The Classical Second Law of Thermodynamics April 17





- Slides: 42

The Classical Second Law of Thermodynamics April 17, 2012 Prof. Sanghee Kim 1 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

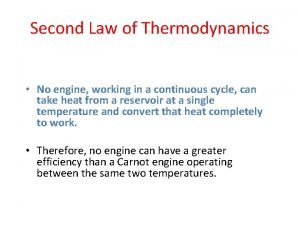
First Law : energy conservation in any ordinary process (no restriction on the direction of heat and work flow) • ex) A hot cup of coffee cools by virtue of heat transfer to the surroundings, • but heat will not flow from the cooler surroundings to the hotter cup of coffee. processes proceed in a certain direction Second Law : existence of such a restriction (difference in quality bwn heat & work ) 2 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

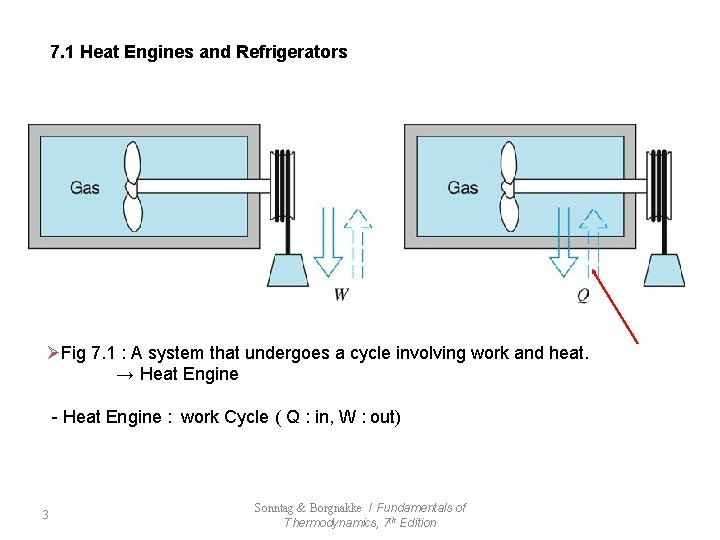
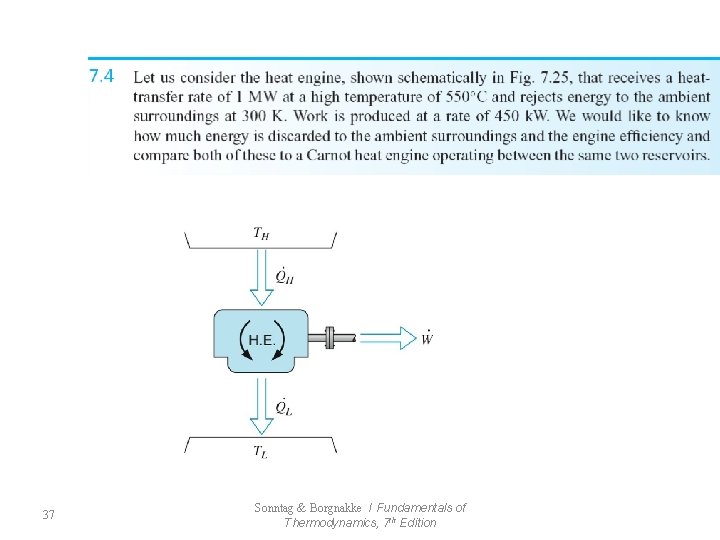
7. 1 Heat Engines and Refrigerators ØFig 7. 1 : A system that undergoes a cycle involving work and heat. → Heat Engine - Heat Engine : work Cycle ( Q : in, W : out) 3 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

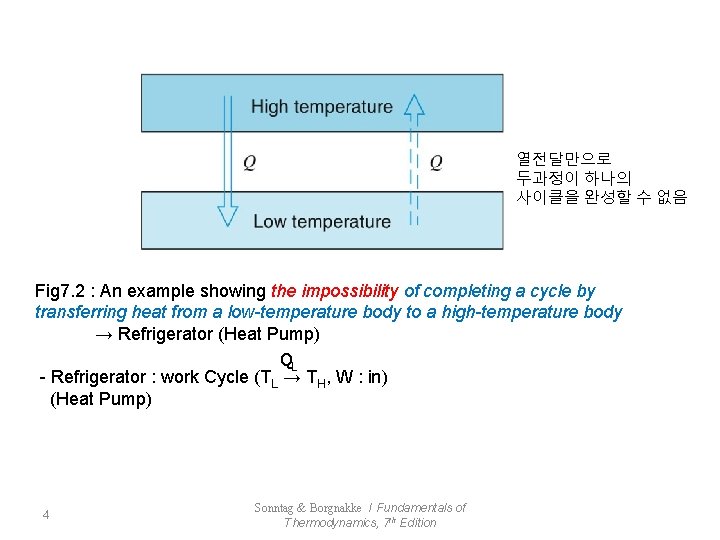
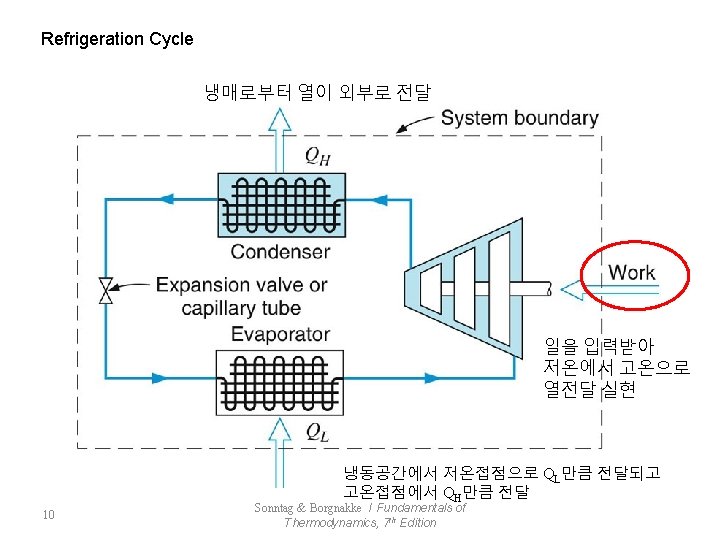
열전달만으로 두과정이 하나의 사이클을 완성할 수 없음 Fig 7. 2 : An example showing the impossibility of completing a cycle by transferring heat from a low-temperature body to a high-temperature body → Refrigerator (Heat Pump) QL - Refrigerator : work Cycle (TL → TH, W : in) (Heat Pump) 4 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

