TissueEngineered Skeletal Muscle Stimulator Syed Asaad Hussain Spencer




![Muscle Formation from Cells 7 [1] Burks, T. N. , Cohn, R. D. “Role Muscle Formation from Cells 7 [1] Burks, T. N. , Cohn, R. D. “Role](https://slidetodoc.com/presentation_image_h/d1a8e66484930b37c50ec51b9b9bb4b6/image-7.jpg)
![Current Devices [1] [2] [1] [3] 9 [1] Vandenburgh, H. H. , Hatfaludy, S. Current Devices [1] [2] [1] [3] 9 [1] Vandenburgh, H. H. , Hatfaludy, S.](https://slidetodoc.com/presentation_image_h/d1a8e66484930b37c50ec51b9b9bb4b6/image-9.jpg)



![Vibration Driven [1] 18 [1] Milano, Shaun. (2014). Allegro Micro. Systems, LLC. Worcester Polytechnic Vibration Driven [1] 18 [1] Milano, Shaun. (2014). Allegro Micro. Systems, LLC. Worcester Polytechnic](https://slidetodoc.com/presentation_image_h/d1a8e66484930b37c50ec51b9b9bb4b6/image-18.jpg)

















![Appendix: Syringe Pump Relations 55 [1] Page, R. (2015). Client Statement. Worcester Polytechnic Institute Appendix: Syringe Pump Relations 55 [1] Page, R. (2015). Client Statement. Worcester Polytechnic Institute](https://slidetodoc.com/presentation_image_h/d1a8e66484930b37c50ec51b9b9bb4b6/image-55.jpg)
![Appendix: Syringe Pump Relations • 56 [1] Page, R. (2015). Client Statement. Worcester Polytechnic Appendix: Syringe Pump Relations • 56 [1] Page, R. (2015). Client Statement. Worcester Polytechnic](https://slidetodoc.com/presentation_image_h/d1a8e66484930b37c50ec51b9b9bb4b6/image-56.jpg)
![Appendix: Drug Approval Process 58 [1] Crasto, A. 2014. The FDA’s Drug Review Process Appendix: Drug Approval Process 58 [1] Crasto, A. 2014. The FDA’s Drug Review Process](https://slidetodoc.com/presentation_image_h/d1a8e66484930b37c50ec51b9b9bb4b6/image-58.jpg)


![Appendix: Key Characteristics of Muscular Tissue Stimulation Anchorage Fiber Alignment [1] Brock, R. “NIH Appendix: Key Characteristics of Muscular Tissue Stimulation Anchorage Fiber Alignment [1] Brock, R. “NIH](https://slidetodoc.com/presentation_image_h/d1a8e66484930b37c50ec51b9b9bb4b6/image-65.jpg)
![Appendix: Tissue Physiology 66 [1] Muscle Physiology | Muscle Tissue Physiology | Tutorials & Appendix: Tissue Physiology 66 [1] Muscle Physiology | Muscle Tissue Physiology | Tutorials &](https://slidetodoc.com/presentation_image_h/d1a8e66484930b37c50ec51b9b9bb4b6/image-66.jpg)

- Slides: 68


Tissue-Engineered Skeletal Muscle Stimulator Syed Asaad Hussain Spencer Ryan Keilich Stephanie Jo Lindow Shreyas Renganathan

Overview • • • 3 • Background • • Significance Drug Approval Process Background Research Current Devices • • Problem Statement Goals Objectives and Constraints Design Specifications • Design Evaluations • Final Design Diagrams • • Design Validation Demonstration (Video) Project Direction Alternative Designs Final Design Proof-of-concept Conclusions & Recommendations

Background 4

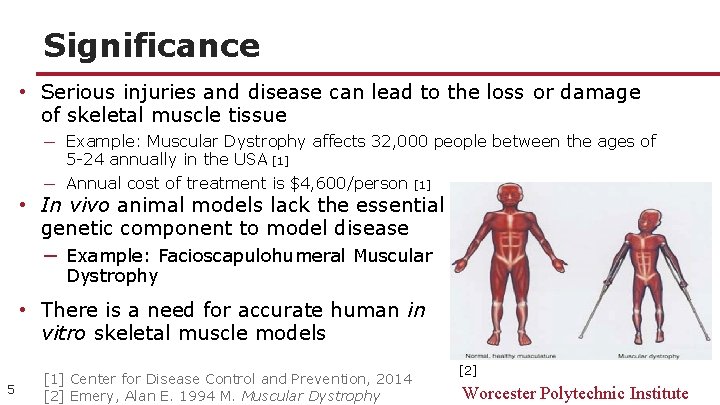
Significance • Serious injuries and disease can lead to the loss or damage of skeletal muscle tissue ─ Example: Muscular Dystrophy affects 32, 000 people between the ages of 5 -24 annually in the USA [1] ─ Annual cost of treatment is $4, 600/person [1] • In vivo animal models lack the essential genetic component to model disease ─ Example: Facioscapulohumeral Muscular Dystrophy • There is a need for accurate human in vitro skeletal muscle models 5 [1] Center for Disease Control and Prevention, 2014 [2] Emery, Alan E. 1994 M. Muscular Dystrophy [2] Worcester Polytechnic Institute

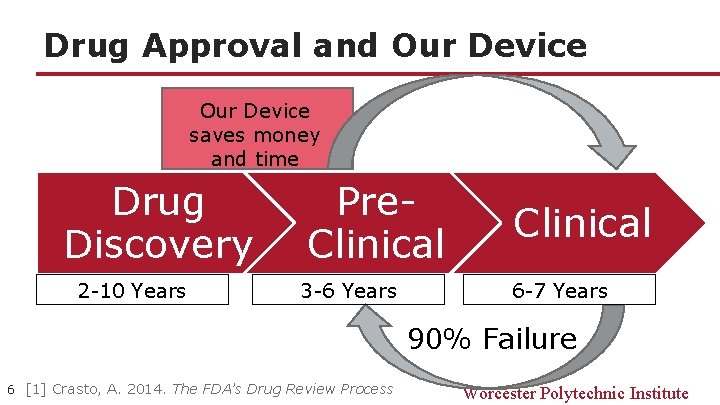
Drug Approval and Our Device saves money and time Drug Discovery 2 -10 Years Pre. Clinical 3 -6 Years Clinical 6 -7 Years 90% Failure 6 [1] Crasto, A. 2014. The FDA’s Drug Review Process Worcester Polytechnic Institute
![Muscle Formation from Cells 7 1 Burks T N Cohn R D Role Muscle Formation from Cells 7 [1] Burks, T. N. , Cohn, R. D. “Role](https://slidetodoc.com/presentation_image_h/d1a8e66484930b37c50ec51b9b9bb4b6/image-7.jpg)
Muscle Formation from Cells 7 [1] Burks, T. N. , Cohn, R. D. “Role of TGF-β signaling in inherited and acquired myopathies” Skeletal Muscle. 2011. May 4; 1(1), 1 -19. [2] Gilles AR, Lieber RL. Structure and function of the skeletal muscle Worcester extracellular matrix. Muscle Nerve. 2011; 44: 318 -331. Polytechnic Institute

