Classification of Matter States of Matter Solids Particles











- Slides: 24

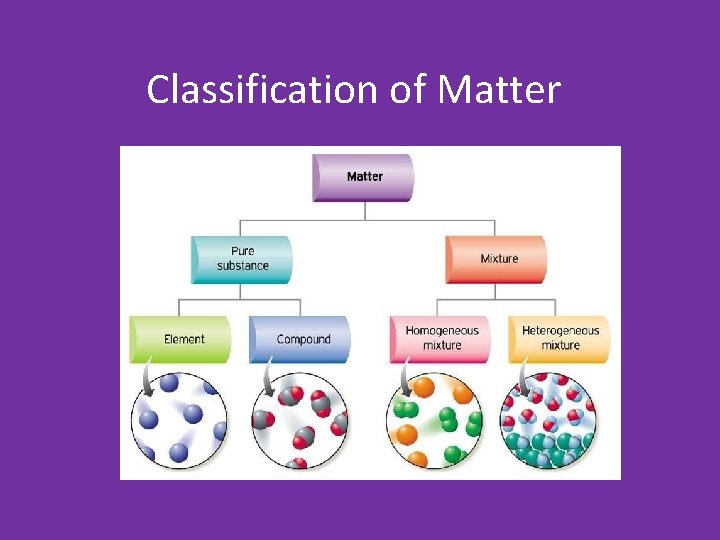
Classification of Matter

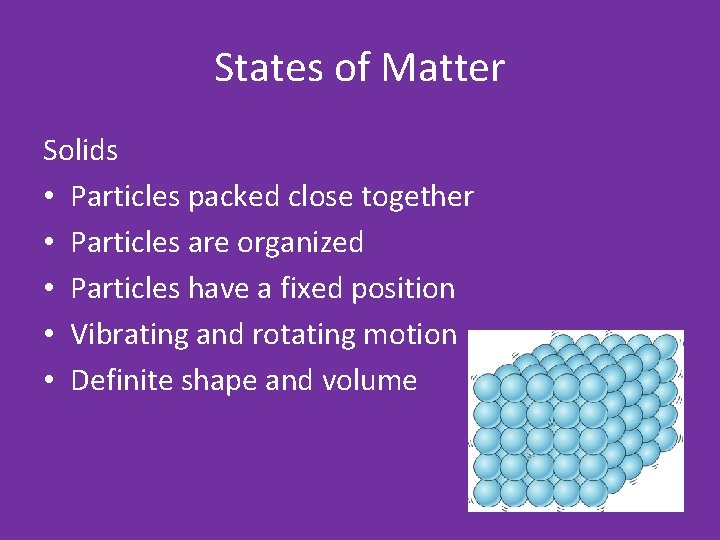
States of Matter Solids • Particles packed close together • Particles are organized • Particles have a fixed position • Vibrating and rotating motion • Definite shape and volume

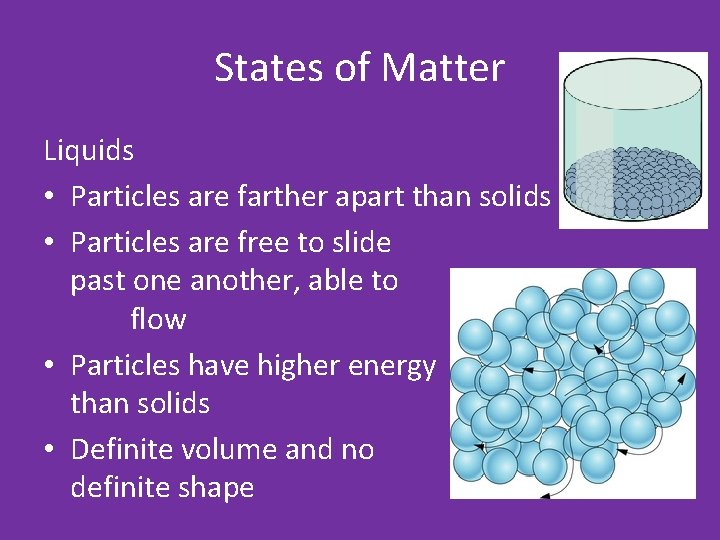
States of Matter Liquids • Particles are farther apart than solids • Particles are free to slide past one another, able to flow • Particles have higher energy than solids • Definite volume and no definite shape