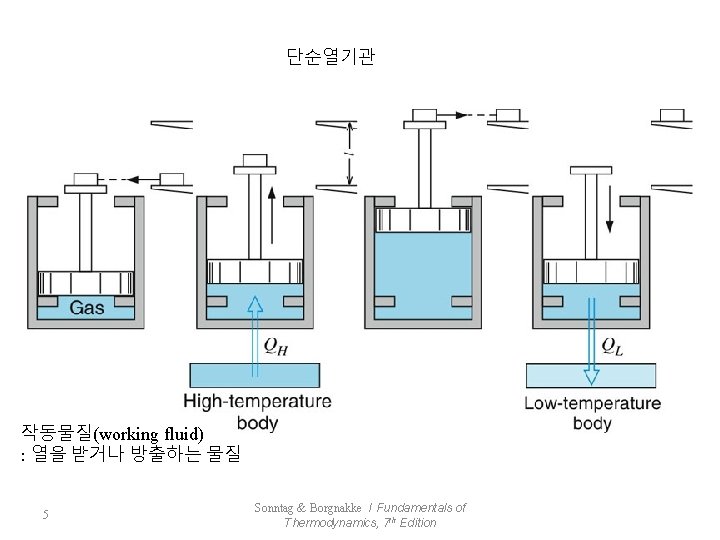
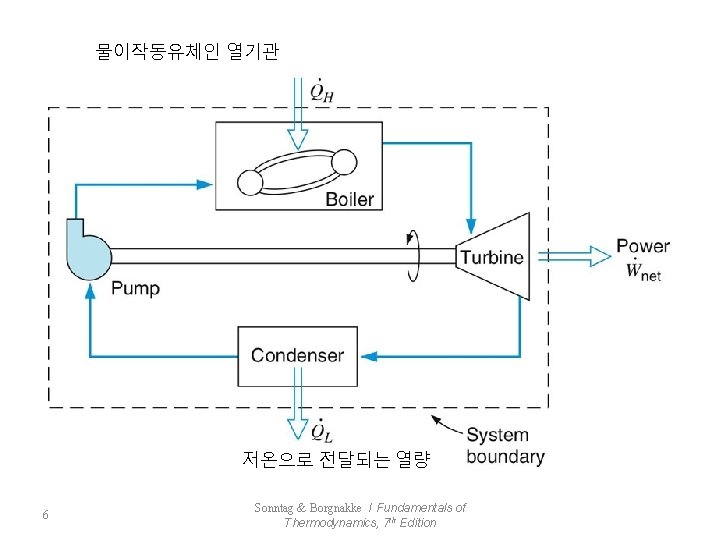
단순열기관 작동물질(working fluid) : 열을 받거나 방출하는 물질 5 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

물이작동유체인 열기관 저온으로 전달되는 열량 6 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

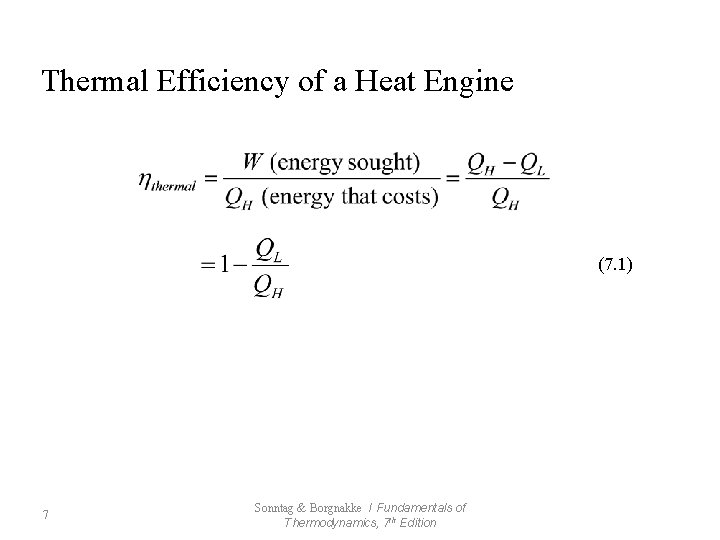
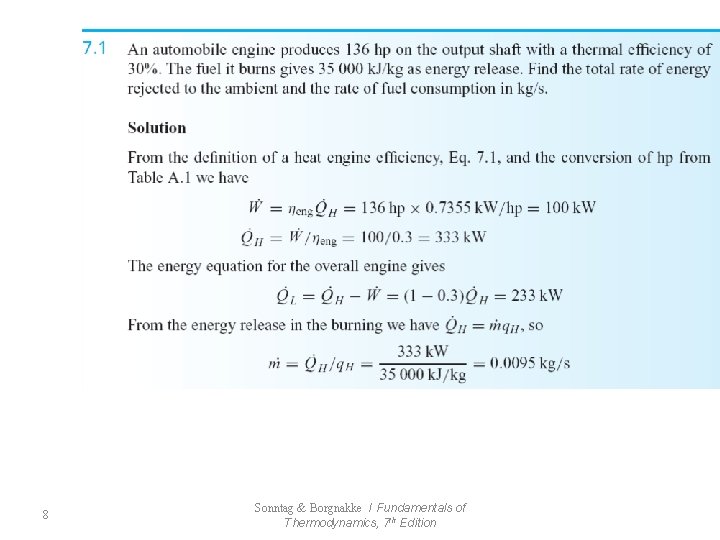
Thermal Efficiency of a Heat Engine (7. 1) 7 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

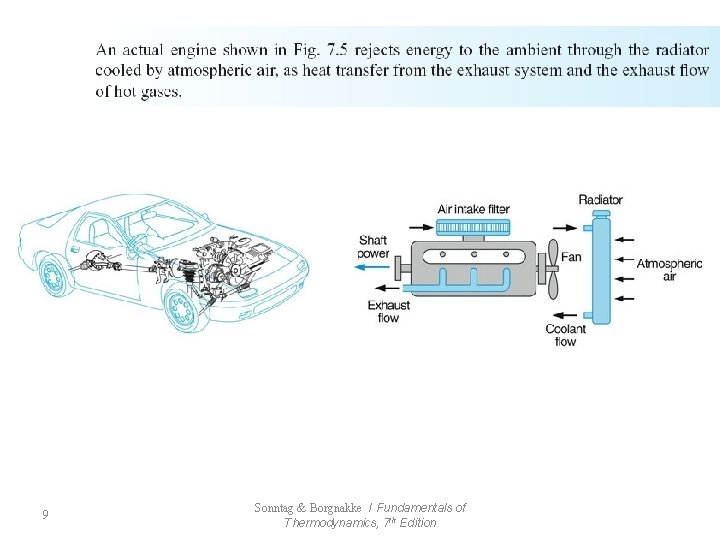
8 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

