Sentinel events and near miss reporting analysis and
































- Slides: 44

Sentinel events and near miss reporting, analysis and prevention The Good Hospital Practice Training Series 2009 The Medical City

• All hospitals have sentinel events. • The difference between an excellent hospital and a poor one is that the excellent hospital continuously tries to improve its systems and processes to understand eventually reduce the number of its sentinel events.

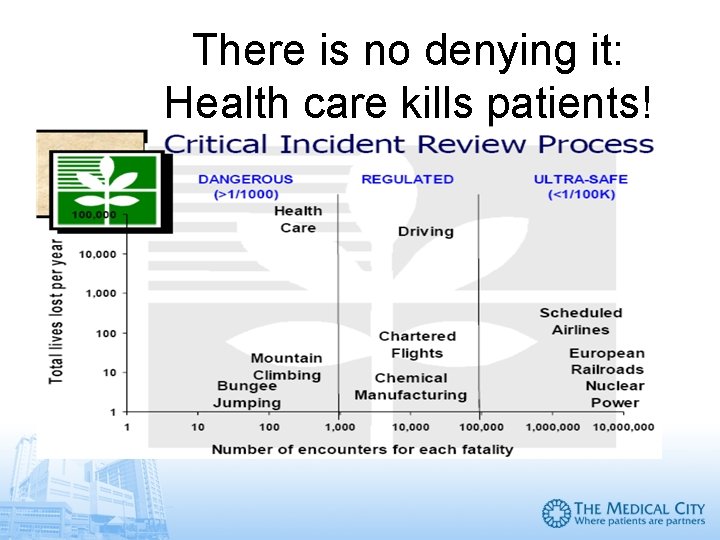
There is no denying it: Health care kills patients!

Why should we make our hospital practice safer? Because • our reputation, • our earnings, and • our continued credentialing and privileging depend on the quality and safety of our medical practice. And because the well-being and the lives of our patients depend on safe and high quality care. .

The JCI Sentinel Events Policy Goals 1. To improve patient care, treatment and services and prevent sentinel events 2. To focus organizational attention to a sentinel event’s root causes and change systems and processes to prevent its recurrence 3. To increase general knowledge of sentinel events, their causes and prevention through data collection 4. To maintain public confidence in accreditation

What is a sentinel event? • An unexpected occurrence involving death, physical or psychological injury or the risk thereof (any process variation for which recurrence carries a significant risk of a serious adverse outcome) • Sentinel means a signal for immediate investigation and response • Sentinel event is not the same as medical error. Patients may experience sentinel events as part of their disease process or high-risk treatments. Even if some error has occurred, it may not have caused the sentinel event. • But sentinel events can result from errors of omission or commission

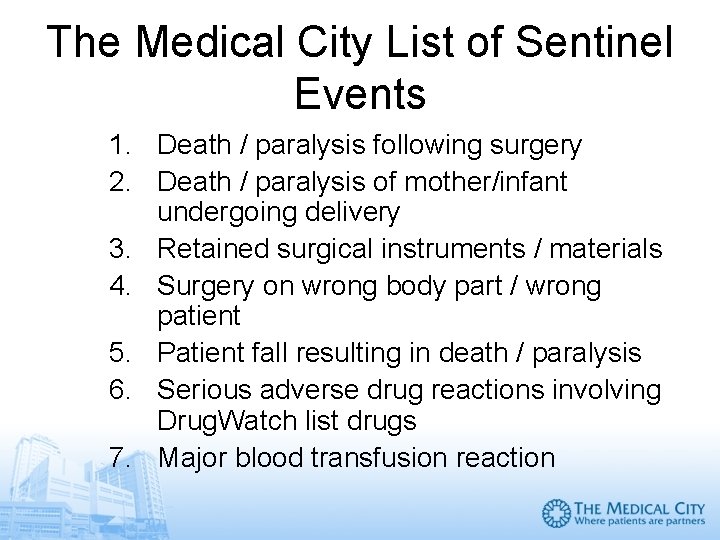
The Medical City List of Sentinel Events 1. Death / paralysis following surgery 2. Death / paralysis of mother/infant undergoing delivery 3. Retained surgical instruments / materials 4. Surgery on wrong body part / wrong patient 5. Patient fall resulting in death / paralysis 6. Serious adverse drug reactions involving Drug. Watch list drugs 7. Major blood transfusion reaction

