Tools for Pharmacovigilance and Cohort Event Monitoring Magnus
















































- Slides: 77

Tools for Pharmacovigilance and Cohort Event Monitoring Magnus Wallberg Senior Systems Architect M Sc Engineering Physics Dar Es Salaam November 26 th, 2009 magnus. wallberg@who-umc. org

Agenda • Where does Cohort Event Monitoring fit – Walk through of other pharmacovigilance methods • Spontaneous reporting – The “Vigis” - Vigi. Base, Vigi. Search/Vigi. Mine and Vigi. Flow • Analysis of longitudinal data (patient records) • Comparison, including CEM – Cohort Event Monitoring • Method • Tool requirements • Cem. Flow Magnus Wallberg, UMC

Pharmacovigilance methods Magnus Wallberg, UMC

Spontaneous reporting • The most common way of performing pharmacovigilance today • … Magnus Wallberg, UMC

Analysis of patient records • A project ongoing at the UMC to analyse longitudinal data (Clinical Insight) • Based on patient record data – Method developed on different but similar datasets – Can be adapted for more generalized datasets • Prototype already available in the UMC research and signal departments Magnus Wallberg, UMC

Cohort Event Monitoring • … Magnus Wallberg, UMC

Different focus (simplified) • Spontaneous reporting – Vigi. Search/Vigi. Mine/Vigi. Flow – Focus on ADRs • Patient records – Focus on patients • Cohort Event Monitoring – Cem. Flow – Focus on drugs – More about Cem. Flow soon… Magnus Wallberg, UMC

Different perspectives Magnus Wallberg, UMC

Cohort Event Monitoring Magnus Wallberg, UMC

Overall objective • Achieve maximum benefit, least harm, for patients Magnus Wallberg, UMC

How? • Monitor a specific medicine, substance or group of medicines by – Collecting: • All data – Events, patient details, concomitant medications, outcomes… • For “all” patients – In the Cohort – Analyze • To get risk profiles and other statistical data – Produce recommendations Magnus Wallberg, UMC

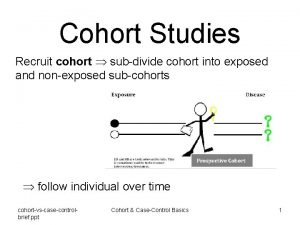
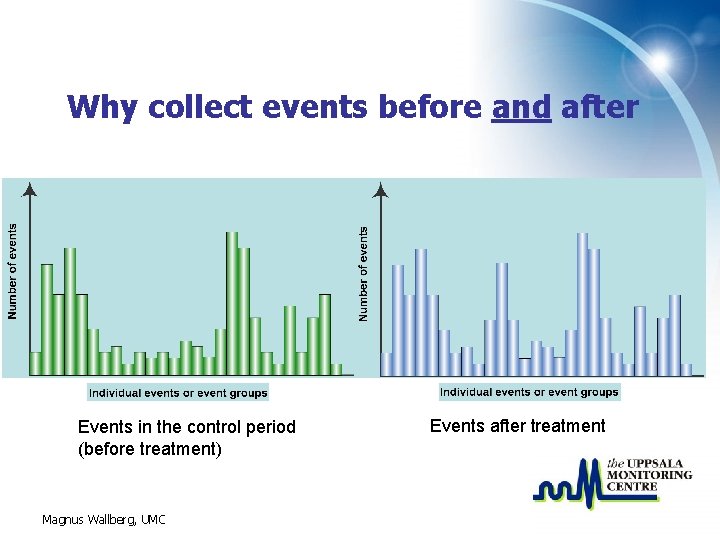
What is Cohort Event Monitoring CEM • In Cohort Event Monitoring (CEM) a group (cohort) of patients are monitored while treated with a specific medicine (or group of medicines). • All events in a control period before and during treatment shall be recorded. Magnus Wallberg, UMC

Why collect events before and after Events in the control period (before treatment) Magnus Wallberg, UMC Events after treatment

Why collect events before and after Magnus Wallberg, UMC

Objectives Should be fairly well known by now… but to summarize: • Characterise known reactions • Measure risk • Detect signals of unrecognised reactions • Detect Interactions • Identify risk factors like Age, Gender, Dose… • Assess safety in pregnancy & lactation • Detect inefficacy Magnus Wallberg, UMC

Stratification possibilities Magnus Wallberg, UMC

Selection of cohort • The cohort should be picked without biases among “all” patients being treated. – For example, all patients visiting the clinic on Tuesdays and Wednesdays that have been prescribed the monitored drug • All patients, falling into the rules of the cohort setup, must be enrolled (to avoid biases) • Continue the enrolment until the predefined size of the Cohort is reached Magnus Wallberg, UMC

This is a “cohort”… Magnus Wallberg, UMC

What to record • • All new Events even if common & minor Change in a pre-existing condition Abnormal changes in laboratory tests Accidents All deaths with date & cause Concomitant medications Concomitant diseases Lost to follow up!! Magnus Wallberg, UMC

Events = reactions + incidents • Reactions – definite – probable – possible • Incidents (background noise) – unlikely – Unclassified (conditional) Magnus Wallberg, UMC

A tool for CEM – web based IT support Magnus Wallberg, UMC

A tool for CEM – different focus • The focus of a CEM tool is different from a spontaneous reporting tool like Vigi. Flow – Patient, not report • More patient details – There is always at least one drug but usually not a reaction (however – many events) – There is more data to collect so the interface must be simple to use • Preferably more information in each chapter and fewer chapters than in Vigi. Flow Magnus Wallberg, UMC