9 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition


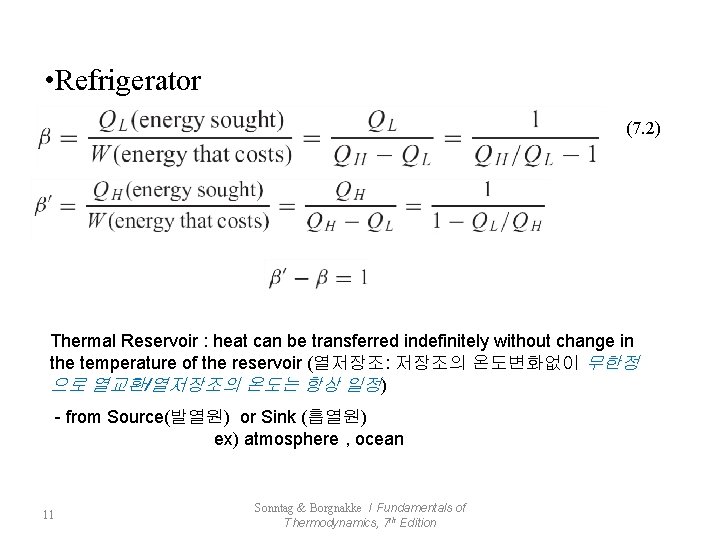
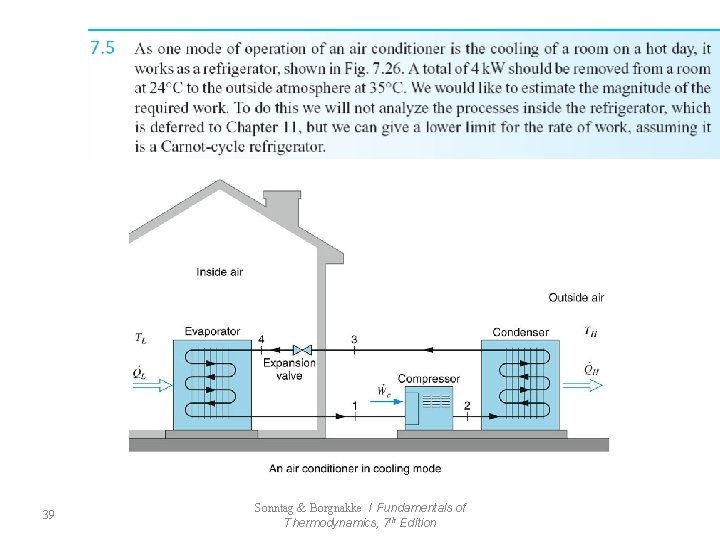
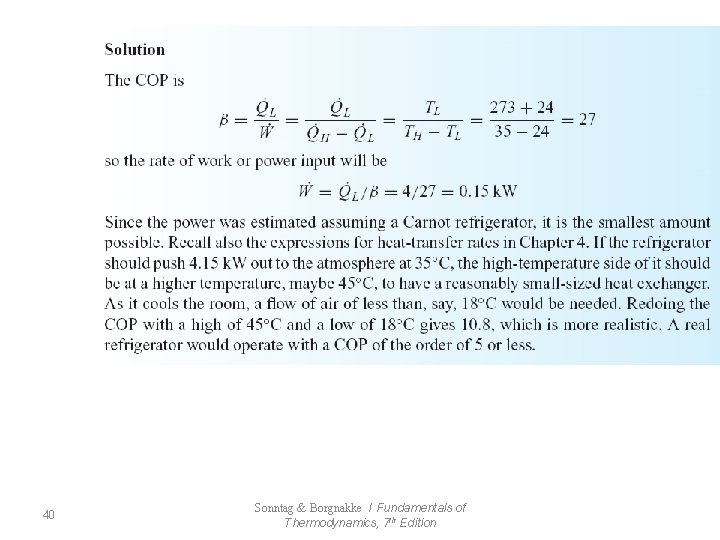
• Refrigerator (7. 2) Thermal Reservoir : heat can be transferred indefinitely without change in the temperature of the reservoir (열저장조: 저장조의 온도변화없이 무한정 으로 열교환/열저장조의 온도는 항상 일정) - from Source(발열원) or Sink (흡열원) ex) atmosphere , ocean 11 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

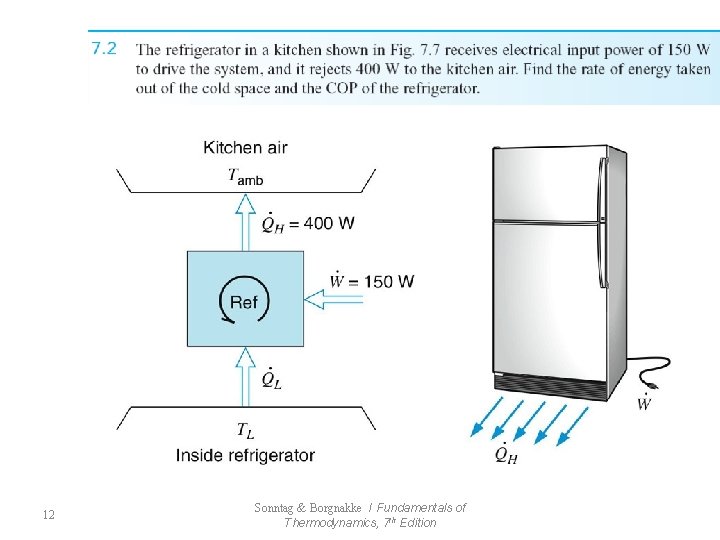
12 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

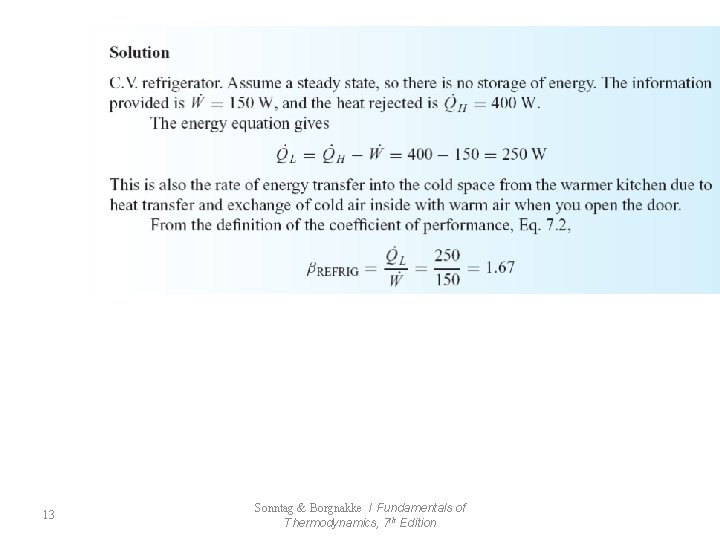
13 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

