Pharmacovigilance Programme Of India Dr Reshmi TR Pharmacovigilance




















- Slides: 23

Pharmacovigilance Programme Of India Dr. Reshmi. TR

Pharmacovigilance Pharmakon Drug, Vigilare to be observant Continuous monitoring for unwanted effects of marketed drugs • WHO definition • Science and activities relating to Detection, Assessment, Understanding , Prevention of adverse effects and any other drug related problems

History • 10 th century Salerno Medical School • “whosoever shall sell any noxious drug shall be hanged” 18 th century William Withering 1 st systematic paper in medicinal drug with detailed description of ADR associated with Digitalis

20 th century Thalidomide disaster

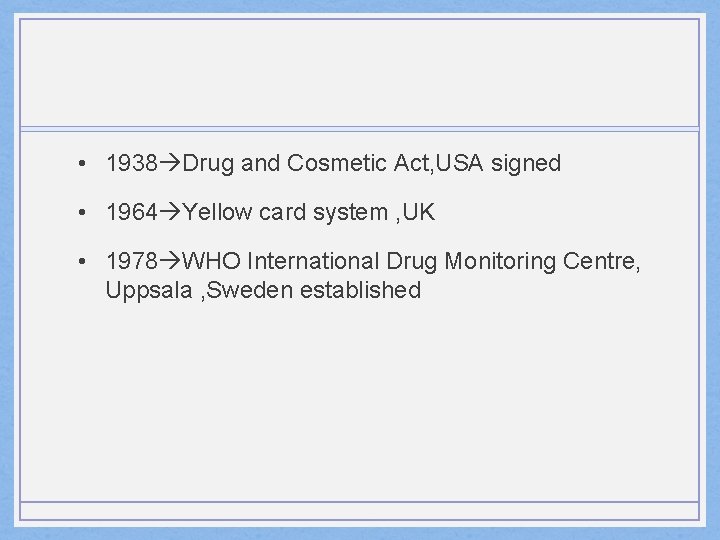
• 1938 Drug and Cosmetic Act, USA signed • 1964 Yellow card system , UK • 1978 WHO International Drug Monitoring Centre, Uppsala , Sweden established

WHO-UMC • Suggested guidelines for setting up Pharmacovigilance centre in every country • Uppsala Monitoring System , Sweden manages international drug monitoring

PVPI • Pharmacovigilance programme of India • July, 2010 at AIIMS, Delhi • April, 2011 shifted to IPC, Ghaziabad • Forward data to WHO-UMC through software Vigiflow

Mission Ensure that benefit outweigh risk

Objective • To create nation wide system for safety reporting • Analyse risk benefit ratio • Promote rational use of medicines

Goal • Expansion of Pharmacovigilance programme to all healthcare institutions • Develop reporting culture

Activities involved in Pharmacovigilance • Post marketing surveillance • Periodic Safety Update Report • During the first 4 years of marketing a new drug the manufacturer has to submit PSUR every 6 months for first 2 years and then once annually for the next 2 years • Dissemination of ADR data drug alert • Changes in labelling of medicines statutary warning /withdrawal of drug

Reporting Øwww. ipc. gov. in Øpvpi. ipcindia@gmail. com Ø 1800 -180 -3024 (9 am to 5. 30 pm)

Types of Reporting • Active Surveillance • Passive Surveillance • Suspected adverse drug reaction reporting form



Causality assessment • Structured and standardized assessment of ADRs as reported through ICSR (Individual Case Safety Report ) for the likelihood of involvement of suspected drug in causing particular event in a given patient.