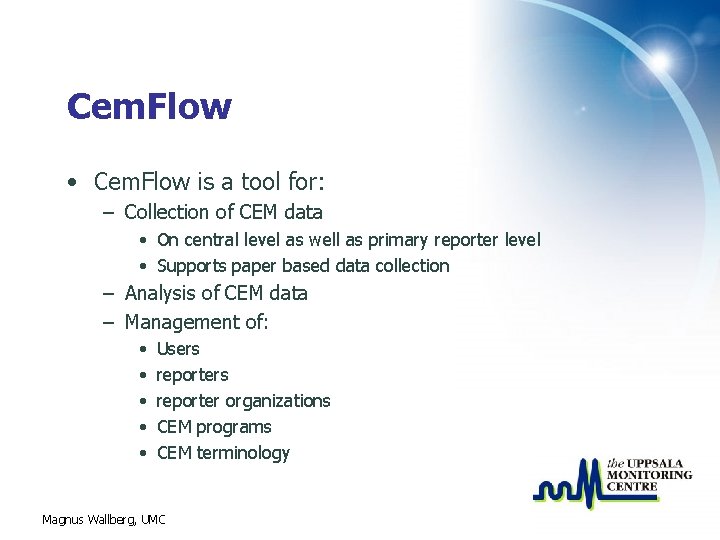
Cem. Flow • Cem. Flow is a tool for: – Collection of CEM data • On central level as well as primary reporter level • Supports paper based data collection – Analysis of CEM data – Management of: • • • Users reporter organizations CEM programs CEM terminology Magnus Wallberg, UMC

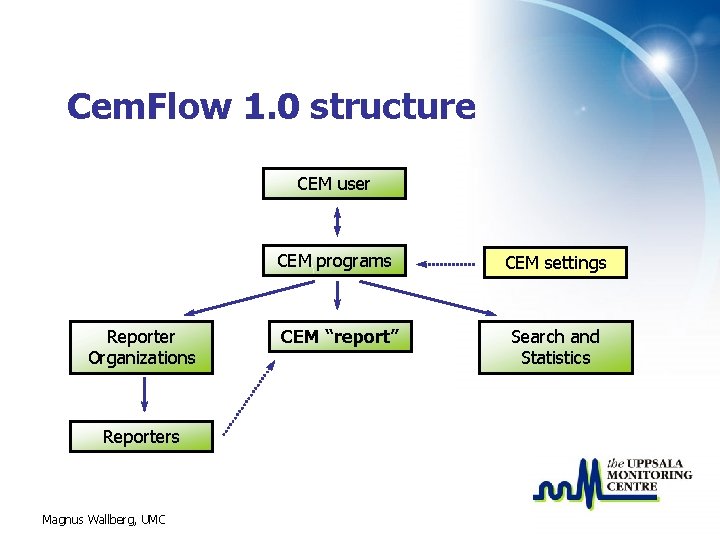
Cem. Flow 1. 0 structure CEM user CEM programs Reporter Organizations Reporters Magnus Wallberg, UMC CEM “report” CEM settings Search and Statistics

Reporter • A reporter is added to the system and referenced on the report via a reporter lookup tool • A reporter should belong to a reporter organization/clinic • A reporter can not log on to the Cem. Flow system – is not a Cem. Flow user Magnus Wallberg, UMC Shani Mwaluka – Mnazi Mmoja Health Centre, Dar es Salaam

Reporter organization • A reporter organization in CEM is for example a clinic/hospital where data for a CEM program is collected • A reporter “should” be connected to a reporter organization • A reporter organization belongs to a CEM program Magnus Wallberg, UMC

Search and Statistics • The Search and Statistics tool provides standard analysis tools and export functionality • Predefined filters and stratifications are available • Will need further research when more data is available Magnus Wallberg, UMC

Filters and stratifications • It is possible to stratify events based on – Sex – Age group – … and more will come • In addition – statistics will be available based on – Concomitant medications – Concomitant diseases – … Magnus Wallberg, UMC

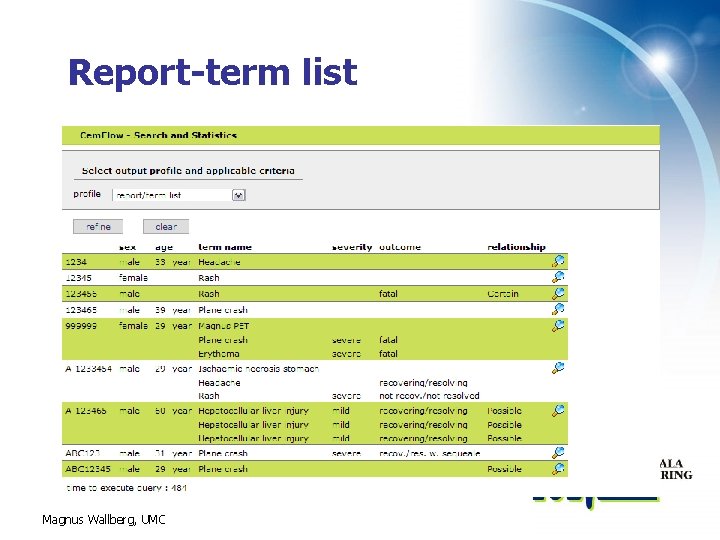
Search and Statistics – cont • Search results are currently presented as figures • In the near future statistics will also be: – Represented in graphs – Possible to export as Excel for local refinement Magnus Wallberg, UMC