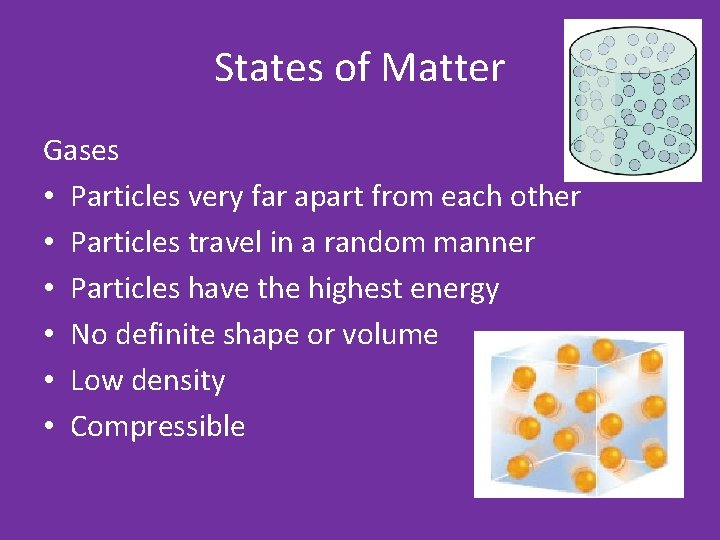
States of Matter Gases • Particles very far apart from each other • Particles travel in a random manner • Particles have the highest energy • No definite shape or volume • Low density • Compressible

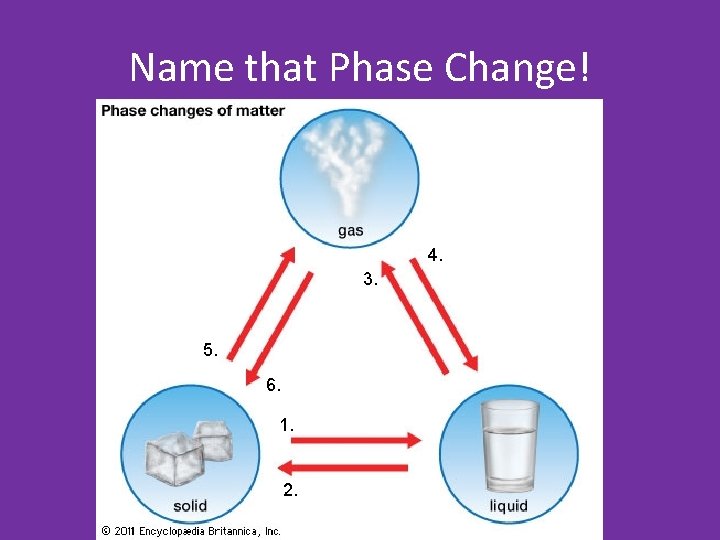
Name that Phase Change! 4. 3. 5. 6. 1. 2.

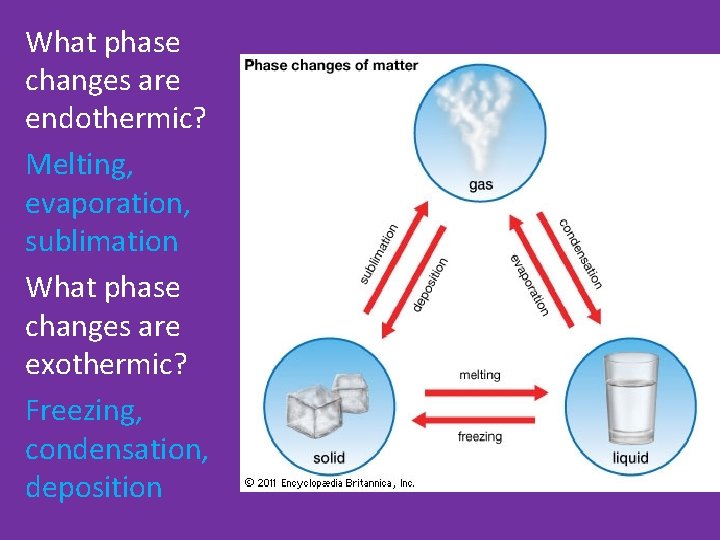
Phases Changes • Changes that absorb energy endothermic are called _______. • Changes that release energy exothermic are called _______.