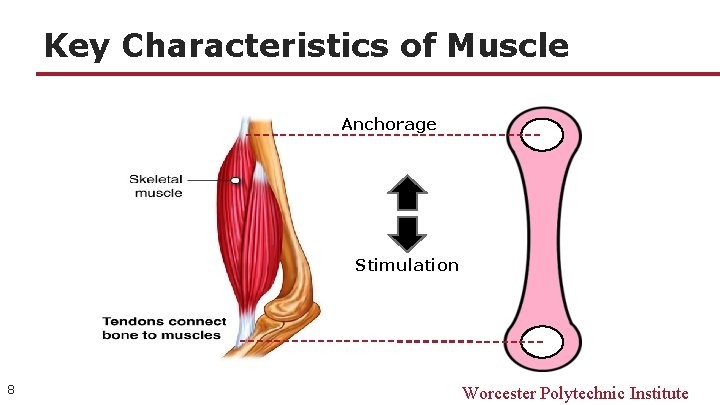
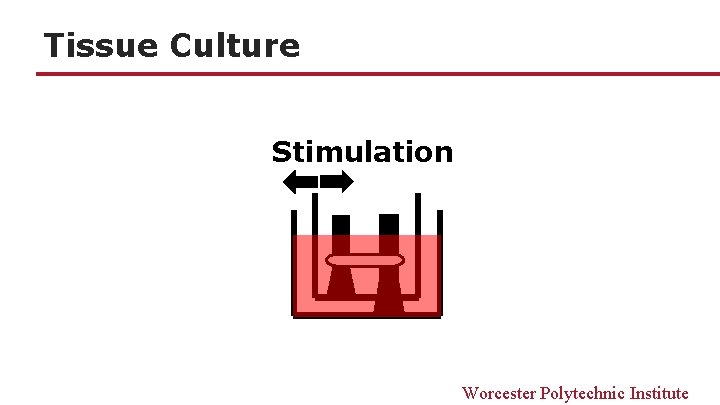
Key Characteristics of Muscle Anchorage Stimulation 8 Worcester Polytechnic Institute
![Current Devices 1 2 1 3 9 1 Vandenburgh H H Hatfaludy S Current Devices [1] [2] [1] [3] 9 [1] Vandenburgh, H. H. , Hatfaludy, S.](https://slidetodoc.com/presentation_image_h/d1a8e66484930b37c50ec51b9b9bb4b6/image-9.jpg)
Current Devices [1] [2] [1] [3] 9 [1] Vandenburgh, H. H. , Hatfaludy, S. , Karlisch, P. , & Shansky, J. (1991). [2] Powell, C. A. , Smiley, B. L. , Mills, J. , & Vandenburgh, H. H. (2002). [3] Christ, G. (2013). U. S. Patent No. 20130197640. Washington, DC: U. S. Worcester Polytechnic Institute

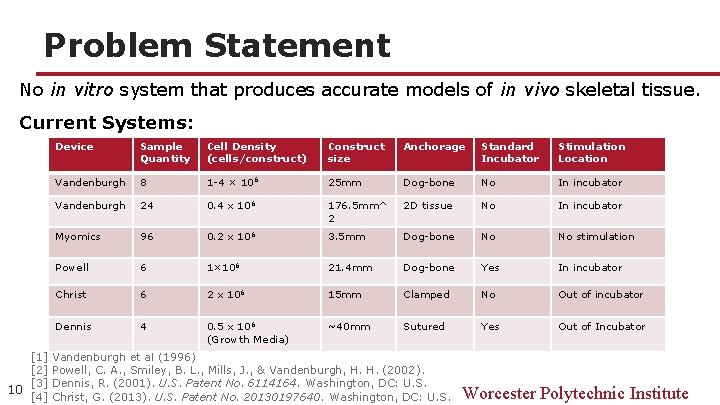
Problem Statement No in vitro system that produces accurate models of in vivo skeletal tissue. Current Systems: Device Sample Quantity Cell Density (cells/construct) Construct size Anchorage Standard Incubator Stimulation Location Vandenburgh 8 1 -4 × 106 25 mm Dog-bone No In incubator Vandenburgh 24 0. 4 x 106 176. 5 mm^ 2 2 D tissue No In incubator Myomics 96 0. 2 x 106 3. 5 mm Dog-bone No No stimulation Powell 6 1× 106 21. 4 mm Dog-bone Yes In incubator Christ 6 2 x 106 15 mm Clamped No Out of incubator Dennis 4 0. 5 x 106 (Growth Media) ~40 mm Sutured Yes Out of Incubator [1] Vandenburgh et al (1996) [2] Powell, C. A. , Smiley, B. L. , Mills, J. , & Vandenburgh, H. H. (2002). [3] Dennis, R. (2001). U. S. Patent No. 6114164. Washington, DC: U. S. 10 [4] Christ, G. (2013). U. S. Patent No. 20130197640. Washington, DC: U. S. Worcester Polytechnic Institute

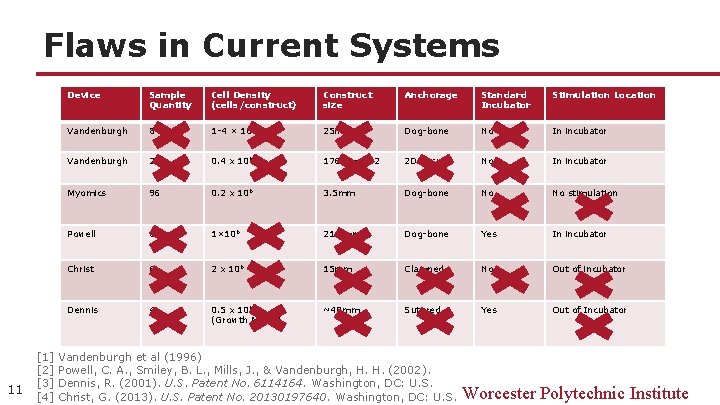
Flaws in Current Systems 11 Device Sample Quantity Cell Density (cells/construct) Construct size Anchorage Standard Incubator Stimulation Location Vandenburgh 8 1 -4 × 106 25 mm Dog-bone No In incubator Vandenburgh 24 0. 4 x 106 176. 5 mm^2 2 D tissue No In incubator Myomics 96 0. 2 x 106 3. 5 mm Dog-bone No No stimulation Powell 6 1× 106 21. 4 mm Dog-bone Yes In incubator Christ 6 2 x 106 15 mm Clamped No Out of incubator Dennis 4 0. 5 x 106 (Growth Media) ~40 mm Sutured Yes Out of Incubator [1] Vandenburgh et al (1996) [2] Powell, C. A. , Smiley, B. L. , Mills, J. , & Vandenburgh, H. H. (2002). [3] Dennis, R. (2001). U. S. Patent No. 6114164. Washington, DC: U. S. [4] Christ, G. (2013). U. S. Patent No. 20130197640. Washington, DC: U. S. Worcester Polytechnic Institute