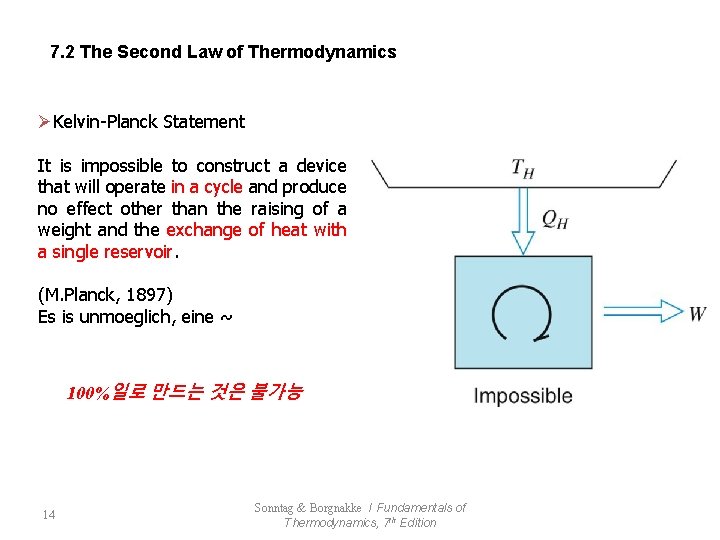
7. 2 The Second Law of Thermodynamics ØKelvin-Planck Statement It is impossible to construct a device that will operate in a cycle and produce no effect other than the raising of a weight and the exchange of heat with a single reservoir. (M. Planck, 1897) Es is unmoeglich, eine ~ 100%일로 만드는 것은 불가능 14 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

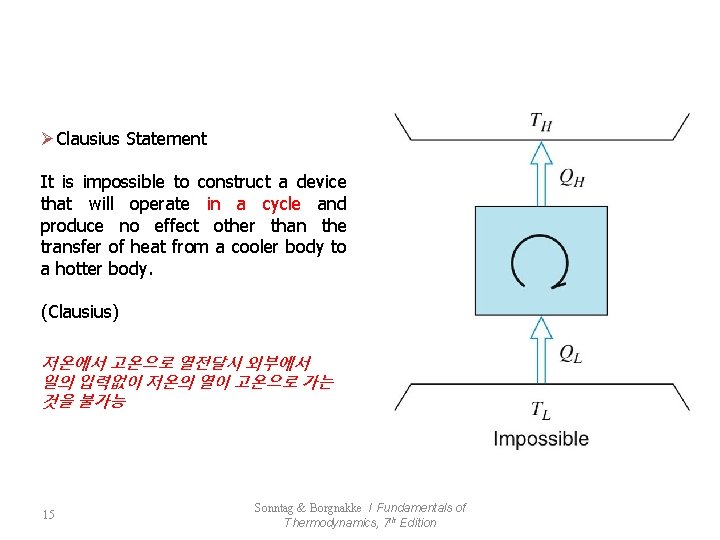
ØClausius Statement It is impossible to construct a device that will operate in a cycle and produce no effect other than the transfer of heat from a cooler body to a hotter body. (Clausius) 저온에서 고온으로 열전달시 외부에서 일의 입력없이 저온의 열이 고온으로 가는 것을 불가능 15 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

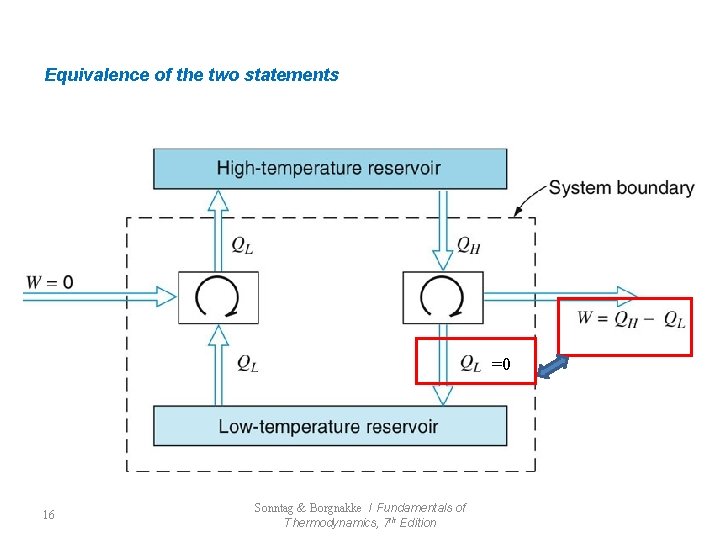
Equivalence of the two statements =0 16 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

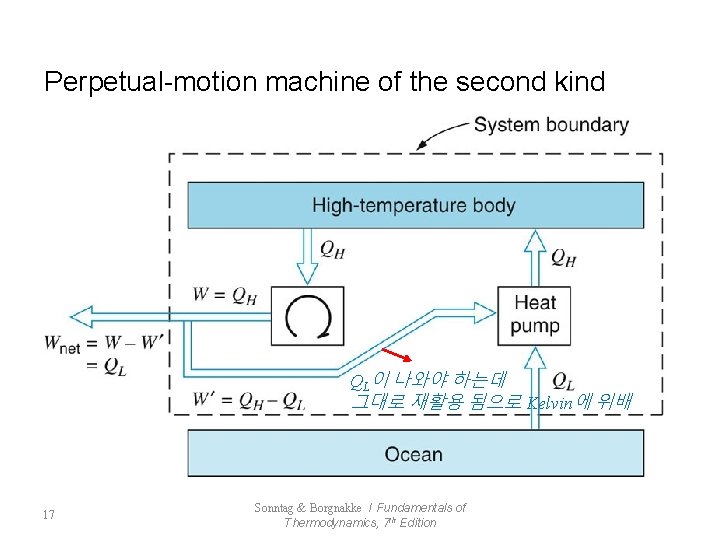
Perpetual-motion machine of the second kind QL이 나와야 하는데 그대로 재활용 됨으로 Kelvin에 위배 17 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