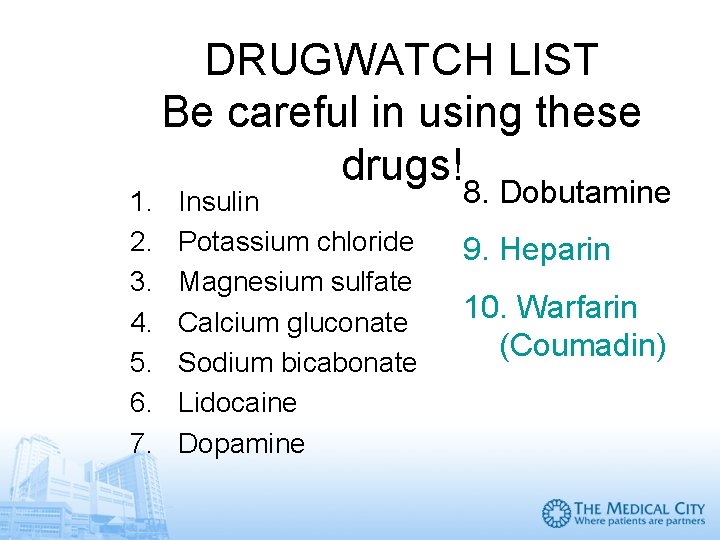
1. 2. 3. 4. 5. 6. 7. DRUGWATCH LIST Be careful in using these drugs! Insulin Potassium chloride Magnesium sulfate Calcium gluconate Sodium bicabonate Lidocaine Dopamine 8. Dobutamine 9. Heparin 10. Warfarin (Coumadin)

The Medical City list of adverse events 1. Infection control indicators 6. Deaths from the following conditions 1. 1 hospital care related infections (ventilator associated pneumonia, 6. 1 Acute myocardial infarction Foley catheter associated UTI, blood 6. 2 Congestive heart failure stream infections from central venous 6. 3 Gastrointestinal hemorrhage lines, surgical site infections) 6. 4 Hip fracture 1. 2 needlestick injuries 6. 5 Pneumonia 2. Heel hematoma after blood 6. 6 Stroke extraction in neonates 7. Surgical incidents 3. Self-extubation in intubated 7. 1 Postoperative hemorrhage or patients hematoma 4. Unanticipated death, cardiorespiratory arrest or loss of 7. 2 Postoperative infection (localized and systemic) major function in any admitted patient 8. Aspiration incidents in patients on tube feeding 5. Unplanned re-admission into the hospital, ICU, NICU or OR

When do you report • Actual Event - when a sentinel event or adverse event actually happens • Near Miss Events – when a sentinel event almost happened but was caught in the nick of time; a process deviation that did not affect outcome, but a recurrence carries significant chance of a serious adverse outcome • Unsafe Conditions – that might predispose to the sentinel event, including – Medical Device Issues – Medical Care issues – Nursing Care issues

When do you report For example, for medication incident • Actual Event - when a wrong dose of insulin is given • Near Miss Events – when a wrong dose of insulin was caught just before administration • Unsafe Conditions – – Medical Device Issues – faulty insulin pump – Medical Care issues – illegible orders – Nursing Care issues – poorly labeled insulin vials

Why report near misses? Sentinel events Adverse events Near misses, other accidents and occurrences Errors that lack an adverse outcome are called near misses or precursor events. They have the same root causes as sentinel events. Most plane crashes are preceded by near misses that have been ignored. Thus, we should report near misses.

