Pharmacovigilance in Uganda PHARMACOVIGILANCE TRAINING DARESSALAAM 22 ND















- Slides: 18

Pharmacovigilance in Uganda PHARMACOVIGILANCE TRAINING DAR-ES-SALAAM 22 ND NOVEMBER 2009 2/26/2021 NATIONAL DRUG AUTHORITY - UGANDA 1

Presentation outline q National Pharmacovigilance centre and capacity – Objectives – Progress – Challenges – Future plans q PV in Public health programs q Most commonly used ARVs q Problems of top concern q Training 2 NATIONAL DRUG AUTHORITY - UGANDA 26 -2 -2021

UGANDA – THE PEARL OF AFRICA 2/26/2021 NATIONAL DRUG AUTHORITY - UGANDA

National Pharmacovigilance Center (NPC) q. Overseen by the Head, Drug Information Department of National Drug Authority q. Guided by the Pharmacovigilance committee of the Board q. Composed of 8 personnel. 4 NATIONAL DRUG AUTHORITY - UGANDA 26 -2 -2021

NPC – 2 q. Was established in 2005 q. Passive reporting system q. The 83 rd member country to the WHO Collaborating Centre on International Drug Monitoring. 5 NATIONAL DRUG AUTHORITY - UGANDA 26 -2 -2021

Objectives of the NPC q. To coordinate Pharmacovigilance activities in the country – collect, analyze and evaluate Adverse Drug Reaction reports from Regional Centers and the field on human and veterinary drugs. q. To collaborate with Uppsala Monitoring Centre. q. Promote exchange of drug information with Drug Information Centers within and outside the country. 6 NATIONAL DRUG AUTHORITY - UGANDA 26 -2 -2021

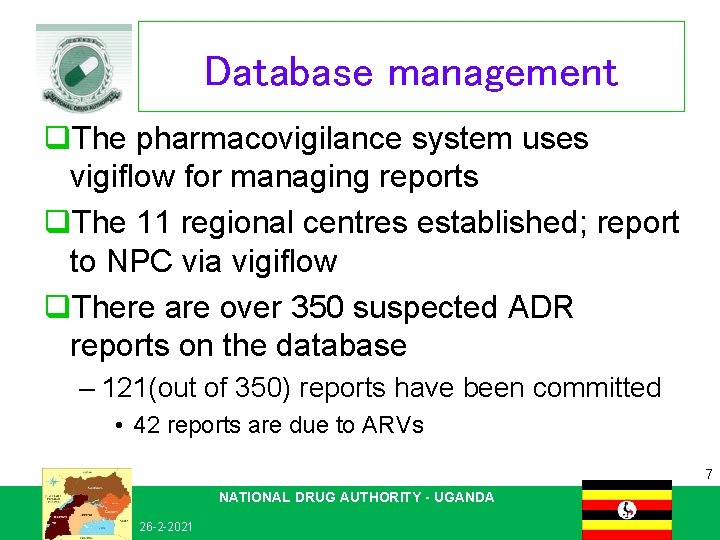
Database management q. The pharmacovigilance system uses vigiflow for managing reports q. The 11 regional centres established; report to NPC via vigiflow q. There are over 350 suspected ADR reports on the database – 121(out of 350) reports have been committed • 42 reports are due to ARVs 7 NATIONAL DRUG AUTHORITY - UGANDA 26 -2 -2021

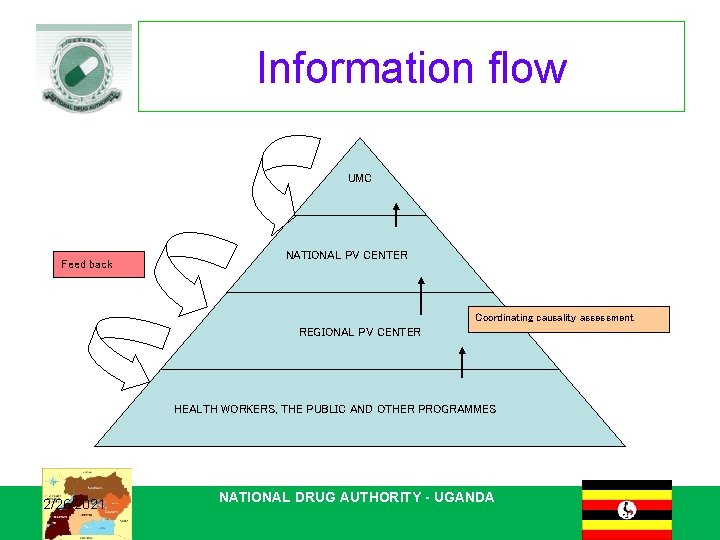
Information flow UMC Feed back NATIONAL PV CENTER Coordinating causality assessment REGIONAL PV CENTER HEALTH WORKERS, THE PUBLIC AND OTHER PROGRAMMES 2/26/2021 NATIONAL DRUG AUTHORITY - UGANDA