• Causality is assessed based on: • Temporal relationship Time sequence of event relation to drug administration • Previous knowledge Whether drug produced the event in earlier cases • Dechallenge: Event subsided on stopping the drug • Rechallenge: Event reappeared on readminstration of the drug


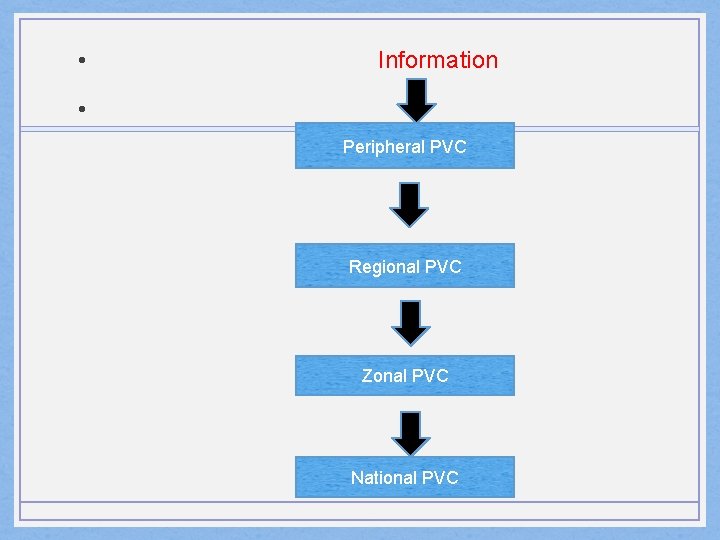
• Information • Peripheral PVC Regional PVC Zonal PVC National PVC


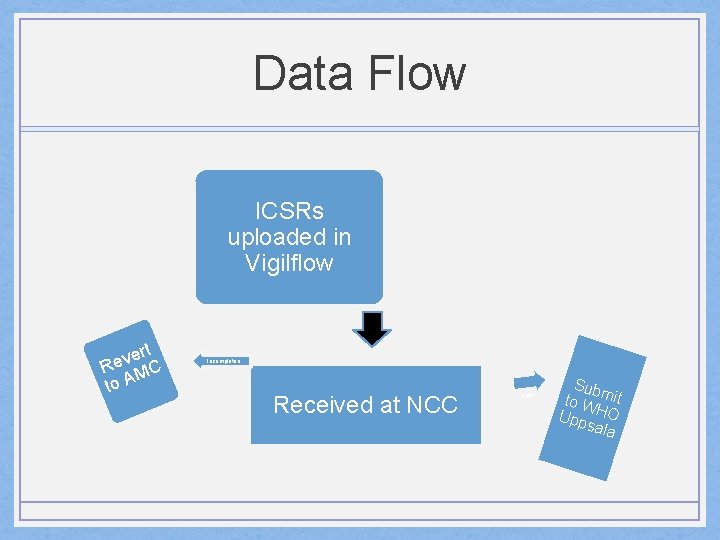
Data Flow ICSRs uploaded in Vigilflow Incompletee Received at NCC valid rt e v Re MC to A Sub to W mit Upp HO sala

Utilisation of Data • Risk management • Drug regulation • Education

 Dr reshmi nath
Dr reshmi nath National programmes
National programmes Rch phase 1
Rch phase 1 Pharmacovigilance definition
Pharmacovigilance definition Mru medication authority
Mru medication authority Indegene pharmacovigilance
Indegene pharmacovigilance Netherlands pharmacovigilance centre lareb
Netherlands pharmacovigilance centre lareb Cem in pharmacovigilance
Cem in pharmacovigilance Pharmacovigilance signal detection methods
Pharmacovigilance signal detection methods International pharmacovigilance centre
International pharmacovigilance centre Define pharmacovigilance
Define pharmacovigilance Pharmacovigilance kursus
Pharmacovigilance kursus Solicited reports in pharmacovigilance
Solicited reports in pharmacovigilance Aims of pharmacovigilance
Aims of pharmacovigilance Pharmacovigilance compliance
Pharmacovigilance compliance Pvnet pharmacovigilance
Pvnet pharmacovigilance Principles of pharmacovigilance
Principles of pharmacovigilance Pharmacovigilance quality assurance
Pharmacovigilance quality assurance Application of pharmacovigilance in zambia
Application of pharmacovigilance in zambia Cem stands for in pharmacovigilance
Cem stands for in pharmacovigilance Who programme for international drug monitoring
Who programme for international drug monitoring Programme approach
Programme approach Study programme example
Study programme example Programme relations publiques
Programme relations publiques