What phase changes are endothermic? Melting, evaporation, sublimation What phase changes are exothermic? Freezing, condensation, deposition

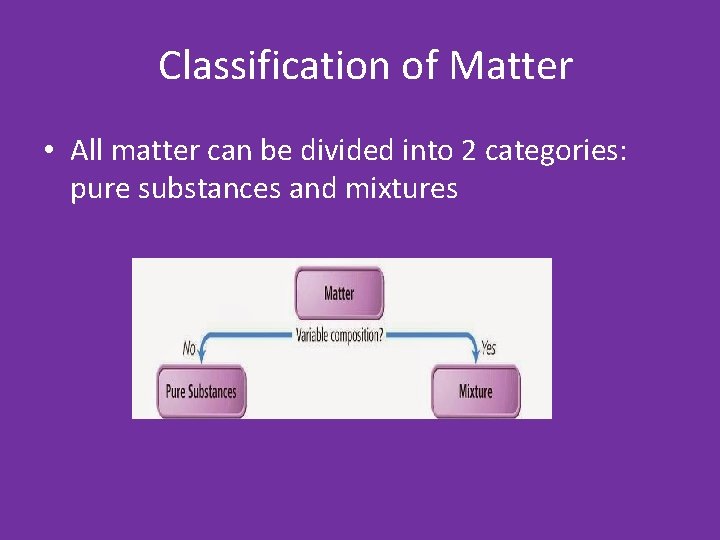
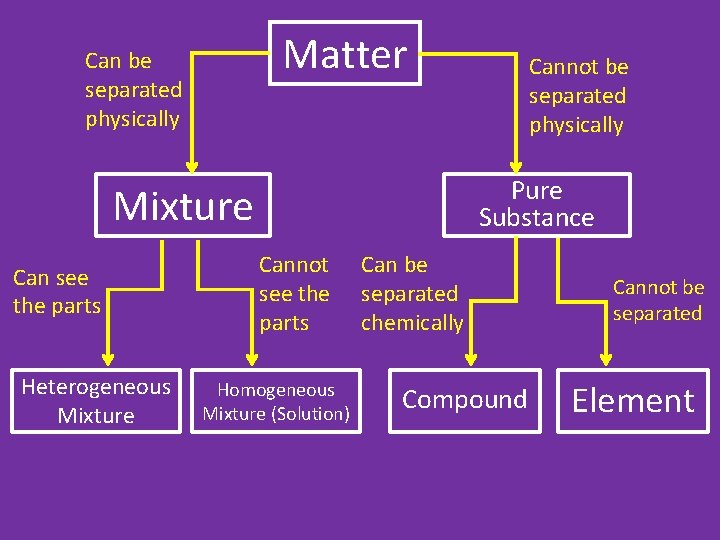
Classification of Matter • All matter can be divided into 2 categories: pure substances and mixtures

Classification of Matter • Pure Substance – matter that is made up of only one type of atom or molecule • Examples: Copper, water, salt, hydrogen

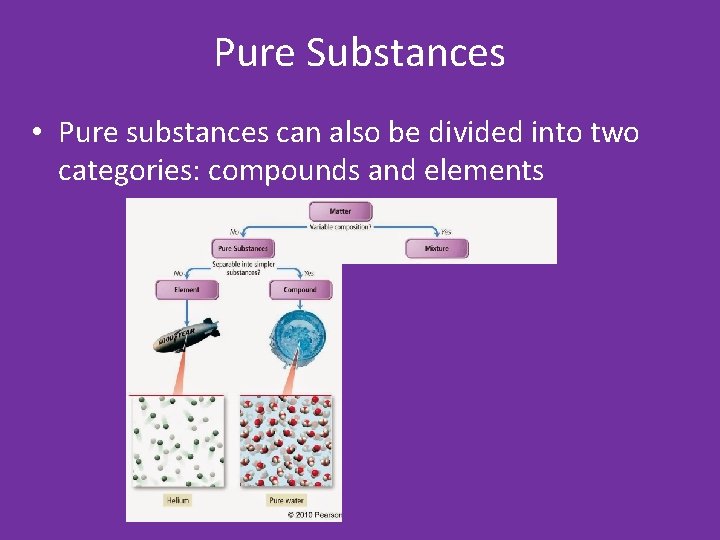
Pure Substances • Pure substances can also be divided into two categories: compounds and elements