Report-term list Magnus Wallberg, UMC

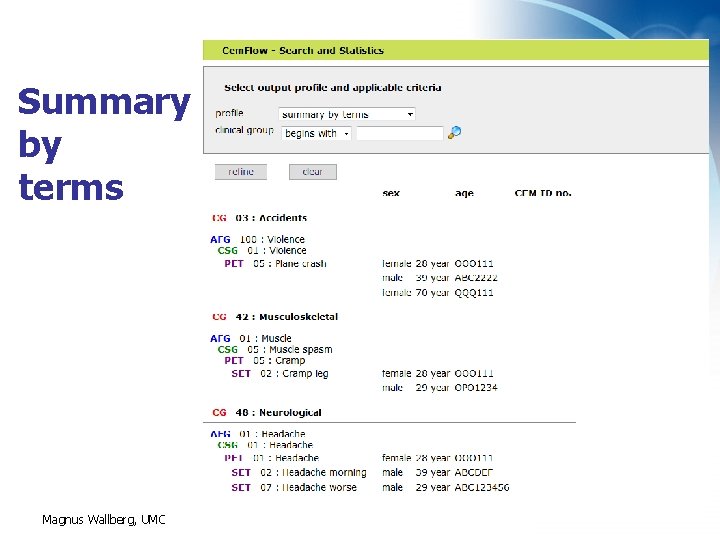
Summary by terms Magnus Wallberg, UMC

Stratification by gender Magnus Wallberg, UMC

Administrative statistics • A sub section of the Search and Statistics tool will provide administrative statistics like: – – Reporting per clinic and reporter Number of reports in the database Number of assessed un-assessed reports … Magnus Wallberg, UMC

CEM terminology Magnus Wallberg, UMC

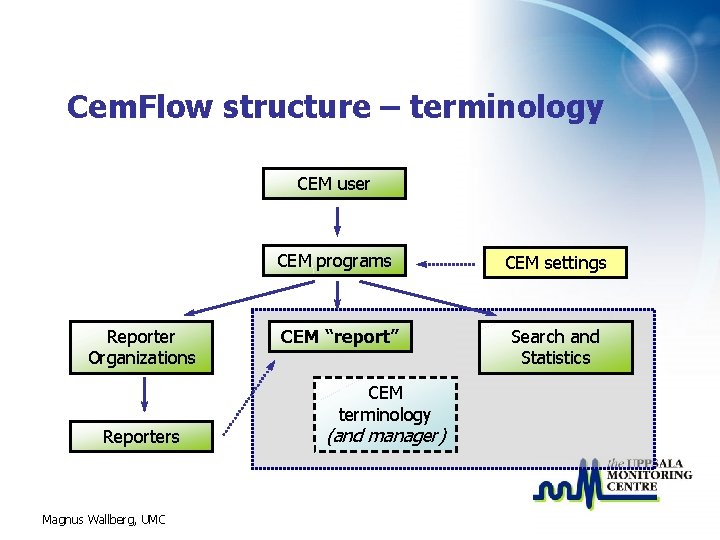
Cem. Flow structure – terminology CEM user CEM programs Reporter Organizations CEM “report” CEM terminology Reporters Magnus Wallberg, UMC (and manager) CEM settings Search and Statistics

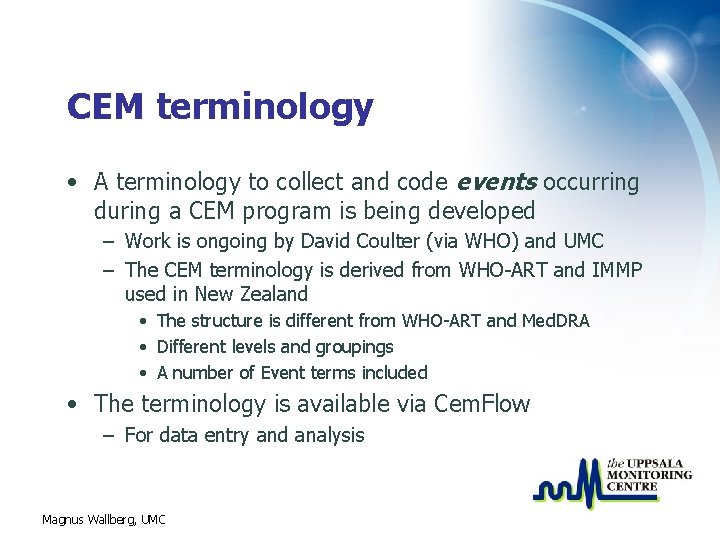
CEM terminology • A terminology to collect and code events occurring during a CEM program is being developed – Work is ongoing by David Coulter (via WHO) and UMC – The CEM terminology is derived from WHO-ART and IMMP used in New Zealand • The structure is different from WHO-ART and Med. DRA • Different levels and groupings • A number of Event terms included • The terminology is available via Cem. Flow – For data entry and analysis Magnus Wallberg, UMC

Why another terminology • One CEM terminology is needed so that different CEM programs can be compared • Other terminologies like WHO-ART and Med. DRA are reaction based – not event based – Many event terms needed can not be coded in WHO-ART or Med. DRA • Definitions needed • When running a CEM program in Africa many new event terms will be needed and efficiently administered – Requires a flexible terminology Magnus Wallberg, UMC

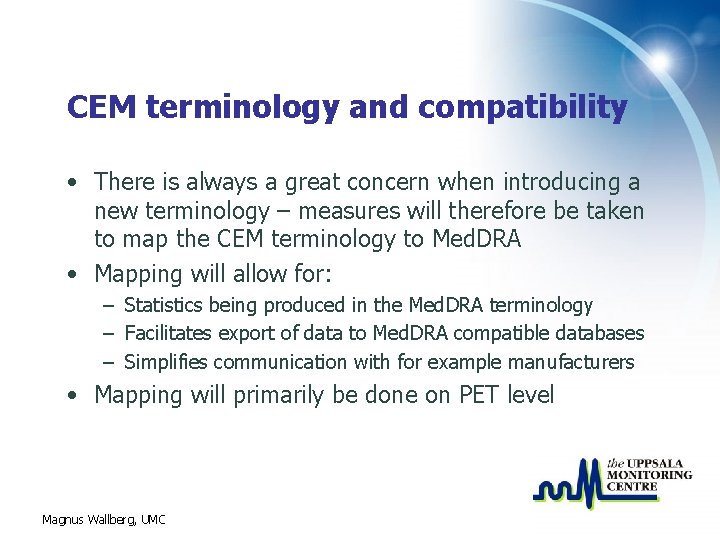
CEM terminology and compatibility • There is always a great concern when introducing a new terminology – measures will therefore be taken to map the CEM terminology to Med. DRA • Mapping will allow for: – Statistics being produced in the Med. DRA terminology – Facilitates export of data to Med. DRA compatible databases – Simplifies communication with for example manufacturers • Mapping will primarily be done on PET level Magnus Wallberg, UMC