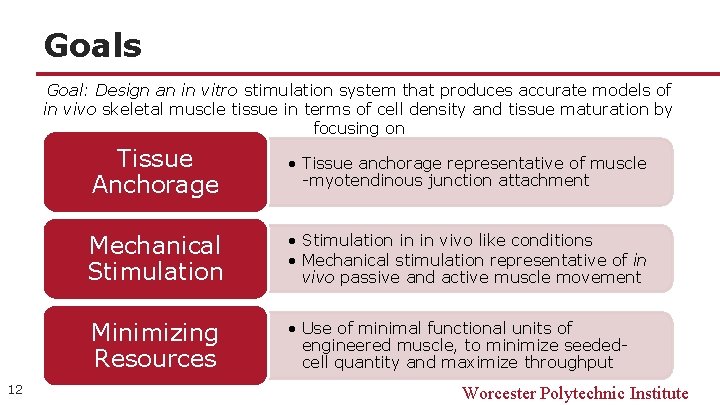
Goals Goal: Design an in vitro stimulation system that produces accurate models of in vivo skeletal muscle tissue in terms of cell density and tissue maturation by focusing on Tissue Anchorage • Tissue anchorage representative of muscle -myotendinous junction attachment Mechanical Stimulation • Stimulation in in vivo like conditions • Mechanical stimulation representative of in vivo passive and active muscle movement Minimizing Resources 12 • Use of minimal functional units of engineered muscle, to minimize seededcell quantity and maximize throughput Worcester Polytechnic Institute

Objectives and Constraints Objectives: Constraints: • Reliability and Consistent Results • Sterilizable • Mechanically Stimulate Tissue • Efficient ─ Reusable ─ High throughput • User Friendliness 13 • Must withstand in vitro culture conditions ─ 37 o. C ─ 99% humidity ─ 5% CO 2 • Non-cytotoxic • Operate while in incubator Worcester Polytechnic Institute

Design Specifications Stimulation Specs: Tissue Culture Specs: • Mechanical Stimulation • Tissue Structure ─ Uniaxial strain ─ Controllable strain between -50% to 50% ─ Static and cyclic capabilities ─ Controllable strain rate ─ Controllable frequency of stimulation 10 ─ Dog-bone shape ─ Minimal Functional Unit § Length: 3 -4 mm § Diameter: 1 mm • Cell Source ─ C 2 C 12 (Myoblast) ─ 500, 000 cells/120 u. L Worcester Polytechnic Institute

Alternative Designs 15

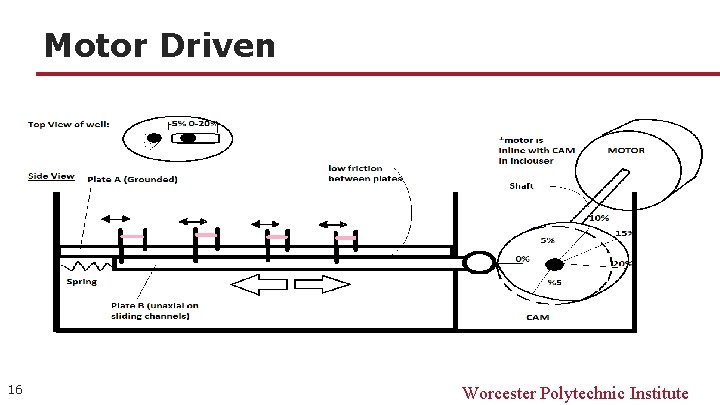
Motor Driven 16 Worcester Polytechnic Institute

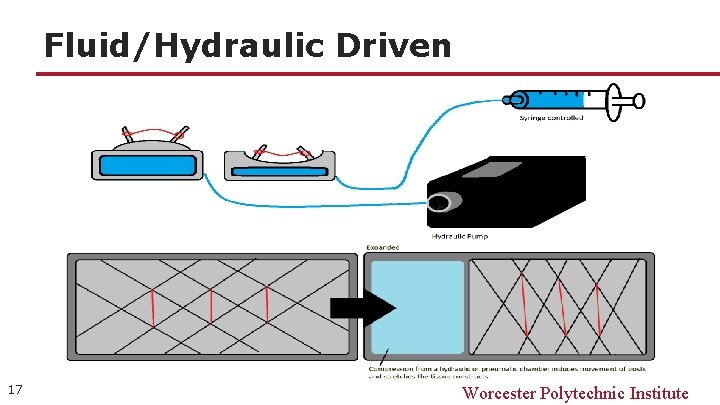
Fluid/Hydraulic Driven 17 Worcester Polytechnic Institute
![Vibration Driven 1 18 1 Milano Shaun 2014 Allegro Micro Systems LLC Worcester Polytechnic Vibration Driven [1] 18 [1] Milano, Shaun. (2014). Allegro Micro. Systems, LLC. Worcester Polytechnic](https://slidetodoc.com/presentation_image_h/d1a8e66484930b37c50ec51b9b9bb4b6/image-18.jpg)
Vibration Driven [1] 18 [1] Milano, Shaun. (2014). Allegro Micro. Systems, LLC. Worcester Polytechnic Institute

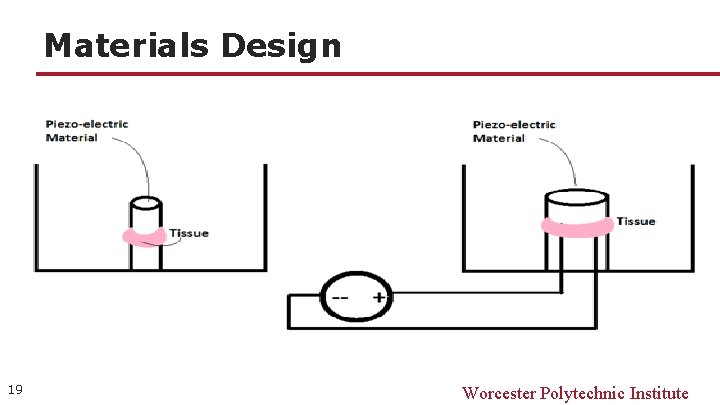
Materials Design 19 Worcester Polytechnic Institute