Pure Substances • Compound – Two or more elements chemically bonded together • Examples: – Carbon dioxide (CO 2) – Water (H 2 O) – Salt (Na. Cl) – Sucrose (C 12 H 22 O 11)

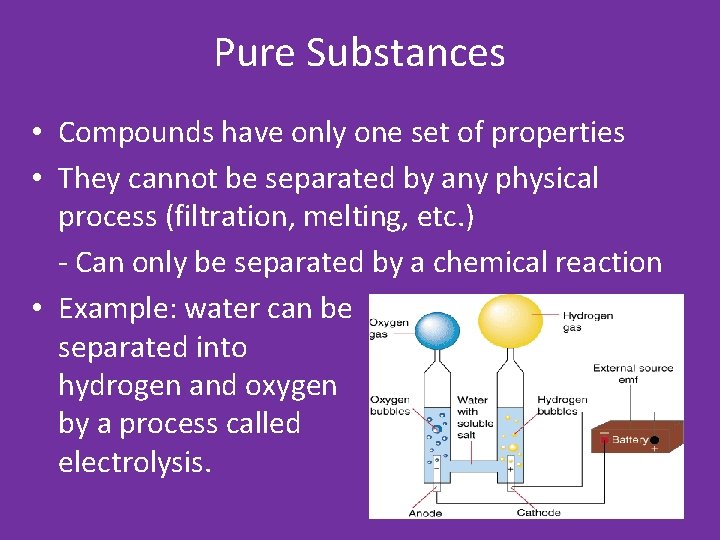
Pure Substances • Compounds have only one set of properties • They cannot be separated by any physical process (filtration, melting, etc. ) - Can only be separated by a chemical reaction • Example: water can be separated into hydrogen and oxygen by a process called electrolysis.

Pure Substances • Elements – Substances made up of only one type of atom - Cannot be separated by any physical OR chemical process • Examples: – Carbon – Helium – Gold

Mixtures • Mixture – Two or more pure substances mixed together – Each substance in the mixture retains its own set of chemical and physical properties. – Unlike pure substances, mixtures can always be separated by physical means.

Mixtures • Example: Copper and zinc can be mixed together to produce brass. • Even though it may look different, it is still copper and zinc. • How could we separate them apart? • Each metal retains its own properties like melting point.

Mixtures If a sample of sand contains iron and salt, how could you separate them from the other minerals?

Mixtures • Heterogeneous mixture – mixture that is not uniform (Hetero: different) - You can see the different parts – Examples: • Sand • Granite • Wood

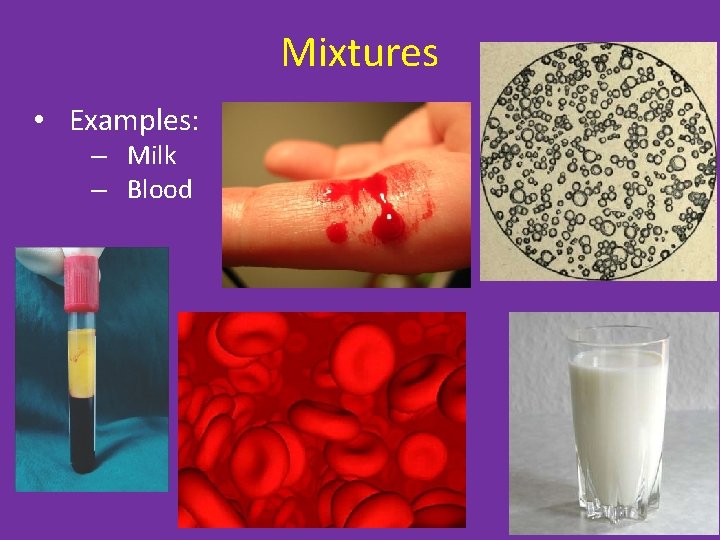
Mixtures • Examples: – Milk – Blood

Mixtures • Homogeneous Mixture (Solution) – mixture that is uniform (Homo: same) – Cannot see the parts – Example: Salt water contains salt and water, but it is uniform (homogeneous). • It can still be separated by physical means Seawater distillation plant

Separating Mixtures • Filtering • Can you filter a salt water solution?