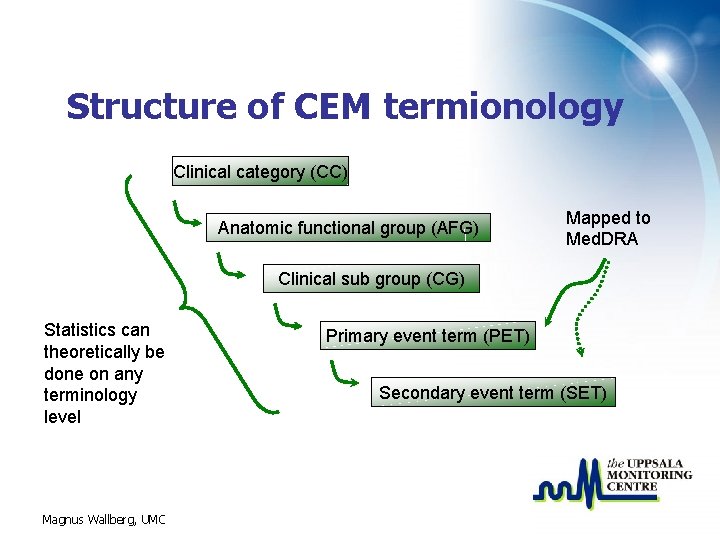
Structure of CEM termionology Clinical category (CC) Anatomic functional group (AFG) Mapped to Med. DRA Clinical sub group (CG) Statistics can theoretically be done on any terminology level Magnus Wallberg, UMC Primary event term (PET) Secondary event term (SET)

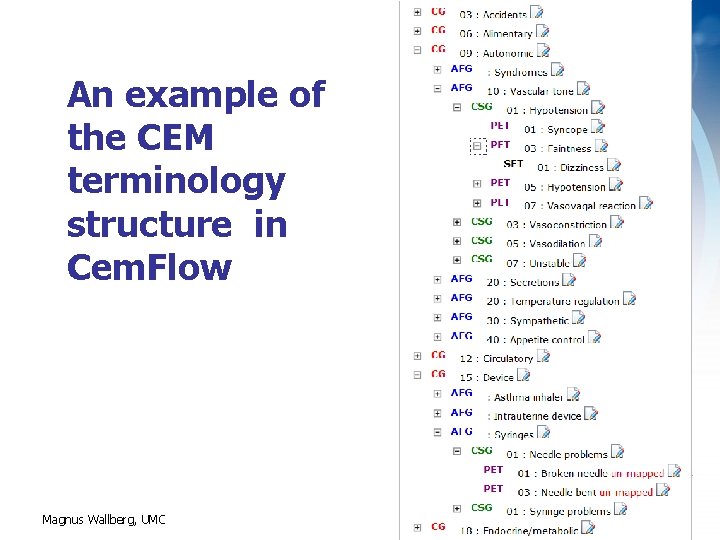
An example of the CEM terminology structure in Cem. Flow Magnus Wallberg, UMC

Why not use Med. DRA or WHO-ART • Another type of grouping is necessary – Different levels – One term can belong in different Clinical Cathegories • And where it is placed is important • Terms are ordered in a clinically meaningful way – Can highlight problem “areas” in a simple way • The number of terms are kept low for simplicity Magnus Wallberg, UMC

CEM terminology • The “same” term (same name) can appear in several “Clinical categories” – Therefore – when coding – the “correct” term must be selected • Each individual term can have a definition attached to simplify the selection process – Definitions will be continuously added and modified Magnus Wallberg, UMC

CEM terminology • The event will be entered as free text by the reporter and connected to a term in the events dictionary by an assessor or reviewer • Coding of the free text events is crucial for the statistical methods to work • It is important that events are coded in “the same way” by all assessors/reviewers Magnus Wallberg, UMC

Terminology manager • To allow for easy maintenance and flexibility of the CEM terminology a terminology manager is available within Cem. Flow – Available for users with special access – Allows for: • • Restructuring of available terms Addition of new terms Mapping of terms to Med. DRA Editing of definitions Magnus Wallberg, UMC

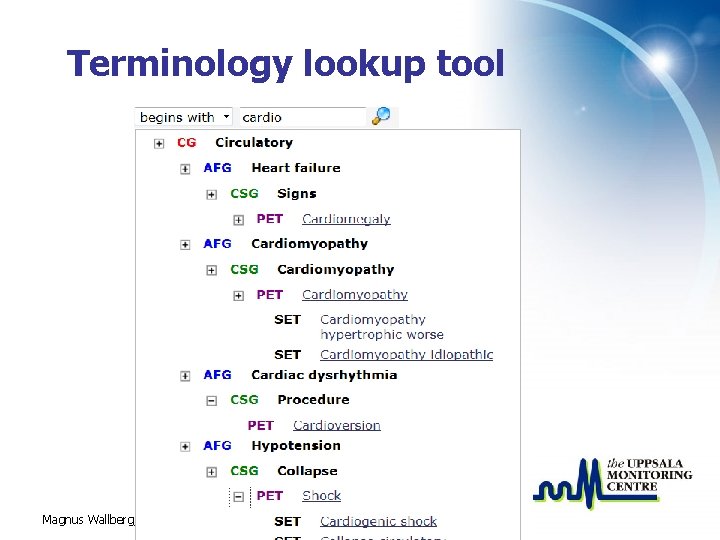
Terminology lookup tool Magnus Wallberg, UMC