The anatomy of a near miss A near miss implies that a sentinel event has nearly occurred. A “near miss” is actually a poor name – a “near hit” would be better. Alternatively, a “good catch” could be used since a catastrophic event has been prevented. A near miss is a cascade of events whereby a sentinel event has been prevented due to a detection and recovery sequence. If either detection or recovery fails, the sentinel event will occur. Thus, detection and recovery play a key role in a near miss. http: //www. krouwerconsulting. com/Essays/Near. Miss. htm

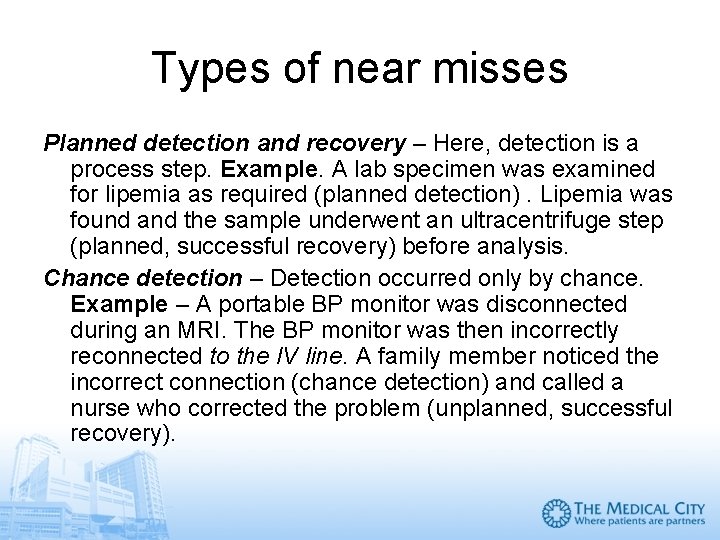
Types of near misses Planned detection and recovery – Here, detection is a process step. Example. A lab specimen was examined for lipemia as required (planned detection). Lipemia was found and the sample underwent an ultracentrifuge step (planned, successful recovery) before analysis. Chance detection – Detection occurred only by chance. Example – A portable BP monitor was disconnected during an MRI. The BP monitor was then incorrectly reconnected to the IV line. A family member noticed the incorrect connection (chance detection) and called a nurse who corrected the problem (unplanned, successful recovery).

Types of near misses Unsafe situation (Accident waiting to happen) – An error event is only recognized as such after a chance detection. Example – Two similar looking medications are next to each other. If an incorrect selection is made, the result could be fatal. Placing the similar medications next to each other can be considered to be a process error event. This error event may be a cause for selection of the incorrect medication. If the wrong medication is selected and this error is detected before administering the medication (chance detection and unplanned, successful recovery), a near miss has occurred.

How risks of sentinel events and near misses can be reduced Reducing risk of sentinel events means reducing the likelihood that the effect of an error event will occur. This can be accomplished by: • reducing the likelihood of an error event • adding or improving a detection step for that error event • adding or improving a recovery step for that error event