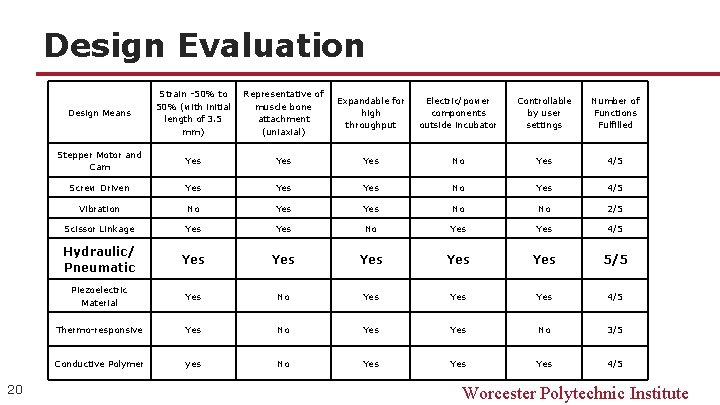
Design Evaluation 20 Design Means Strain -50% to 50% (with initial length of 3. 5 mm) Representative of muscle bone attachment (uniaxial) Expandable for high throughput Electric/power components outside incubator Controllable by user settings Number of Functions Fulfilled Stepper Motor and Cam Yes Yes No Yes 4/5 Screw Driven Yes Yes No Yes 4/5 Vibration No Yes No No 2/5 Scissor Linkage Yes No Yes 4/5 Hydraulic/ Pneumatic Yes Yes Yes 5/5 Piezoelectric Material Yes No Yes Yes 4/5 Thermo-responsive Yes No 3/5 Conductive Polymer yes No Yes Yes 4/5 Worcester Polytechnic Institute

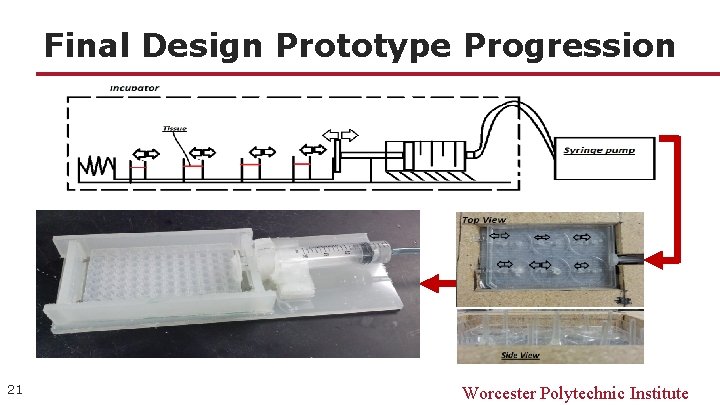
Final Design Prototype Progression 21 Worcester Polytechnic Institute

Final Design 22

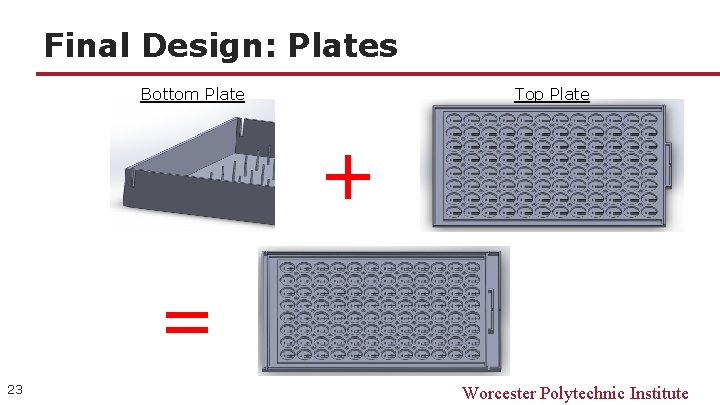
Final Design: Plates Bottom Plate Top Plate + = 23 Worcester Polytechnic Institute

Final Design: Well and Post Design 24 Worcester Polytechnic Institute

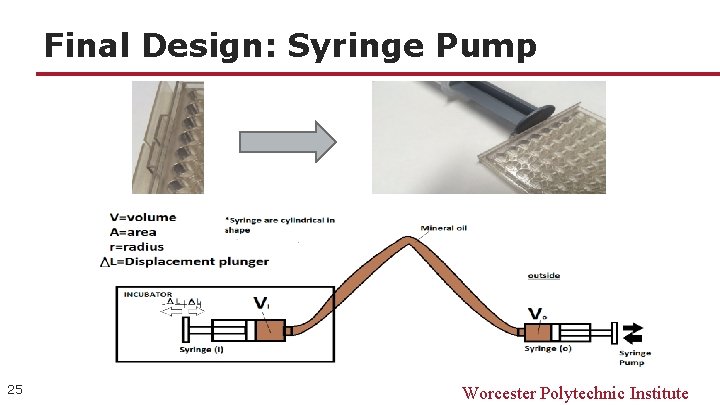
Final Design: Syringe Pump 25 Worcester Polytechnic Institute

Final Design: Complete 26 Worcester Polytechnic Institute

Tissue Culture Flood outer wells with differentiation media heating block (50 o. C) Worcester Polytechnic Institute

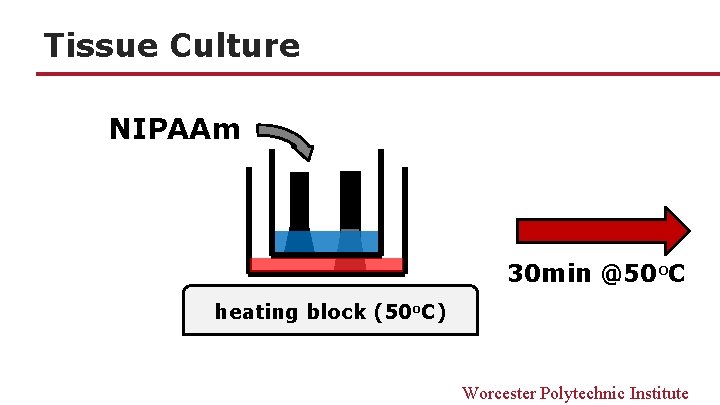
Tissue Culture NIPAAm 30 min @50 o. C heating block (50 o. C) Worcester Polytechnic Institute

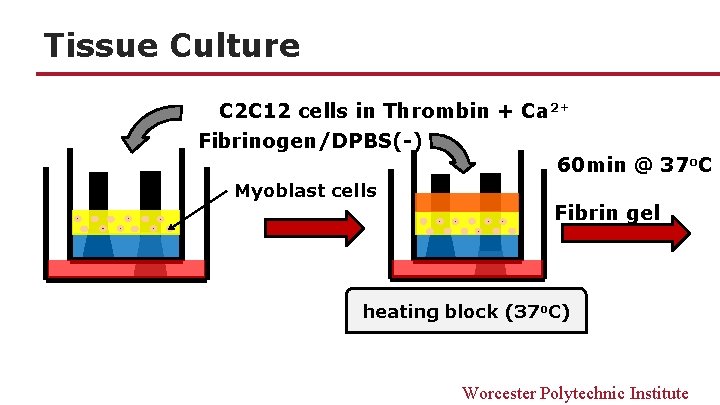
Tissue Culture C 2 C 12 cells in Thrombin + Ca 2+ Fibrinogen/DPBS(-) Myoblast cells 60 min @ 37 o. C Fibrin gel heating block (37 o. C) Worcester Polytechnic Institute

Tissue Culture Construct Formation Tissue Construct 30 Worcester Polytechnic Institute