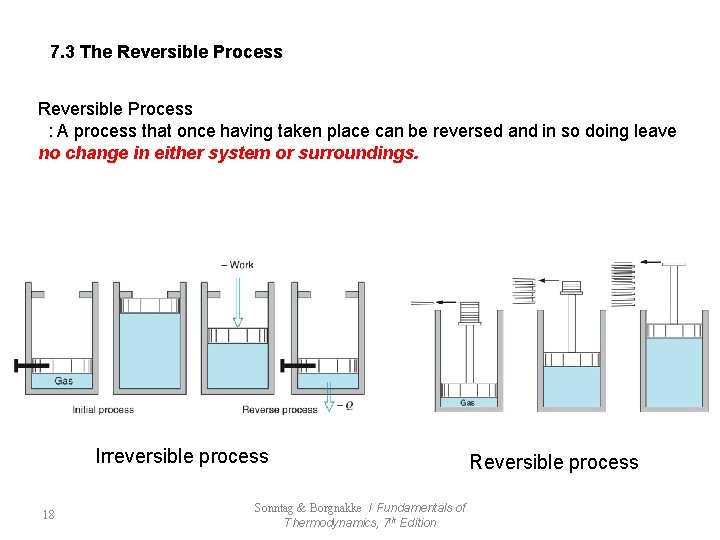
7. 3 The Reversible Process : A process that once having taken place can be reversed and in so doing leave no change in either system or surroundings. Irreversible process 18 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition Reversible process

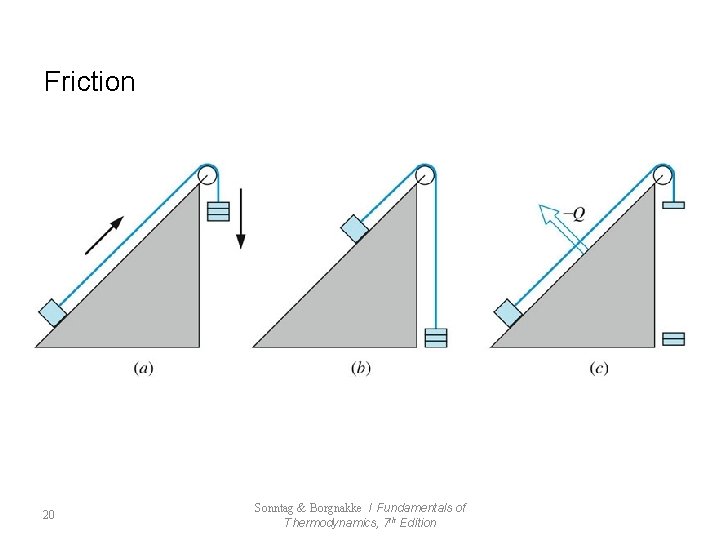
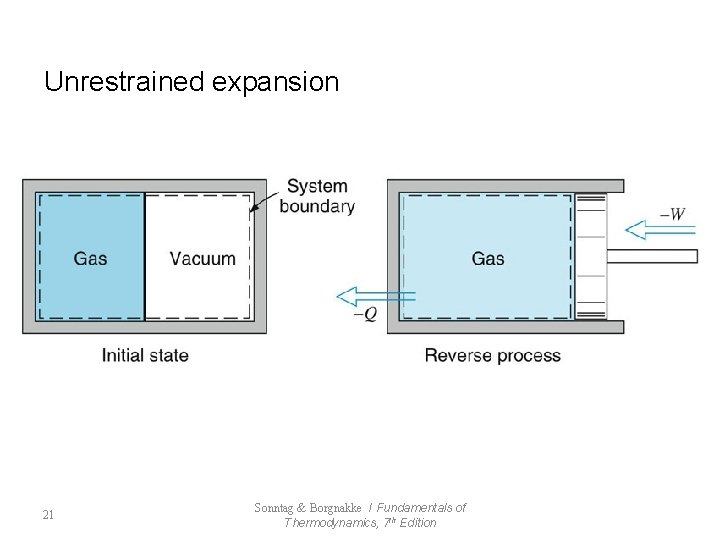
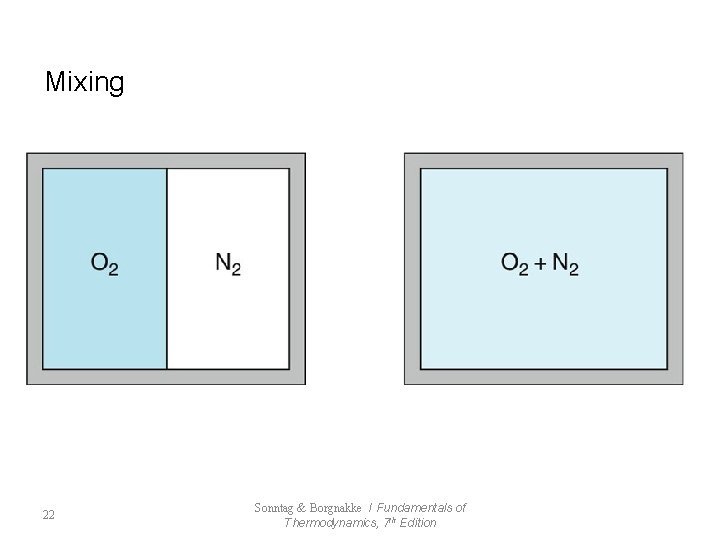
7. 4 Factors that Render Processes Irreversible l Reversible Process - frictionless mechanical process - frictionless adiabatic state change l Irreversible Process : impossible to return to original state (All Natural Process) - Friction - Unrestrained Expansion - Heat transfer Through a Finite Temperature Difference - Mixing of Two Different Substances - Other Factors 19 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

Friction 20 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

Unrestrained expansion 21 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

Mixing 22 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

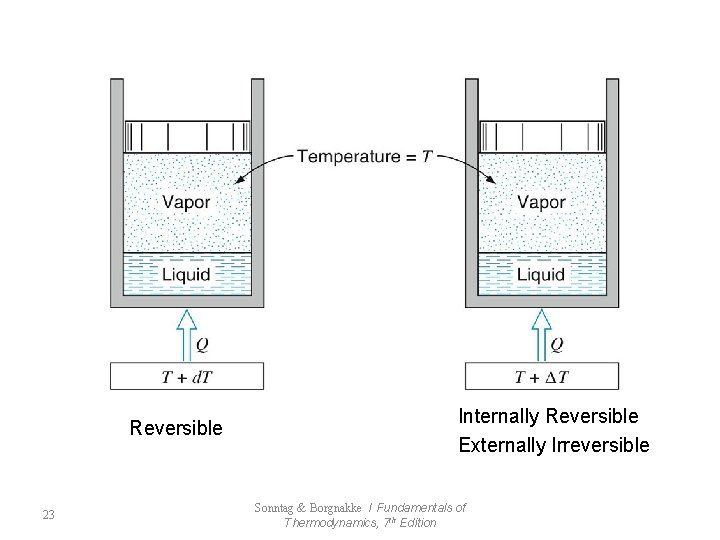
Reversible 23 Internally Reversible Externally Irreversible Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