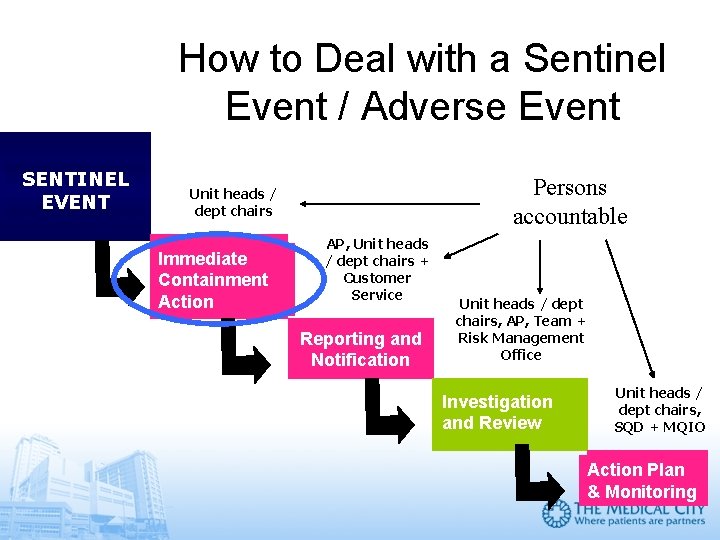
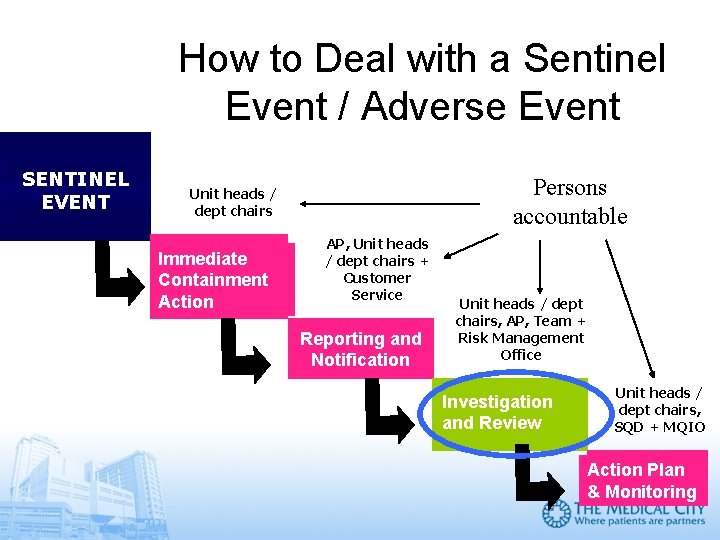
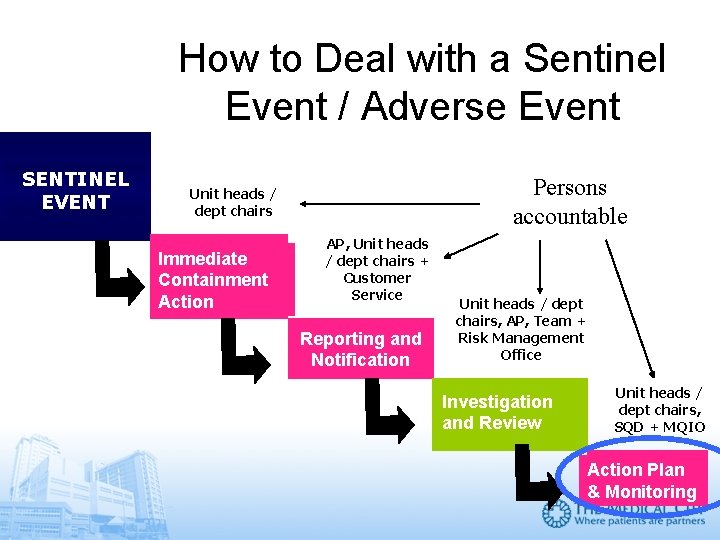
How to Deal with a Sentinel Event / Adverse Event SENTINEL EVENT Persons accountable Unit heads / dept chairs Immediate Containment Action AP, Unit heads / dept chairs + Customer Service Reporting and Notification Unit heads / dept chairs, AP, Team + Risk Management Office Investigation and Review Unit heads / dept chairs, SQD + MQIO Action Plan & Monitoring

How to immediately contain a sentinel event 1. Continue to take CARE of the Patient • Address current health care needs • Obtain necessary referrals and introduce to patient / family all new members of medical care team 2. PRESERVE the Evidence • Sequester all involved machinery (pumps, anesthesia machines) and preserve settings • Sequester all involved medication equipment (syringes, IV tubing, medication vials) • Activate or acquire back-up equipment

How to immediately contain a sentinel event 3. DOCUMENT in the Medical Record • Include only verifiable facts about the event, care given in response and new care plans • DO NOT include subjective feelings or beliefs, events which you did not personally witness, hearsay evidence 4. Plan for timely PATIENT DISCLOSURE • Why disclose? Patients have the right to know. • Who will disclose? The AP is responsible for disclosure. The hospital may form a team to assist him in planning for the disclosure. • When to disclose? As promptly as the patient’s condition will allow. Timely disclosure rebuilds a patient’s trust.