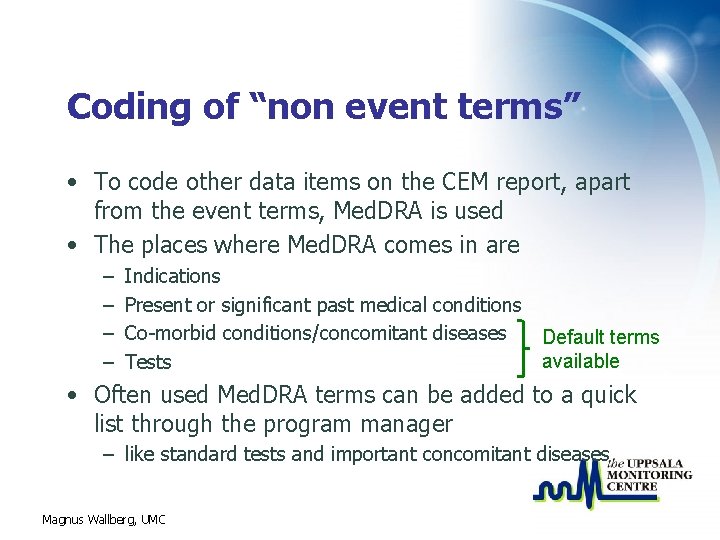
Coding of “non event terms” • To code other data items on the CEM report, apart from the event terms, Med. DRA is used • The places where Med. DRA comes in are – – Indications Present or significant past medical conditions Co-morbid conditions/concomitant diseases Default terms available Tests • Often used Med. DRA terms can be added to a quick list through the program manager – like standard tests and important concomitant diseases Magnus Wallberg, UMC

Hands on Cem. Flow Magnus Wallberg, UMC

Hands on • Introduction and start up of the hands on section. . . Magnus Wallberg, UMC

Magnus Wallberg, UMC

CEM program • A CEM program is the main “entity” of the Cem. Flow tool. – Cem. Flow supports many CEM programs in parallel – All “reports” and reporters belong to a specific program – Search and Statistics are made on reports for a specific program • However, reports from other programs may be used as comparator/baseline data Magnus Wallberg, UMC

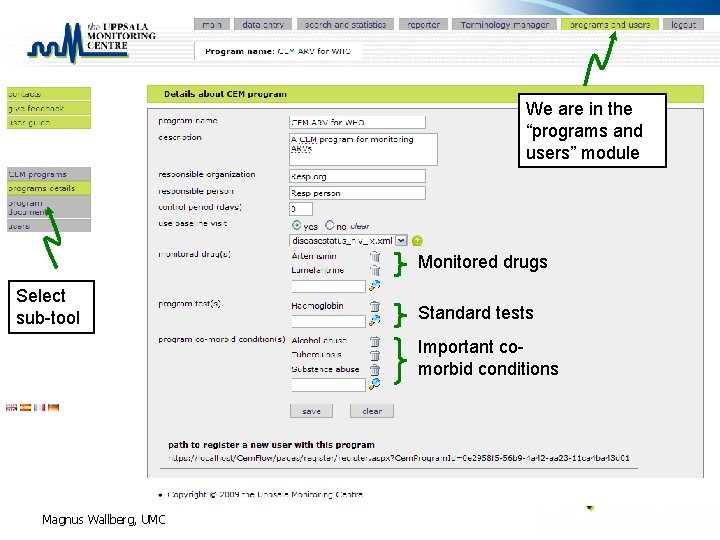
CEM program settings • A CEM program has: – – Organization (“owner” and contact person) Description Documents (like SOPs, Questionnaires and manuals) Settings • • Program drug(s) Definition of control period Predefined laboratory tests Set up of visits – use of base line visit – multiple follow ups • … Magnus Wallberg, UMC

We are in the “programs and users” module Monitored drugs Select sub-tool Standard tests Important comorbid conditions Magnus Wallberg, UMC

Hands on • I will log on to Cem. Flow and set up a new CEM program for this session Magnus Wallberg, UMC

User • The “users” of the Cem. Flow system register themselves and are assigned the access to a CEM program by an administrator. • The users can be: – Assessors at the head organization – Data entry staff – Reporters at regional sites • A user can have access to any number of CEM programs Magnus Wallberg, UMC

Magnus Wallberg, UMC

Hands on • Go to https: //tools. who-umc. org/cemflow • Register with your e-mail and password – Set your country to Andorra • So that you will be easy to find! • When you are registered: – Tell me and I will give you proper access! Magnus Wallberg, UMC

CEM “report” • A CEM “report” is the Cem. Flow equivalent to the CEM questionnaires – All questionnaires collected in one CEM report • Baseline, Pre, Post, Pregnancy and Pregnancy outcome questionnaires • The equivalent to an individual questionnaire is entered as a “visit” with the events as the most important information items (except for baseline visits) • CEM reports are managed through the Data Entry module of Cem. Flow Magnus Wallberg, UMC

