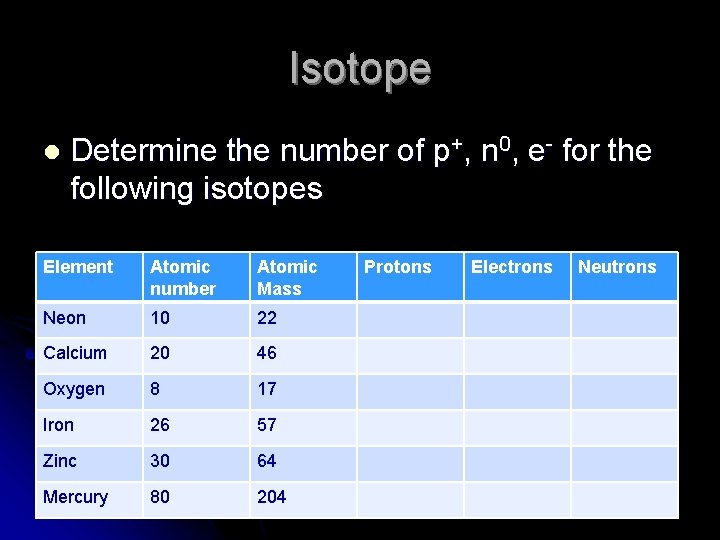
Isotope l Determine the number of p+, n 0, e- for the following isotopes Element Atomic number Atomic Mass Neon 10 22 Calcium 20 46 Oxygen 8 17 Iron 26 57 Zinc 30 64 Mercury 80 204 Protons Electrons Neutrons
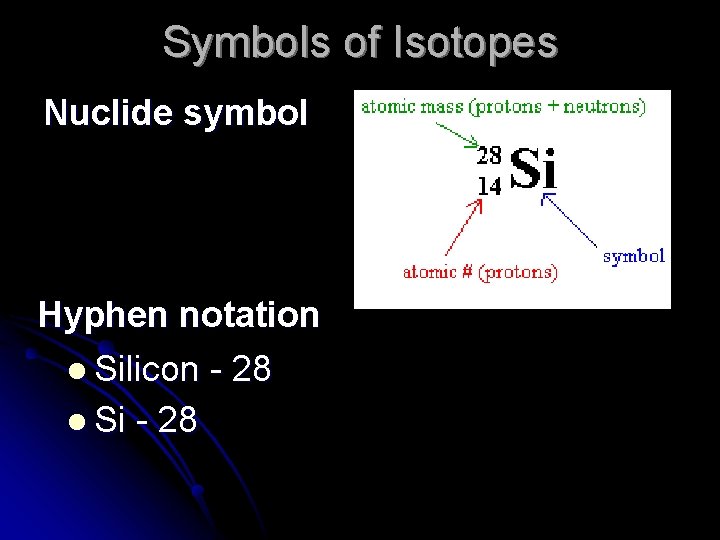
Symbols of Isotopes Nuclide symbol Hyphen notation l Silicon - 28 l Si - 28

Isotopes l Lets look back at potassium Bananas are a great source of potassium. l But which potassium does a banana have? l l All 3

Isotopes l Look at Potassium’s mass on the periodic table l 39. 098 l How amu do we have a mass on the PT of 39. 098?
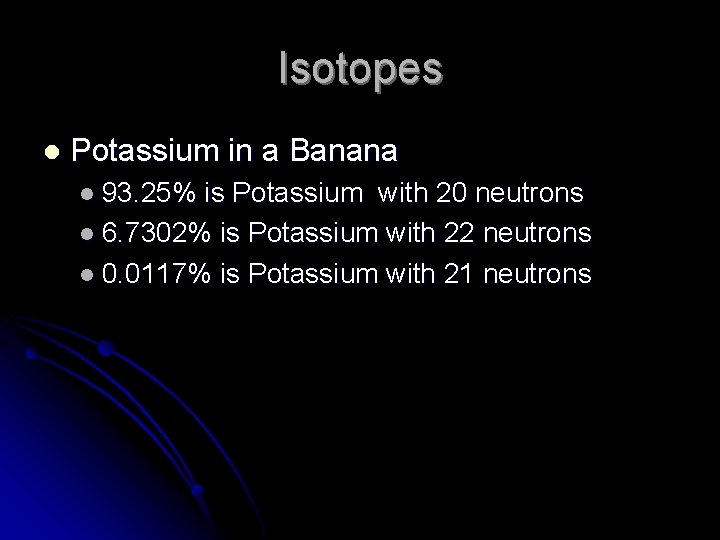
Isotopes l Potassium in a Banana l 93. 25% is Potassium with 20 neutrons l 6. 7302% is Potassium with 22 neutrons l 0. 0117% is Potassium with 21 neutrons

Average Atomic Mass l The mass on the periodic table is l Average atomic mass l What does this mean? l Average of all the masses and their abundance of the isotopes of that element.

Average atomic mass
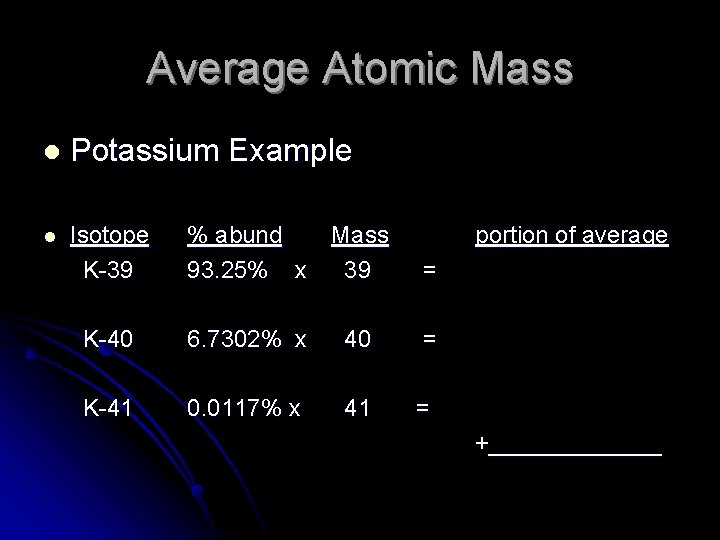
Average Atomic Mass l Potassium Example l Isotope K-39 % abund Mass 93. 25% x 39 portion of average = K-40 6. 7302% x 40 = K-41 0. 0117% x 41 = +_______

Average Atomic Example l From classwork:

Isotope Lab l Math used in this lab – example Let x = Pre 1982 pennies; let y = Post 1982 pennies
- Slides: 17