How to immediately contain a sentinel event Plan for timely PATIENT DISCLOSURE What to disclose? ”Known Facts” • Objective verifiable information, documented in Medical Record • Adequate to ensure patient / family‘s understanding of event • Patient’s likely health outcome and prognosis • Express regret and convey empathy (“We regret that this incident happened. ”) • Avoid speculation and blame

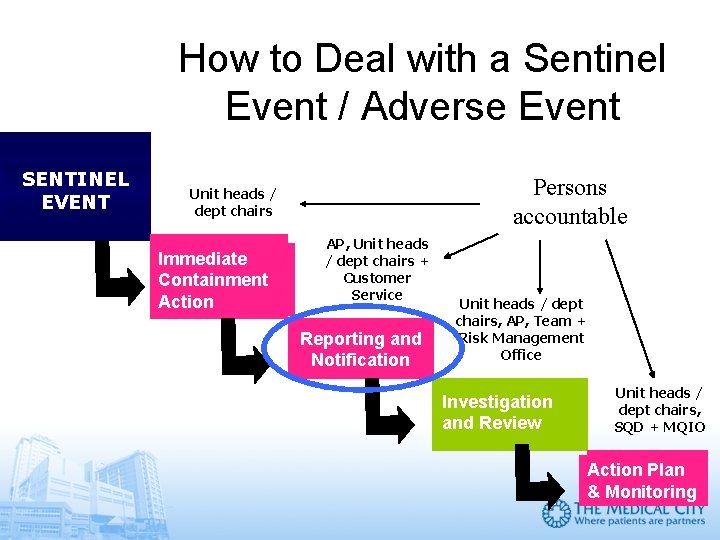
How to Deal with a Sentinel Event / Adverse Event SENTINEL EVENT Persons accountable Unit heads / dept chairs Immediate Containment Action AP, Unit heads / dept chairs + Customer Service Reporting and Notification Unit heads / dept chairs, AP, Team + Risk Management Office Investigation and Review Unit heads / dept chairs, SQD + MQIO Action Plan & Monitoring

How to report a Sentinel Event / Adverse Event REPORT the Event to MQIO by completing a Sentinel Event Report Form within 24 hours. q Do Not Place in Medical Record or Discuss in Medical Record q Do Not Photocopy q An Incident Report may also be required. OR you can simply call the Safety Hotline 8777.

How to Deal with a Sentinel Event / Adverse Event SENTINEL EVENT Persons accountable Unit heads / dept chairs Immediate Containment Action AP, Unit heads / dept chairs + Customer Service Reporting and Notification Unit heads / dept chairs, AP, Team + Risk Management Office Investigation and Review Unit heads / dept chairs, SQD + MQIO Action Plan & Monitoring

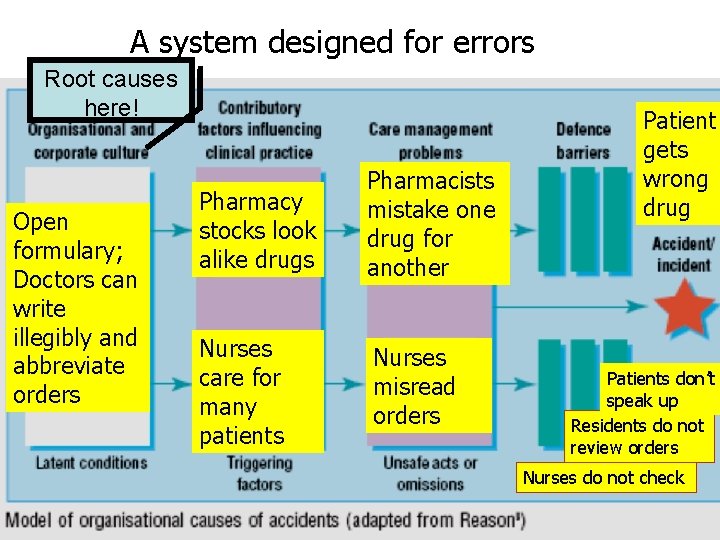
How is a sentinel event investigated? • RMO organizes a small team of leaders to analyze the event with those involved • The focus of the analysis is NOT to assign blame but to search for underlying causes. • Most sentinel events happen not because staff members intend to make mistakes but because there are inadequate systems to make the right thing easy to do (or to make the wrong thing difficult to do). • The search for the root causes of sentinel events is called Root Cause Analysis (RCA).