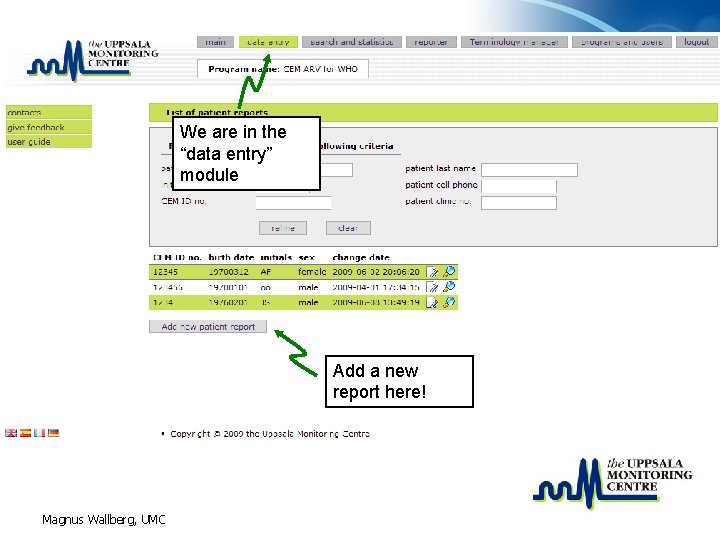
List of CEM reports • To be able to access old reports a report list with a filter is the first view in the data entry area • There are several reasons to open “old reports” – Adding additional information (about for example a follow up visit) – Doing an assessment – Viewing a specific report – … Magnus Wallberg, UMC

We are in the “data entry” module Add a new report here! Magnus Wallberg, UMC

Magnus Wallberg, UMC

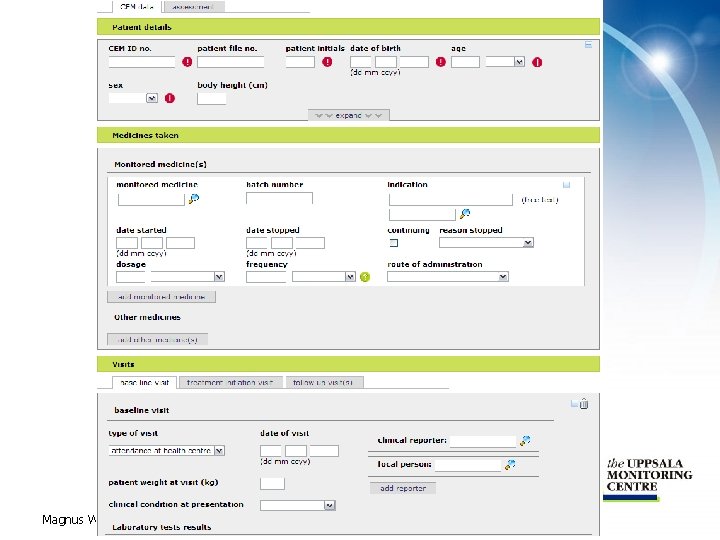
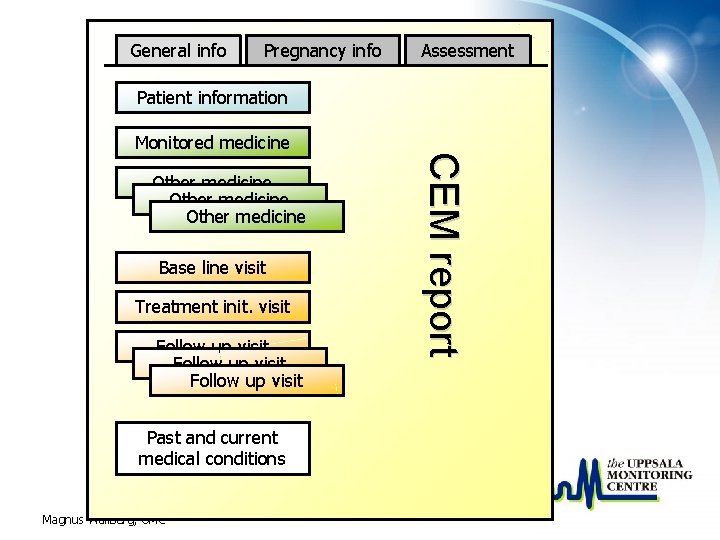
General info Pregnancy info Assessment Patient information Other medicine Base line visit Treatment init. visit Follow up visit Past and current medical conditions Magnus Wallberg, UMC CEM report Monitored medicine

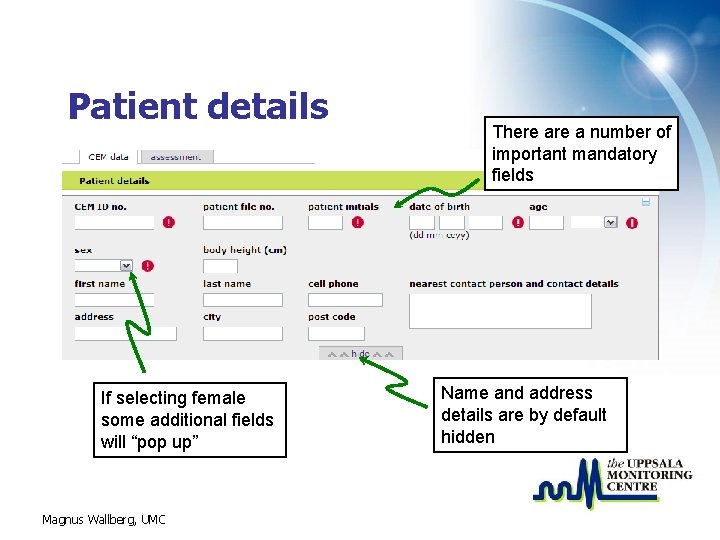
Patient details If selecting female some additional fields will “pop up” Magnus Wallberg, UMC There a number of important mandatory fields Name and address details are by default hidden