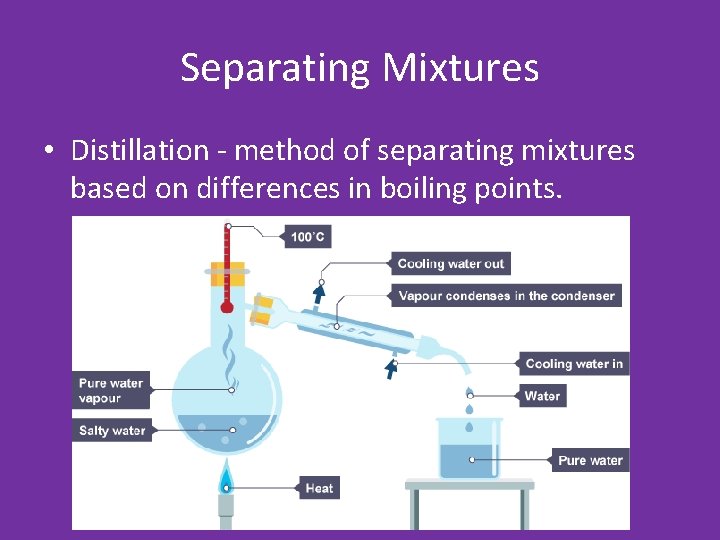
Separating Mixtures • Distillation - method of separating mixtures based on differences in boiling points.

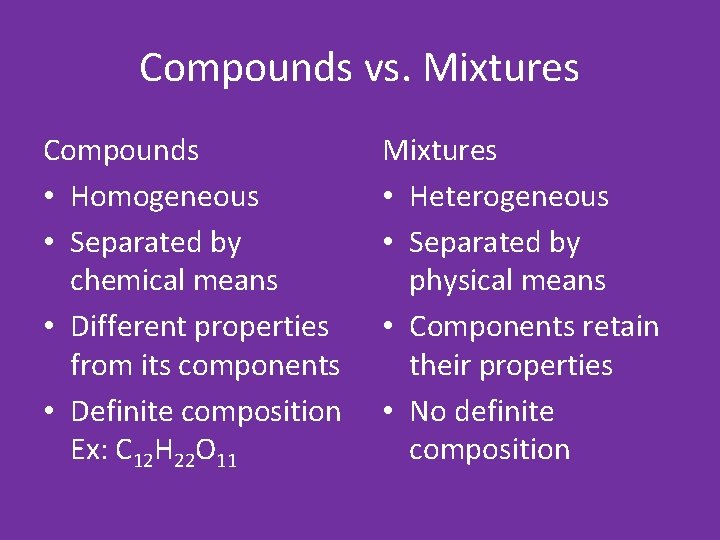
Compounds vs. Mixtures Compounds • Homogeneous • Separated by chemical means • Different properties from its components • Definite composition Ex: C 12 H 22 O 11 Mixtures • Heterogeneous • Separated by physical means • Components retain their properties • No definite composition

Matter Can be separated physically Pure Substance Mixture Can see the parts Heterogeneous Mixture Cannot be separated physically Cannot see the parts Homogeneous Mixture (Solution) Can be separated chemically Compound Cannot be separated Element

 Chapter 11 - states of matter: liquids and solids
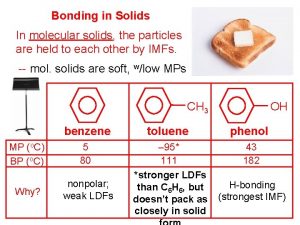
Chapter 11 - states of matter: liquids and solids The attraction between particles gives solids a definite
The attraction between particles gives solids a definite Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Postulate 1
Postulate 1 Section 1 composition of matter
Section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Section 1 composition of matter
Section 1 composition of matter An explanation of how particles in matter behave
An explanation of how particles in matter behave Building block of matter which contains subatomic particles
Building block of matter which contains subatomic particles What is mutual force
What is mutual force Classification of elementary particles
Classification of elementary particles Classification of solids
Classification of solids Slave states free states
Slave states free states Southern states of america
Southern states of america Tyranny
Tyranny Whats the study of matter and energy
Whats the study of matter and energy Four phases of matter
Four phases of matter Four states of matter
Four states of matter States of matter
States of matter Thermal energy states of matter
Thermal energy states of matter Changing states of matter
Changing states of matter Phet states of matter basics
Phet states of matter basics 5 states of matter
5 states of matter 3 states of matter venn diagram
3 states of matter venn diagram The kinetic theory of matter states that
The kinetic theory of matter states that