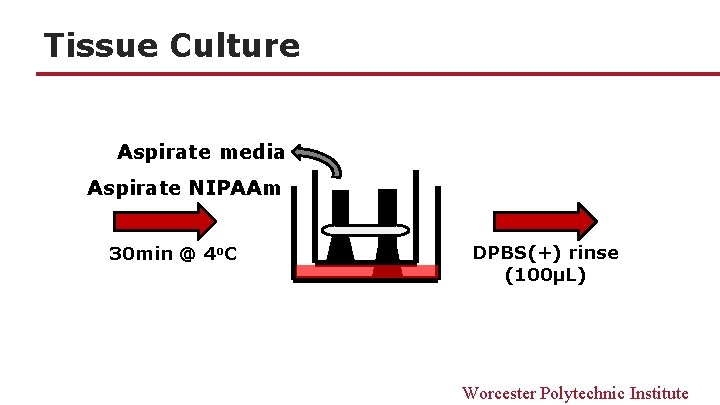
Tissue Culture Aspirate media Aspirate NIPAAm 30 min @ 4 o. C DPBS(+) rinse (100μL) Worcester Polytechnic Institute

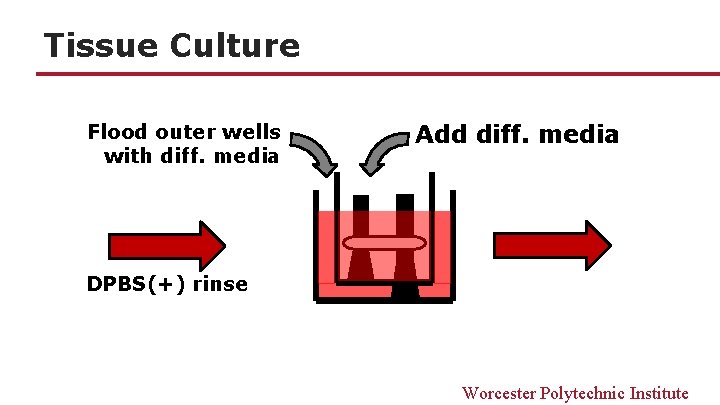
Tissue Culture Flood outer wells with diff. media Add diff. media DPBS(+) rinse Worcester Polytechnic Institute

Tissue Culture Stimulation Worcester Polytechnic Institute

Tissue Culture: Flow Diagram Flood outer wells with diff. media C 2 C 12 cells in Thrombin + Ca 2+ NIPAAm heating block (50 o. C) Myoblast cells 30 min @50 o. C Fibrinogen Construct Formation Flood gently (diff. media) Tissue Construct 30 min @ 4 o. C Aspirate media Aspirate NIPAAm Overnight incubation (37 o. C) Flood outer wells with diff. media 60 min @ 37 o. C Fibrin gel Add diff. media heating block (37 o. C) Stimulation DPBS(+) rinse 34 Worcester Polytechnic Institute

Gold Standard Comparison 35 Parameter MQP Myomics Sample Quantity 96 96 Cell Density (cells/volume) 5 x 105 / 120 μL (C 2 C 12) 2 x 105 / 120 μL (primary myoblasts) Construct size 3. 5 mm Anchorage Dog-bone Stimulation Location In incubator n/a Number of Samples Stimulated at a Time 96 0 Worcester Polytechnic Institute

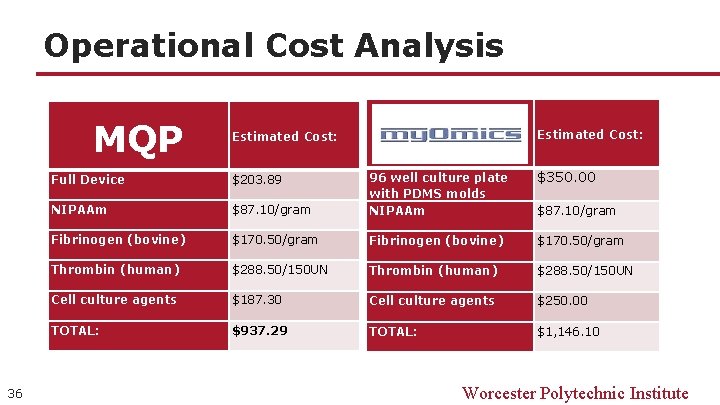
Operational Cost Analysis MQP 36 Estimated Cost: $350. 00 $87. 10/gram 96 well culture plate with PDMS molds NIPAAm Fibrinogen (bovine) $170. 50/gram Thrombin (human) $288. 50/150 UN Cell culture agents $187. 30 Cell culture agents $250. 00 TOTAL: $937. 29 TOTAL: $1, 146. 10 Full Device $203. 89 NIPAAm $87. 10/gram Worcester Polytechnic Institute

Strain/Volume Relationship Amount Strained by Volume Change 0. 4 0. 3 Strain 0. 2 -0. 6 0. 1 -0. 4 -0. 2 0 0 0. 2 0. 4 0. 6 -0. 1 -0. 2 -0. 3 predicted behavior dry media -0. 4 37 ∆Volume [m. L] Worcester Polytechnic Institute

Video Demonstration 38 Worcester Polytechnic Institute

Proof of Concept Device Verification 39

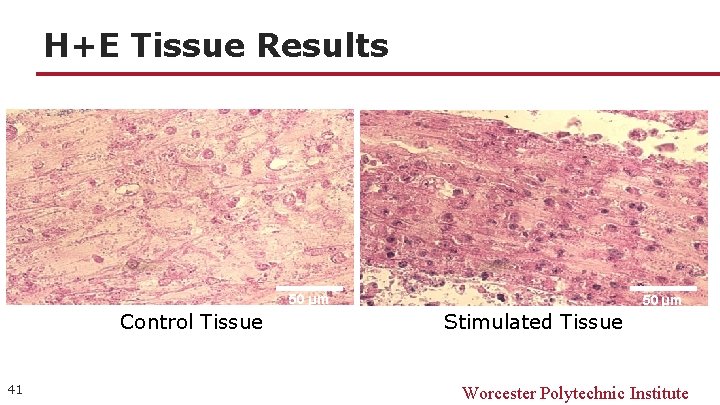
Tissue Results • Mechanical strain tests ─ ─ Exercise: 5%-15% 15 minutes 1 cycle/minute 4 days • Stimulated vs. Control ─ H+E Stain ─ Myosin Stain 40 Worcester Polytechnic Institute

H+E Tissue Results 50 µm Control Tissue 41 50 µm Stimulated Tissue Worcester Polytechnic Institute

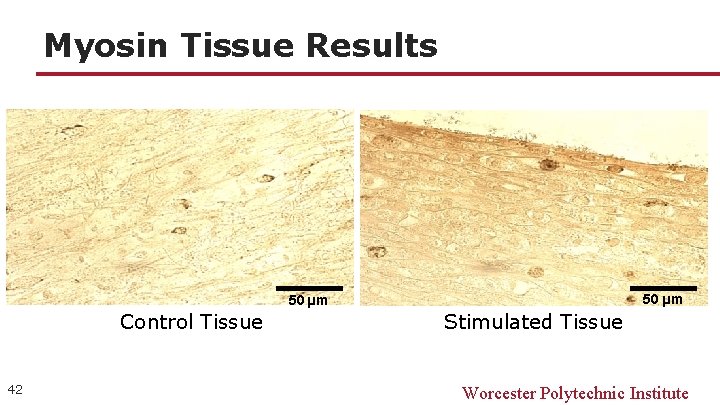
Myosin Tissue Results 50 µm Control Tissue 42 Stimulated Tissue Worcester Polytechnic Institute