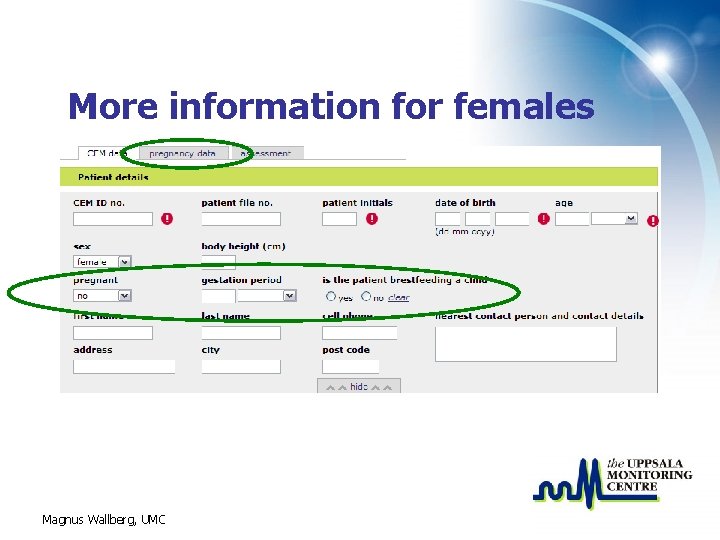
More information for females Magnus Wallberg, UMC

Hands on • Create a new ”CEM report” • Add patient information – The patient shall be a female – Note tha edditional fields for a female patient • Add drugs with details to the drug list – One monitored drug – One other drug • Collapse the entered drugs with the ”-”-sign Magnus Wallberg, UMC

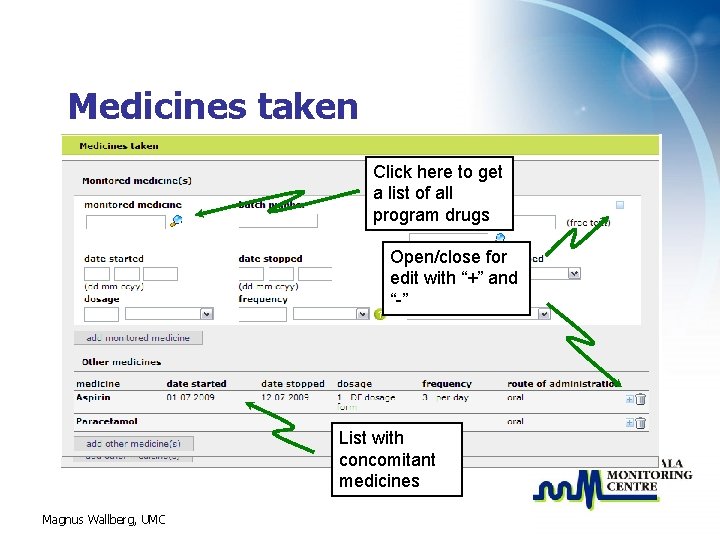
Medicines taken Click here to get a list of all program drugs Open/close for edit with “+” and “-” List with concomitant medicines Magnus Wallberg, UMC

Visits • There are three types of visits – Base line visit – only one • Only used if “Use baseline visit” is ticked in the program administrator – Treatment initiation visit – only one – Follow up visit – more than one can be added • The visits are grouped in tabs – Base line visit tab, treatment initiation visit tab and follow up visit(s) tab • The most important is the follow up visit – shown on next slide Magnus Wallberg, UMC

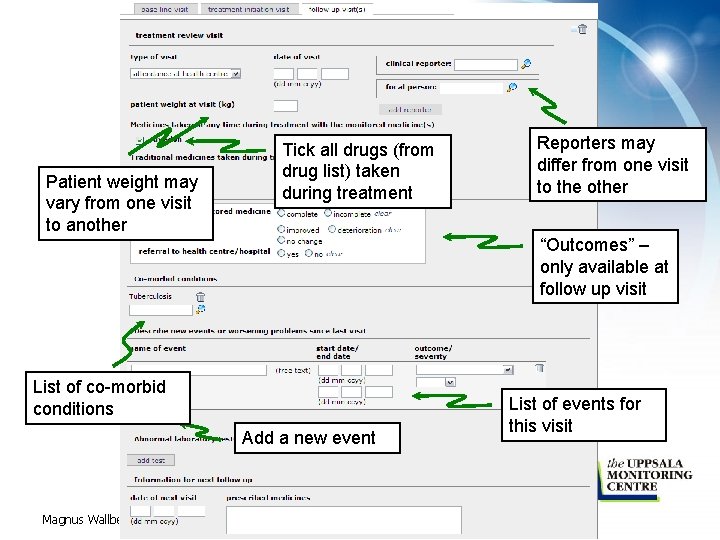
Patient weight may vary from one visit to another Tick all drugs (from drug list) taken during treatment Reporters may differ from one visit to the other “Outcomes” – only available at follow up visit List of co-morbid conditions Add a new event Magnus Wallberg, UMC List of events for this visit

Hands on • Add one base line visit with data • Add one treatment initiation visit with data – Add at least one test • Add one follow up visit – Add at least two events Magnus Wallberg, UMC

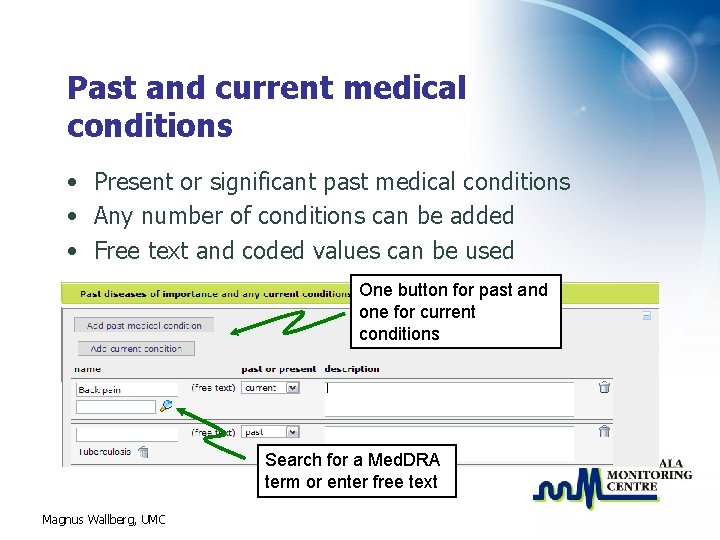
Past and current medical conditions • Present or significant past medical conditions • Any number of conditions can be added • Free text and coded values can be used One button for past and one for current conditions Search for a Med. DRA term or enter free text Magnus Wallberg, UMC