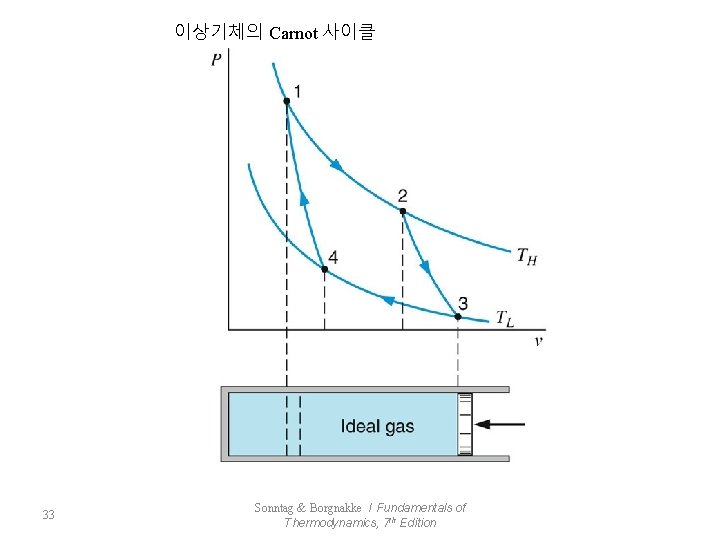
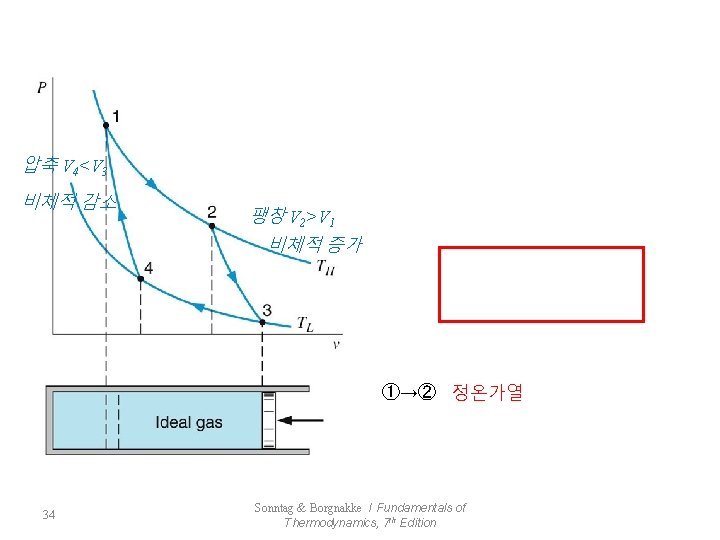
7. 5 The Carnot Cycle - Reversible : the most efficient cycle that can operate between two constant-temperature reservoirs 가역과정에서 가장 효율이 높은 사이클 1 : rev. isothermal process, QH is transferred to the system 2 : rev. adiabatic process, Working Fluid : TH→TL 3 : rev. isothermal process, QL is rejected from the system 4 : rev. adiabatic process, Working Fluid : TL→TH 24 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

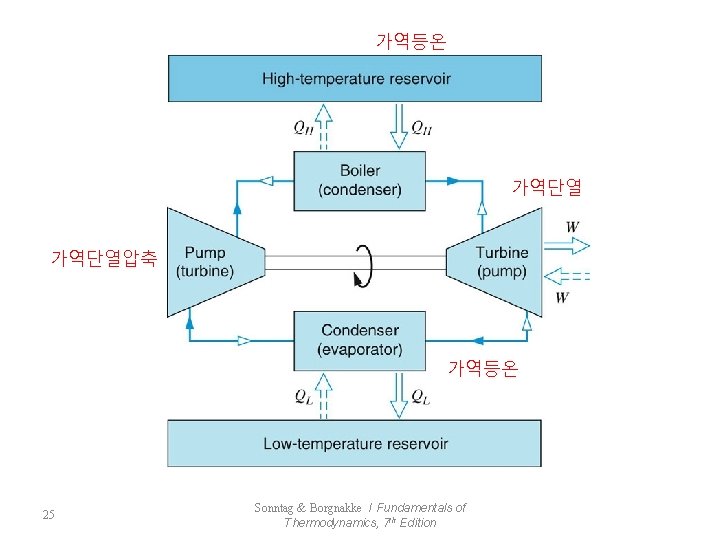
가역등온 가역단열압축 가역등온 25 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition


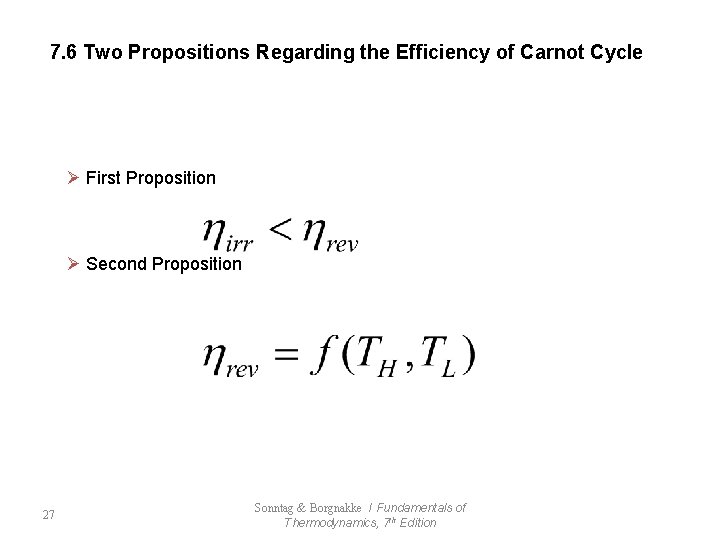
7. 6 Two Propositions Regarding the Efficiency of Carnot Cycle Ø First Proposition Ø Second Proposition 27 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