Conclusions & Recommendations 43

Conclusions DEVICE ü ü Produces reliable and consistent results Uniaxial mechanical stimulation of tissue Efficient: High Throughput and Reusable User friendliness Market Comparison ü ü ü Higher functionality Device: $203<Others: $350 Higher content testing No need for live animal model Observation of human tissue interaction Deliverable: A mechanical stimulator of skeletal muscle tissue that produces tissue samples with improved fiber alignment, increased fiber density, and more accurate modeling of in vivo tissue when compared to nonstimulated in vitro tissue constructs. 44 Worcester Polytechnic Institute

Recommendations Device Recommendations: Test Recommendations: • Perform more stimulation • Replace post to PDMS experiments and statistically • Replace MED 610 validate results • Use more rigid tubing to • Adapt to engineered tissue reduce error by cell self-assembly ─ Increased fiber density • Electrical stimulation component ─ More alike native tissue • Optimize media composition ─ Tissue maturation ─ Hypertrophy ─ Fiber size 45 Worcester Polytechnic Institute

Questions? 46

References • Page, R. (2014). Client Statement. • “Tissue engineering and regenerative medicine”, 2012. Osaka Institute of Technology, from http: //www. oit. ac. jp/english/engineering/img/bio. Engneer/pht_bio_02. jpg Guilak et al. (2003). Functional Tissue Engineering. Springer-Verlag New York, Inc. Aschettino, M. , Delfosse, S. , Larson, K. , Quinn, C. (2011). Bio. Mimetic. Skeletal Muscle Tissue Model. Major Qualifying Project. https: //www. wpi. edu/Pubs/E-project/Available/E-project-042512122709/unrestricted/RLP-1101_Biomimetic_Skeletal_Muscle_Tissue_Model. pdf Milano, Shaun. (2014). Allegro Micro. Systems, LLC. Vandenburgh, H. , Tatto, M. D. , Shansky, J. , Lemaire, J. , Chang, A. , Payumo, F. , . . . & Raven, L. (1996). Tissue-engineered skeletal muscle organoids for reversible gene therapy. Human gene therapy, 7(17), 2195 -2200. http: //online. liebertpub. com/doi/pdf/10. 1089/hum. 1996. 7. 17 -2195 Vandenburgh, H. H. , Hatfaludy, S. , Karlisch, P. , & Shansky, J. (1991). Mechanically induced alterations in cultured skeletal muscle growth. Journal of biomechanics, 24, 91 -99. Powell, C. A. , Smiley, B. L. , Mills, J. , & Vandenburgh, H. H. (2002). Mechanical stimulation improves tissue-engineered human skeletal muscle. American Journal of Physiology-Cell Physiology, 283(5), C 1557 -C 1565. Radisic, M. (2005). U. S. Patent No. 20050112759. Washington, DC: U. S. Dennis, R. (2001). U. S. Patent No. 6114164. Washington, DC: U. S. Christ, G. (2013). U. S. Patent No. 20130197640. Washington, DC: U. S. • • • 47 Worcester Polytechnic Institute

Appendices 48

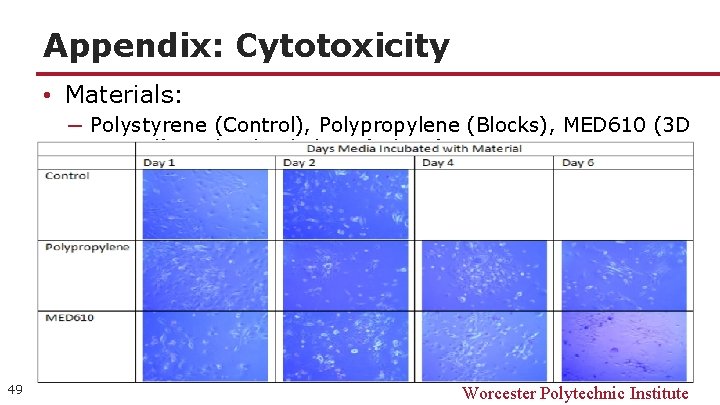
Appendix: Cytotoxicity • Materials: ─ Polystyrene (Control), Polypropylene (Blocks), MED 610 (3 D Printed), and Polyethylene (tubing) 49 Worcester Polytechnic Institute

50 cu ba in s te d) cu ba in s . d) at e cu ba in s ay ay D 61 0 (6 ED M D 61 0 (4 ED M ay D 61 0 (2 ED M ay in D c. . In s (6 D ay e In s (4 D ay . . cu. In c. . s ay in D 1 ) ol 2 (c on tr ro l) on t (c e 2 D ay ( e ( 1 61 0 ED M le n py ro ly p Po ne py le ro ly p Po ( e re n ne le py yp ro Po l ty ly s Po re n ty ly s Po Percentage Alive Appendix: Cytotoxicity Graphed Percentage of Cells Alive after 7 Days of Media Exposure 100 90 80 70 60 50 40 30 20 10 0 Media Treatment Experimental groups compared via ANOVA analysis p-value: 0. 092 Worcester Polytechnic Institute

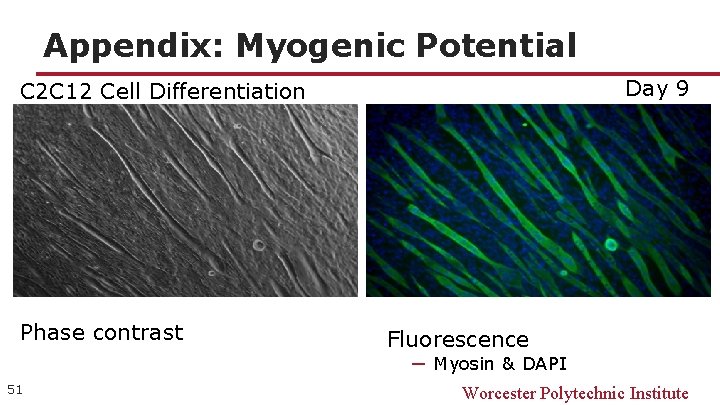
Appendix: Myogenic Potential Day 9 C 2 C 12 Cell Differentiation Phase contrast Fluorescence ─ Myosin & DAPI 51 Worcester Polytechnic Institute