Hands on • Add one past and one current medical condition Magnus Wallberg, UMC

What is happening now and onward Magnus Wallberg, UMC

Current CEM activities • Two CEM programs are currently running – Tanzania • • Piloting of questionnaires and method CEM launch 17 th of Mars 2009 Adjustments done as result of lessons learnt from pilot The second phase has been initiated in Dar Es Salaam Magnus Wallberg, UMC

Magnus Wallberg, UMC

Tanzania Magnus Wallberg, UMC

Current CEM activities • Two CEM programs are currently running – Tanzania • • Piloting of questionnaires and method CEM launch 17 th of Mars 2009 Adjustments done as result of lessons learnt from pilot The second phase has been initiated in Dar Es Salaam – Nigeria • A first pilot have been run with approximately 3000 patients • A scale up is coming up Magnus Wallberg, UMC

Magnus Wallberg, UMC

WHO Collaborating Centre for International Drug Monitoring Box 1051, SE - 751 40 Uppsala Sweden Tel +46 18 65 60 60, Fax +46 18 65 60 88 E-mail: info@who-umc. org Website: www. who-umc. org
 Cem in pharmacovigilance
Cem in pharmacovigilance Cohort event monitoring
Cohort event monitoring Retrospective cohort study
Retrospective cohort study Pregnancy and infant cohort monitoring and evaluation
Pregnancy and infant cohort monitoring and evaluation Jamasoft
Jamasoft Pharmacovigilance quality assurance
Pharmacovigilance quality assurance Mru medication authority
Mru medication authority Aims of pharmacovigilance
Aims of pharmacovigilance Pvnet pharmacovigilance
Pvnet pharmacovigilance Cem stands for in pharmacovigilance
Cem stands for in pharmacovigilance Netherlands pharmacovigilance centre lareb
Netherlands pharmacovigilance centre lareb Solicited reports in pharmacovigilance
Solicited reports in pharmacovigilance Principles of pharmacovigilance
Principles of pharmacovigilance Objectives of pharmacovigilance
Objectives of pharmacovigilance International pharmacovigilance centre
International pharmacovigilance centre Pharmacovigilance signal detection methods
Pharmacovigilance signal detection methods Pharmacovigilance compliance
Pharmacovigilance compliance Application of pharmacovigilance in zambia
Application of pharmacovigilance in zambia Indegene pharmacovigilance
Indegene pharmacovigilance Kålbrok
Kålbrok Compound probability examples
Compound probability examples Longitudinal prospective study
Longitudinal prospective study Difference between case control and cohort study
Difference between case control and cohort study Difference between monitoring and evaluation
Difference between monitoring and evaluation Near miss analysis
Near miss analysis Independent event vs dependent event
Independent event vs dependent event Independent event vs dependent event
Independent event vs dependent event Describe table of contents
Describe table of contents Peta konsep news item
Peta konsep news item Newsworthy event (s) background event (s) sources
Newsworthy event (s) background event (s) sources Gastrocnemius muscle origin and insertion
Gastrocnemius muscle origin and insertion Cohort study example
Cohort study example Cohort study
Cohort study Case series
Case series Puldow cross
Puldow cross Retrospective cohort vs case series
Retrospective cohort vs case series Retrospective cohort study vs case control
Retrospective cohort study vs case control Quasi experimental study
Quasi experimental study Types of cohort studies
Types of cohort studies Retrospective cohort study
Retrospective cohort study Cohort model
Cohort model Summary lean startup
Summary lean startup Demographic cohort
Demographic cohort Cohort effects definition
Cohort effects definition Cohort model
Cohort model Cohort study community medicine
Cohort study community medicine Bias in cohort studies
Bias in cohort studies Cohort effects definition
Cohort effects definition Cohort effects definition
Cohort effects definition Golden cohort
Golden cohort Students cohort
Students cohort Cohort adalah
Cohort adalah Cohort study definition
Cohort study definition Cohort based courses
Cohort based courses Icarus first cohort
Icarus first cohort Kritik jurnal
Kritik jurnal Bgp monitoring software
Bgp monitoring software Siebel performance monitoring tools
Siebel performance monitoring tools Cisco ucs traffic monitoring
Cisco ucs traffic monitoring Openstack monitoring tools
Openstack monitoring tools Open systems nsm network security monitoring
Open systems nsm network security monitoring Traditional media monitoring tools
Traditional media monitoring tools Contract monitoring tools
Contract monitoring tools Continuous auditing continuous monitoring
Continuous auditing continuous monitoring Negative pressure room
Negative pressure room Acme en telesistole
Acme en telesistole Magnus lindgren tryggare sverige
Magnus lindgren tryggare sverige Magnus grøsfjeld
Magnus grøsfjeld Thy leg
Thy leg Principles of bobath approach
Principles of bobath approach Magnus hedin biometria
Magnus hedin biometria Deerus deafus
Deerus deafus Mds klassifikation
Mds klassifikation Magnus effect equation
Magnus effect equation Comparativo di bonus
Comparativo di bonus Adductor magnus nerve supply
Adductor magnus nerve supply Adductor hiatus
Adductor hiatus Magnus eriksson miun
Magnus eriksson miun