PV in Public health programs q. There is some involvement of personnel in the national public health programs q. Some Treatment Programs do monitor adverse events – Plans to collaborate with these programs are under way q. The NPC has incorporated a session on safety monitoring on ARVs in the training of trainers’ sessions 2/26/2021 NATIONAL DRUG AUTHORITY - UGANDA

The suspected ADR report form 2/26/2021 NATIONAL DRUG AUTHORITY - UGANDA

Challenges q There is still low awareness on ADR reporting which calls for more sensitization q Wrong perception of PV - i. e as a program (with financial emoluments) not a practice! q Human resource – PV activities are done alongside other regulatory and routine activities q Functionality of the established centers is compromised by low motivation 11 NATIONAL DRUG AUTHORITY - UGANDA 2/26/2021 26 -2 -2021

Future plans … 1 q Continue with sensitisation (with emphasis to key stakeholders) q Start CEM as a means of post market surveillance especially for diseases of public health concern q Work with NGOs and health consumers advocacy groups in the community q Orientation of NPC staff at a fully functional Pharmacovigilance centre 12 NATIONAL DRUG AUTHORITY - UGANDA 26 -2 -2021

Most commonly used ARVs q. Stavudine q. Lamivudine q. Nevirapine q. Efavirenz q. Zidovudine q. Kaletra 2/26/2021 NATIONAL DRUG AUTHORITY - UGANDA

Most commonly used ARVs-2 q. Abacavir q. Didanosine q. Tenofovir q. Atazanasvir q. Emtricitabine 2/26/2021 NATIONAL DRUG AUTHORITY - UGANDA

ARV toxicity problems of top concern q. Steven Johnson reaction to NVP and cotrimoxazole q. NVP related Hepatotoxicity q. Anaemia with AZT q. Peripheral neuropathy d 4 T 2/26/2021 NATIONAL DRUG AUTHORITY - UGANDA

Trained professionals q. The course material used in training is on general pharmacovigilance q 330 health professionals are trained (Over 1000 sensitized) 2/26/2021 NATIONAL DRUG AUTHORITY - UGANDA

ACKNOWLEDGMENT q Ministry of Health q World Health Organisation – UMC, WHO Uganda, WHO AFRO/HQ • For technical support • For financial support, especially in enabling NDA to participate in this training – Other national centres • Participation and Quick response to our queries on Vigimed network q United States Agency for International Development • PEPFAR, PMI for partnering with NDA Pharmacovigilance and quality control activities 17 NATIONAL DRUG AUTHORITY - UGANDA 26 -2 -2021

2/26/2021 NATIONAL DRUG AUTHORITY - UGANDA
 Uganda aids rate
Uganda aids rate Islamic microfinance in uganda
Islamic microfinance in uganda 551555
551555 Sdf uganda
Sdf uganda Tyler godoff
Tyler godoff Burmese tiger pit
Burmese tiger pit Malaria in uganda facts
Malaria in uganda facts Kajmansi o. vs canada
Kajmansi o. vs canada Psa meaning in accounting
Psa meaning in accounting Ministry of east african community affairs uganda
Ministry of east african community affairs uganda Examples of class c drugs in uganda
Examples of class c drugs in uganda Uganda cooperative transport union limited
Uganda cooperative transport union limited African tea uganda
African tea uganda Community policing in uganda
Community policing in uganda Features of marine insurance
Features of marine insurance Challenges of budget implementation in uganda
Challenges of budget implementation in uganda Tea growing in uganda
Tea growing in uganda Sdgca
Sdgca Nche uganda
Nche uganda