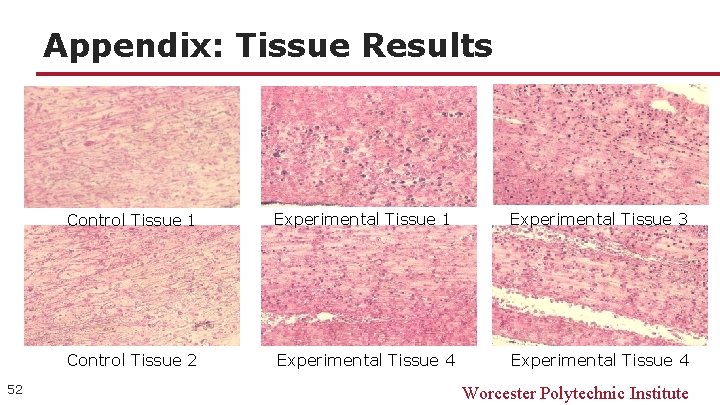
Appendix: Tissue Results 52 Control Tissue 1 Experimental Tissue 3 Control Tissue 2 Experimental Tissue 4 Worcester Polytechnic Institute

Appendix: Tissue results analysis 53 Control Tissue 1 Experimental Tissue 3 Control Tissue 2 Experimental Tissue 4 Worcester Polytechnic Institute

Appendix: Thermal Expansion Testing 54 Worcester Polytechnic Institute
![Appendix Syringe Pump Relations 55 1 Page R 2015 Client Statement Worcester Polytechnic Institute Appendix: Syringe Pump Relations 55 [1] Page, R. (2015). Client Statement. Worcester Polytechnic Institute](https://slidetodoc.com/presentation_image_h/d1a8e66484930b37c50ec51b9b9bb4b6/image-55.jpg)
Appendix: Syringe Pump Relations 55 [1] Page, R. (2015). Client Statement. Worcester Polytechnic Institute
![Appendix Syringe Pump Relations 56 1 Page R 2015 Client Statement Worcester Polytechnic Appendix: Syringe Pump Relations • 56 [1] Page, R. (2015). Client Statement. Worcester Polytechnic](https://slidetodoc.com/presentation_image_h/d1a8e66484930b37c50ec51b9b9bb4b6/image-56.jpg)
Appendix: Syringe Pump Relations • 56 [1] Page, R. (2015). Client Statement. Worcester Polytechnic Institute

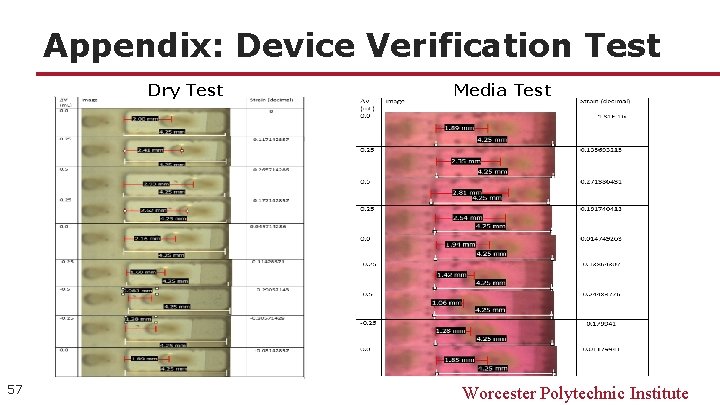
Appendix: Device Verification Test Dry Test 57 Media Test Worcester Polytechnic Institute
![Appendix Drug Approval Process 58 1 Crasto A 2014 The FDAs Drug Review Process Appendix: Drug Approval Process 58 [1] Crasto, A. 2014. The FDA’s Drug Review Process](https://slidetodoc.com/presentation_image_h/d1a8e66484930b37c50ec51b9b9bb4b6/image-58.jpg)
Appendix: Drug Approval Process 58 [1] Crasto, A. 2014. The FDA’s Drug Review Process Worcester Polytechnic Institute

Appendix: Marketability Impact Cheaper and more efficient means of testing Researchers and Pharmaceutical Companies Stakeholders No live animal or human testing required Observation of human tissue interactions Patients with Muscular Diseases Improved treatment methods for diseases Worcester Polytechnic Institute

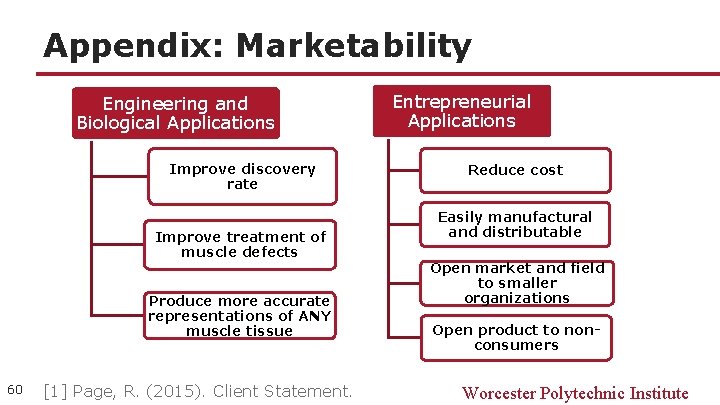
Appendix: Marketability Engineering and Biological Applications Improve discovery rate Improve treatment of muscle defects Produce more accurate representations of ANY muscle tissue 60 [1] Page, R. (2015). Client Statement. Entrepreneurial Applications Reduce cost Easily manufactural and distributable Open market and field to smaller organizations Open product to nonconsumers Worcester Polytechnic Institute

Appendix: Objective Tree Stimulator Reliability Function Consistent Results Stimulation User Friendly Efficiency Minimal Functional Unit Reusable High Throughput Accuracy Culture in Device Automated Programmable Mechanical Dogbone Uniaxial Cyclic and Static Worcester Polytechnic Institute

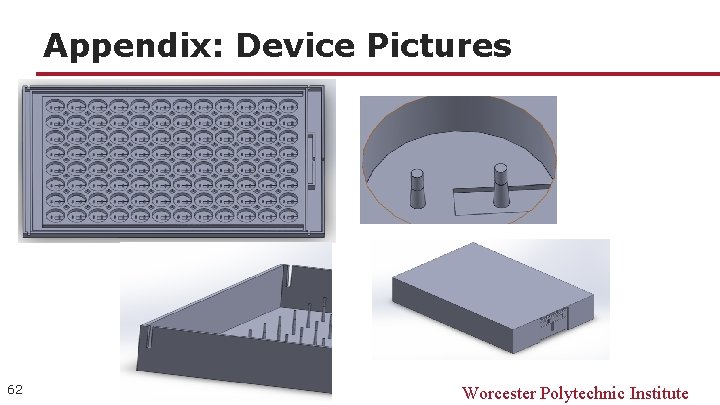
Appendix: Device Pictures 62 Worcester Polytechnic Institute

Appendix: Laboratory Protocols • Diagram from main presentation • Page Lab cell protocols 63 [1] Page, R. (2015). Client Statement. Strain regimen • 1 -2% strain at 1 cycle per minute for 15 minutes on stim day 1 • 2 -4% strain at 1 cycle per 2 minutes for 15 minutes on stim day 2 • 2 -4% strain at 1 cycle per minute for 15 minutes on stim day 3 Worcester Polytechnic Institute