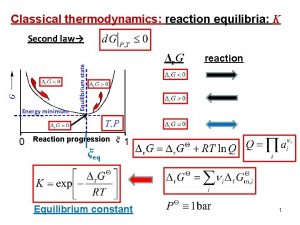
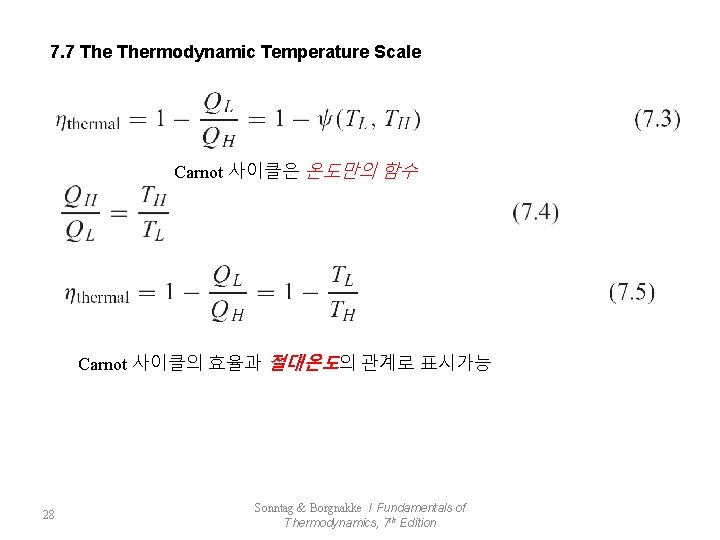
7. 7 Thermodynamic Temperature Scale Carnot 사이클은 온도만의 함수 Carnot 사이클의 효율과 절대온도의 관계로 표시가능 28 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

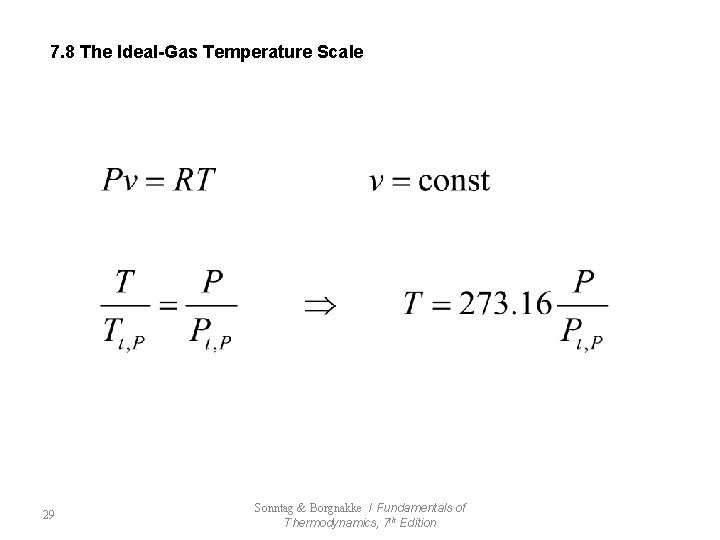
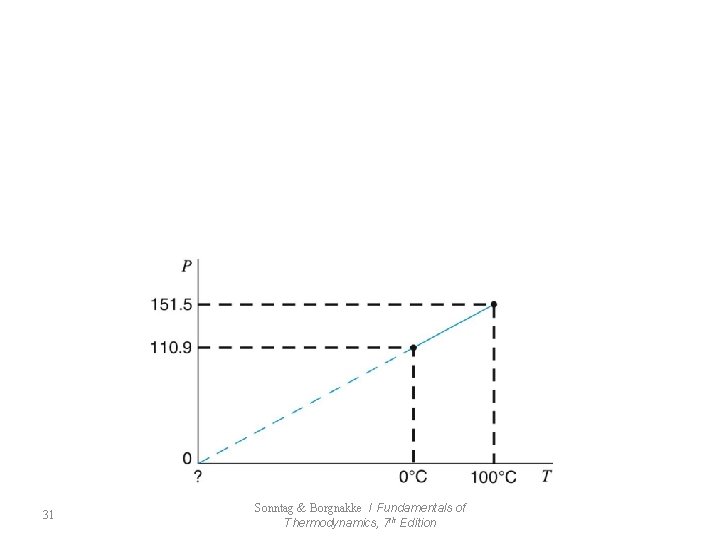
7. 8 The Ideal-Gas Temperature Scale 29 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

30 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

31 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

32 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

이상기체의 Carnot 사이클 33 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

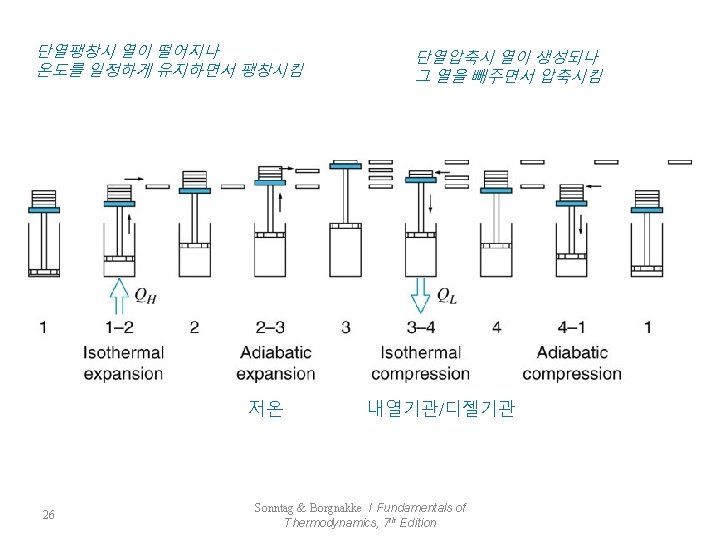
압축 V 4<V 3 비체적 감소 팽창 V 2>V 1 비체적 증가 ①→② 정온가열 34 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

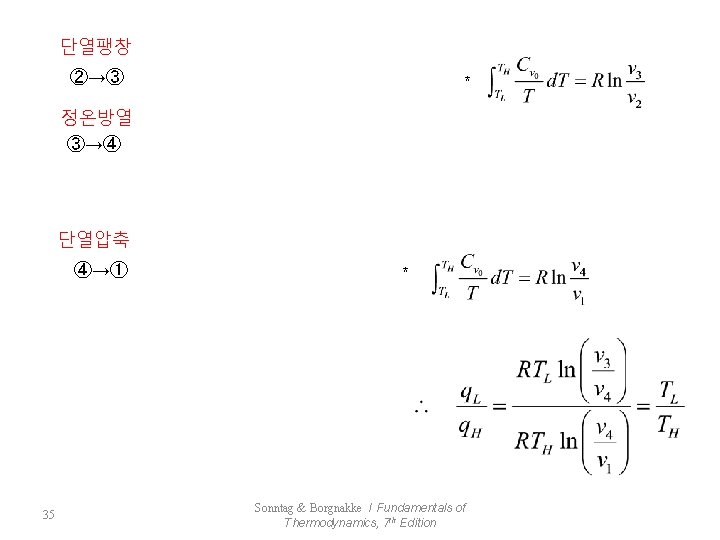
단열팽창 ②→③ * 정온방열 ③→④ 단열압축 ④→① 35 * Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