A system designed for errors Root causes here! Open formulary; Doctors can write illegibly and abbreviate orders Pharmacy stocks look alike drugs Pharmacists mistake one drug for another Nurses care for many patients Nurses misread orders Patient gets wrong drug Patients don’t speak up Residents do not review orders Nurses do not check

Types of process variation • For a sentinel event to occur there must be a deviation or variation from the desired process • Common-cause variation is intrinsic to the process itself. They cannot be eliminated but they can be reduced. • Special-cause variation occurs because of an unusual external circumstance that affects the process. They should be identified and eliminated. • However, elimintaing a special-cause variation (e. g. , firing an errant employee) will not prevent the recurrence of a sentinel event because the processes that permitted the error are still in place. This is why root causes of a sentinel event must be identified.

The focus of RCA • RCA focuses on redesigning processes to reduce common-cause variations • Special-cause variations in the performance of patient care frequently the result of common-cause variations in organization systems. • This relationship provides the opportunity to decrease the risk of special-cause variations in one process by redesigning the larger system of which it is a part. • For example, improving communication between doctors and nurses, such as routine read-back of orders, will prevent nurses from mistranscribing drugs in SHAMAN.

What is an acceptable RCA? 1. The analysis focuses primarily on systems and processes, not on individual performance 2. The analysis progresses from special causes in clinical processes to common causes in organizational processes 3. The analysis repeatedly digs deeper by asking “Why? ”; then, when answered, “Why? ” again, and so on 4. The analysis identifies changes that could be made in systems and processes (either through redesign or development of new systems or processes) which would reduce the risk of such events occurring in the future 5. The analysis is thorough and credible

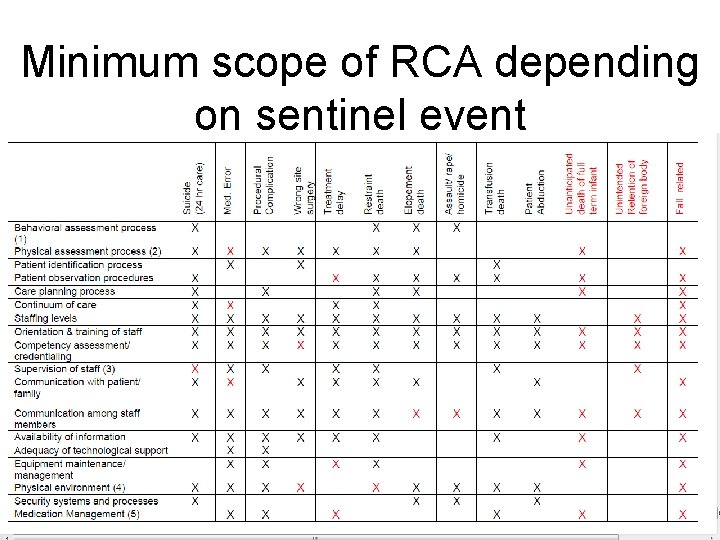
What is a thorough RCA? 1. A determination of the human and other factors most directly associated with the sentinel event and the process(es) and systems related to its occurrence 2. An analysis of the underlying systems and processes through a series of “Why? ” questions to determine where redesign might reduce risk 3. An inquiry into all areas appropriate to the specific type of event (see next slide) 4. An identification of risk points and their potential contributions to this type of event 5. A determination of potential improvement in processes or systems that would tend to decrease the likelihood of such events in the future, or a determination, after analysis, that no such improvement opportunities exist