Appendix: Degenerative Muscle Disease • Diseases: ─ Muscular Dystrophy § Facioscapulohumeral § Duchenne MD ─ Multiple Sclerosis ─ Amyotrophic Lateral Sclerosis ─ Myasthenia Gravis • Common Symptoms: ─ ─ Loss of muscle mass Loss of coordination Signal transduction problems No cure • Average cost of care (MD): ─ $18, 930 per 4 years (per case) 1. Emery, Alan E. 1994 M. Muscular Dystrophy 2. "Muscular Dystrophy: Hope Through Research, " 2013 NINDS. No. 13 -77 Worcester Polytechnic Institute
![Appendix Key Characteristics of Muscular Tissue Stimulation Anchorage Fiber Alignment 1 Brock R NIH Appendix: Key Characteristics of Muscular Tissue Stimulation Anchorage Fiber Alignment [1] Brock, R. “NIH](https://slidetodoc.com/presentation_image_h/d1a8e66484930b37c50ec51b9b9bb4b6/image-65.jpg)
Appendix: Key Characteristics of Muscular Tissue Stimulation Anchorage Fiber Alignment [1] Brock, R. “NIH study uncovers details of early stages in muscle formation and regeneration. May 2013. Worcester Muscle-Tendon Attachment Polytechnic Institute
![Appendix Tissue Physiology 66 1 Muscle Physiology Muscle Tissue Physiology Tutorials Appendix: Tissue Physiology 66 [1] Muscle Physiology | Muscle Tissue Physiology | Tutorials &](https://slidetodoc.com/presentation_image_h/d1a8e66484930b37c50ec51b9b9bb4b6/image-66.jpg)
Appendix: Tissue Physiology 66 [1] Muscle Physiology | Muscle Tissue Physiology | Tutorials & Quizzes. "Muscle Physiology | Muscle Tissue Physiology | Tutorials & Quizzes. Worcester Polytechnic Institute

Appendix: Constraints (incubator conditions) • Device needs to withstand incubation • Muscle cells passively contract and rupture • Cells culture complications ─ Protein denaturation § Limits temperature § p. H § cell density ─ Viability range § Temperature § p. H § Density Worcester Polytechnic Institute

Appendix H: Raw data from tests 68 Worcester Polytechnic Institute
 Wpi syringe pump
Wpi syringe pump Pena muscle stimulator
Pena muscle stimulator Smooth muscle contraction vs skeletal muscle contraction
Smooth muscle contraction vs skeletal muscle contraction Iceberg competenze
Iceberg competenze Iceberg competency model (spencer & spencer)
Iceberg competency model (spencer & spencer) Modello iceberg di spencer e spencer
Modello iceberg di spencer e spencer Modello iceberg di spencer e spencer
Modello iceberg di spencer e spencer Spencer spencer
Spencer spencer Spencer and spencer 1993
Spencer and spencer 1993 Informer stimulator function
Informer stimulator function Boston scientific spinal cord stimulator
Boston scientific spinal cord stimulator Alan asaad
Alan asaad Walid asaad
Walid asaad Skeletal muscle
Skeletal muscle Microscopic anatomy of skeletal muscle
Microscopic anatomy of skeletal muscle Skeletal muscke
Skeletal muscke Skeletal muscle relaxants classification
Skeletal muscle relaxants classification Cardiac muscle tissue
Cardiac muscle tissue Characteristics of skeletal smooth and cardiac muscle
Characteristics of skeletal smooth and cardiac muscle Skeletal muscle cylindrical
Skeletal muscle cylindrical Function of skeletal muscle
Function of skeletal muscle Cardiac muscle cross section
Cardiac muscle cross section Skeletal muscle relaxants classification
Skeletal muscle relaxants classification Functions of skeletal muscle
Functions of skeletal muscle Sarcoplasmic
Sarcoplasmic Muscle diagram
Muscle diagram Structure of a skeletal muscle fiber
Structure of a skeletal muscle fiber Cross bridge anatomy
Cross bridge anatomy Myofiber vs myofibril
Myofiber vs myofibril Figure 10-1 skeletal muscle fiber
Figure 10-1 skeletal muscle fiber Macro structure of muscle
Macro structure of muscle Function of skeletal muscle
Function of skeletal muscle Non depolarizing muscle relaxant classification
Non depolarizing muscle relaxant classification Skeletal muscle tissue structure
Skeletal muscle tissue structure Skeletal muscle function
Skeletal muscle function Skeletal muscle tissue description
Skeletal muscle tissue description Microscopic anatomy of skeletal muscle figure 6-2
Microscopic anatomy of skeletal muscle figure 6-2 Skeletal muscle
Skeletal muscle Terminal cisterna
Terminal cisterna Myosin in smooth muscle
Myosin in smooth muscle Skeletal muscle relaxants classification
Skeletal muscle relaxants classification Function of muscle
Function of muscle Is skeletal muscle an organ
Is skeletal muscle an organ Lesson 5.1 the organization of a skeletal muscle
Lesson 5.1 the organization of a skeletal muscle Structure of skeletal muscle
Structure of skeletal muscle 4 muscles of pharynx
4 muscles of pharynx Comparison of skeletal cardiac and smooth muscle
Comparison of skeletal cardiac and smooth muscle Skeletal muscle organisation
Skeletal muscle organisation Pseudostratified vs simple columnar
Pseudostratified vs simple columnar Skeletal muscle relaxants classification
Skeletal muscle relaxants classification Cardiac muscle cell vs skeletal
Cardiac muscle cell vs skeletal Microscopic anatomy of skeletal muscle figure 6-2
Microscopic anatomy of skeletal muscle figure 6-2 Comparison of skeletal cardiac and smooth muscle
Comparison of skeletal cardiac and smooth muscle Chapter 6 the muscular system figure 6-9
Chapter 6 the muscular system figure 6-9 Skeletal muscle origin
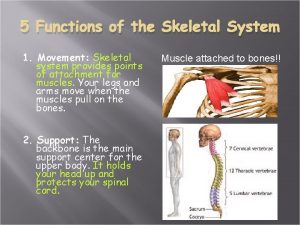
Skeletal muscle origin What is the 5 functions of the skeletal system
What is the 5 functions of the skeletal system Cardiac muscle
Cardiac muscle Characteristics of skeletal smooth and cardiac muscle
Characteristics of skeletal smooth and cardiac muscle Autonomic nervous system skeletal muscle
Autonomic nervous system skeletal muscle Centrally acting skeletal muscle relaxants
Centrally acting skeletal muscle relaxants Muscle histology review
Muscle histology review Contraction
Contraction Five golden rules of skeletal muscle activity
Five golden rules of skeletal muscle activity Trunk muscles
Trunk muscles Loaf muscles of hand
Loaf muscles of hand Skeletal muscle pump
Skeletal muscle pump Thigh cross section
Thigh cross section Epi peri endo
Epi peri endo Types of muscle tissue
Types of muscle tissue