7. 9 Ideal vs. real machines 가역(Carnot)사이클 실제열기관/냉동기/열펌프 효율이 낮음 36 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

37 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

38 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

39 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

40 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

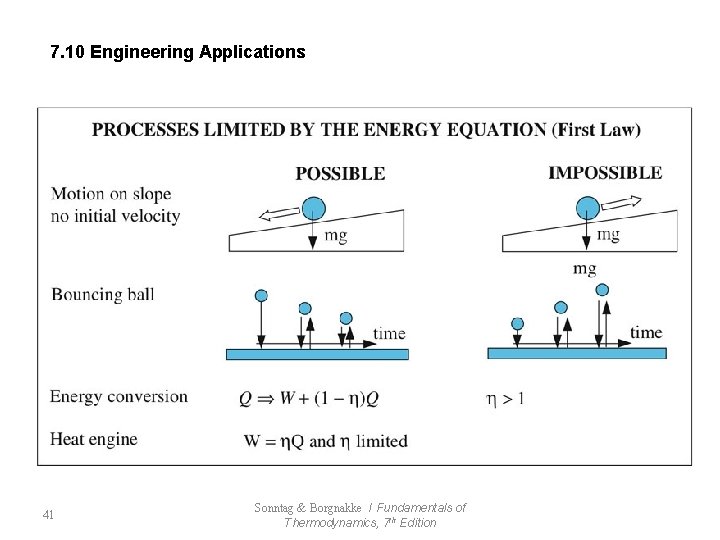
7. 10 Engineering Applications 41 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition

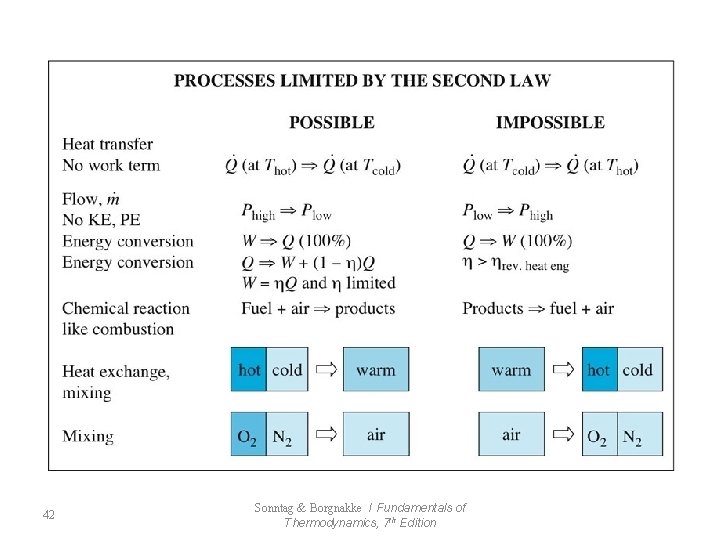
42 Sonntag & Borgnakke / Fundamentals of Thermodynamics, 7 th Edition
 Change in entropy formula
Change in entropy formula State second law of thermodynamics
State second law of thermodynamics P v t relationship in adiabatic process
P v t relationship in adiabatic process Second law of thermodynamics
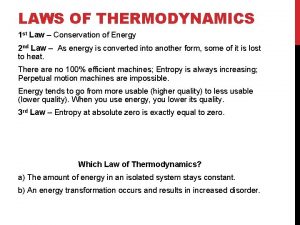
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics 2 nd law of thermodynamics
2 nd law of thermodynamics 2nd law of thermodynamics
2nd law of thermodynamics Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law and second law and third law
Newton's first law and second law and third law Classical thermodynamics
Classical thermodynamics Not a classical accompaniment of poulet maryland
Not a classical accompaniment of poulet maryland 27 miles per gallon into kilometers per liter
27 miles per gallon into kilometers per liter Sssf thermodynamics
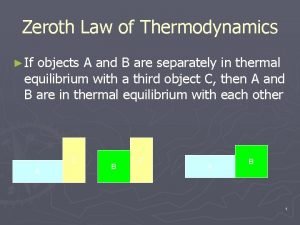
Sssf thermodynamics Zeroth law of thermodynamics
Zeroth law of thermodynamics Newtons third law of thermodynamics
Newtons third law of thermodynamics Zeroth law of thermodynamics statement
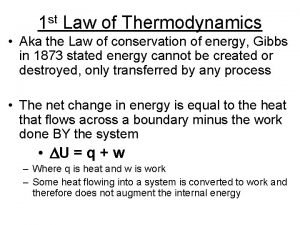
Zeroth law of thermodynamics statement First law of thermodynamics
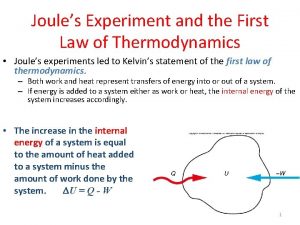
First law of thermodynamics First law of thermodynamics cyclic process
First law of thermodynamics cyclic process First law of thermodynamics joule's experiment
First law of thermodynamics joule's experiment Laws in thermodynamics
Laws in thermodynamics First law applied to flow process
First law applied to flow process What are the laws of thermodynamics
What are the laws of thermodynamics Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law Third law of thermodynamics is depend on
Third law of thermodynamics is depend on Dq=cdt
Dq=cdt First law of thermodynamics sign convention
First law of thermodynamics sign convention 1st law of thermodynamics
1st law of thermodynamics 1st law of thermodynamics
1st law of thermodynamics First law of thermodynamics control mass
First law of thermodynamics control mass Zeroth law of thermodynamics
Zeroth law of thermodynamics First law of thermodynamics
First law of thermodynamics Zeroth law of thermodynamics
Zeroth law of thermodynamics What is the law of thermodynamics
What is the law of thermodynamics Frist law of thermodynamics
Frist law of thermodynamics First law of thermodynamics for ideal gas
First law of thermodynamics for ideal gas V=k/p
V=k/p Constant of avogadro's law
Constant of avogadro's law Constant slope
Constant slope Mendel's second law of independent assortment
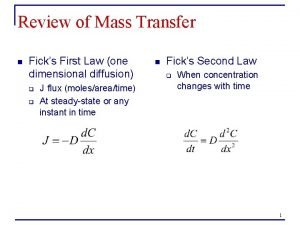
Mendel's second law of independent assortment Fick's second law of diffusion
Fick's second law of diffusion Energy of four forces quick check
Energy of four forces quick check Physics
Physics Physics sjsu
Physics sjsu