Minimum scope of RCA depending on sentinel event

What is a credible RCA? 1. Include participation by the leadership of the organization and by individuals most closely involved in the processes and systems under review 2. Be internally consistent (that is, not contradict itself or leave obvious questions unanswered) 3. Provide an explanation for all findings of “not applicable” or “no problem” 4. Include consideration of any relevant literature

What is Failure Mode and Effects Analysis (FMEA)? • FMEA focuses on projecting what are the steps in a process that are prone to failure and providing barriers to protect patients and staff from these failures. • For example, in drug administration, the failures may include wrong drug ordering, wrong drug computation, wrong encoding, wrong drug dispensing and administration to wrong patient. • Failures are then prioritized based on their negative impact and likelihood of occurrence. • Failure prone steps are then either eliminated or redesigned to make failures less likely or at least more easily detectable.

How FMEA and RCA can eliminate near misses Planned detection and recovery – FMEA analysis seeks to add planned detection and recovery where they were absent or to improve detection and recovery, by asking how can an error event be detected and what is the recovery. Chance detection – During FMEA analysis, the addition of a detection step can be thought of as changing a chance detection to a planned detection. If an error event has occurred and been detected by chance, the addition of this detection as a planned process step would have been achieved through RCA.

How FMEA and RCA can eliminate near misses Unsafe situation – An unsafe situation is an unrecognized error event. By definition if the error event is unrecognized, detection and recovery are unknown. By analyzing the process steps through FMEA, events that were previously unrecognized as potential errors could now be so recognized. Planned detection and recovery steps could then be added. Chance detection implies an unsafe situation - If one considers the BP problem above, one could suggest that having a BP Luer connector that can attach to an IV line is an unsafe situation (e. g. , an error). Starting with that premise there are several possible mitigations including training, warning labels, and different equipment that would prevent the incorrect connection.

How to Deal with a Sentinel Event / Adverse Event SENTINEL EVENT Persons accountable Unit heads / dept chairs Immediate Containment Action AP, Unit heads / dept chairs + Customer Service Reporting and Notification Unit heads / dept chairs, AP, Team + Risk Management Office Investigation and Review Unit heads / dept chairs, SQD + MQIO Action Plan & Monitoring

What is an acceptable action plan? 1. Identifies changes that can be implemented to reduce risk or formulates a rationale for not undertaking such changes 2. Identifies, in situations where improvement actions are planned, who is responsible for implementation, when the action will be implemented (including any pilot testing), and how the effectiveness of the actions will be evaluated

Sentinel event and near miss reporting promotes a culture of patient safety The key to improving safety lies not in changing the human condition, but in changing the conditions under which humans work. Reason J. Human Error. Cambridge, UK: Cambridge University Press; 1990

Sentinel event and near miss reporting promotes a culture of patient safety You can do your share in installing a culture of safety that continuously seeks to minimize hazards and patient harm that may result from the processes of healthcare. We depend on you to • Report sentinel events and near misses • Take part in RCAs and FMEAs • Be part of the action to reduce sentinel events and near misses

Are you a patient safety advocate? 1. Which of the following is/are TMC sentinel event/s: a. Death from acute myocardial infarction b. Ventilator-associated pneumonia c. Wrong patient procedure d. All of the above Answer? 2. You must make a sentinel event report when a. An error has resulted in serious harm to a patient. b. Two unlabelled insulin vials are found next to each other. c. The surgical mark and the actual site of incision do not match. d. All of the above. Answer?

Are you a patient safety advocate? 3. The first step in dealing with a sentinel event is a. Submit a properly filled out report to MQIO. b. Take care of the patient’s health needs. c. Sequester all equipment and drugs. d. All of the above Answer? 4. Patient disclosure must be a. Done after sufficient root cause analysis b. Done when all the facts are in c. Done to enable a patient to continue to participate in her care d. All of the above Answer?

Are you a patient safety advocate? 5. Errors usually happen a. Due to disregard for SOPs b. When system barriers and defenses are breached c. Because of inefficiencies and bad work attitudes. . d. All of the above Answer? 6. We must report near misses because a. They are the precursors of sentinel events b. Their root causes are the same as sentinel events c. Just because they did not harm the patient does not mean they won’t next time d. All of the above Answer?

Are you a patient safety advocate? 7. Which of the following is a reportable unsafe condition? a. OF is administered using a venoset. b. Syrups are given to kids by syringe c. Pharmacy has drugs with similar brand names. d. All of the above. Answer? 8. How does FMEA and RCA eliminate near misses? a. Steps to detect a potential error can be added. b. Detection of an error by chance can be converted to routine detection. c. Recovery from an error can be planned. d. All of the above Answer?

Are you a patient safety advocate? 8 out of 8 – you are a pillar of Check your answers: our safety culture! 6 or 7 out of 8 – you are a 1. C budding role model in 2. D patient safety 4 or 5 out of 8 – you must strive 3. B to be better than the rest; your patients will thank you 4. C 2 or 3 out of 8 – improving your 5. B understanding of patient safety is key to changing 6. D your behavior!* 7. D 0 or 1 out of 8 – let us try again* * Please go over the slides 8. D again.

This SIM Card certifies that ______(please overwrite with your name, thank you)__, MD has successfully completed the Self Instructional Module on Sentinel Events and Near Misses Reporting, Analysis and Prevention (Sgd) Dr Alfredo Bengzon President and CEO (Sgd) Dr Jose Acuin Director, Medical Quality Improvement
 Sentinel event list
Sentinel event list Near miss ppt
Near miss ppt What is an incident
What is an incident Kazaya ramak kala örnekleri
Kazaya ramak kala örnekleri Near miss
Near miss Near miss examples
Near miss examples Near miss
Near miss Miss is
Miss is Electrical near miss
Electrical near miss What is an incident
What is an incident Near miss examples ppt
Near miss examples ppt Near foot near shoulder tackling
Near foot near shoulder tackling Events after the reporting date
Events after the reporting date Mutually exclusive events vs not mutually exclusive events
Mutually exclusive events vs not mutually exclusive events Adoption and foster care analysis and reporting system
Adoption and foster care analysis and reporting system Corporate financial reporting and analysis
Corporate financial reporting and analysis 2001: űrodüsszeia the sentinel
2001: űrodüsszeia the sentinel Sentinel-1 acquisition plan
Sentinel-1 acquisition plan Sentinel outlets meaning
Sentinel outlets meaning Sentinel injuries in infants are
Sentinel injuries in infants are Sentinel loop in python
Sentinel loop in python Sentinel surveillance definition
Sentinel surveillance definition Sentinel node
Sentinel node Sentinel surveillance definition
Sentinel surveillance definition Sentinel loop sign
Sentinel loop sign Sentinel surveillance definition
Sentinel surveillance definition Sentinel controlled loop flowchart
Sentinel controlled loop flowchart Crt 2015
Crt 2015 Sentinel value
Sentinel value Usgs sentinel 2
Usgs sentinel 2 Sentinel repetition
Sentinel repetition Linear search with sentinel
Linear search with sentinel Cut off belirtisi
Cut off belirtisi Circular linked list with header node
Circular linked list with header node The seafarer the wanderer the wife's lament
The seafarer the wanderer the wife's lament How many symbols are in the ffa emblem
How many symbols are in the ffa emblem Lb 2 puskesmas
Lb 2 puskesmas Sentinel manager wonderware
Sentinel manager wonderware Sentinel pile
Sentinel pile Customer first consult
Customer first consult Sentinel control structure
Sentinel control structure History of patient safety
History of patient safety Ffa parliamentarian symbol
Ffa parliamentarian symbol Sentinel lymph node biopsy indications
Sentinel lymph node biopsy indications Bio sentinel
Bio sentinel