STATES OF MATTER REVIEW States of Matter Mass

















- Slides: 28

STATES OF MATTER REVIEW

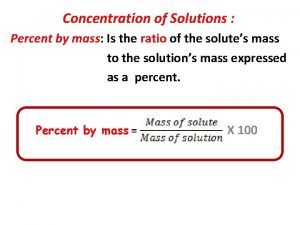
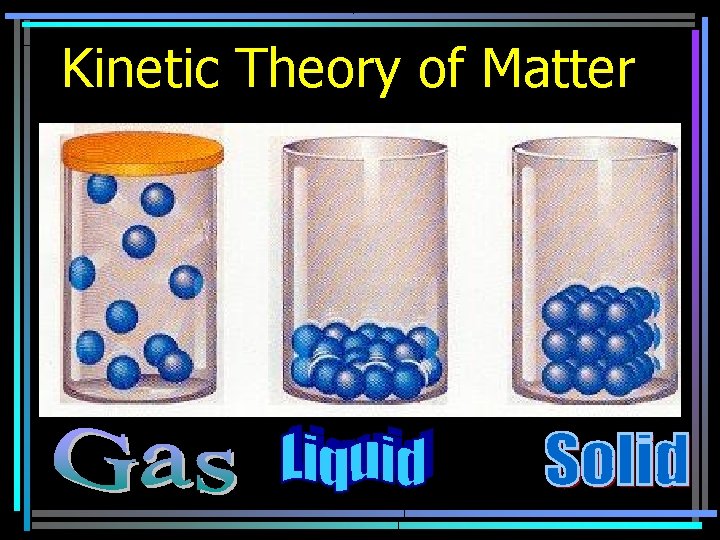
States of Matter • Mass & takes up space • No 2 pieces occupy the same space at the same time • 4 forms - depends on temperature – Solids – Liquids – Gases – Plasma

Solids • Definite volume & shape • Not enough energy to move – Crystalline – repeating geometric patterns – Noncrystalline – amorphous no true form, thick liquids

Liquids • Definite volume • No definite shape – takes shape of container • Particles have enough energy to move – Viscosity – property of how easily liquid flows

Gases • No definite volume or shape • Takes volume & shape of container • Particles have enough energy to overcome attractive forces holding them together & fill the container

Plasma • Like a gas • Lots of energy • Electrically charged, fast moving particles – the sun, space shuttle, lightening


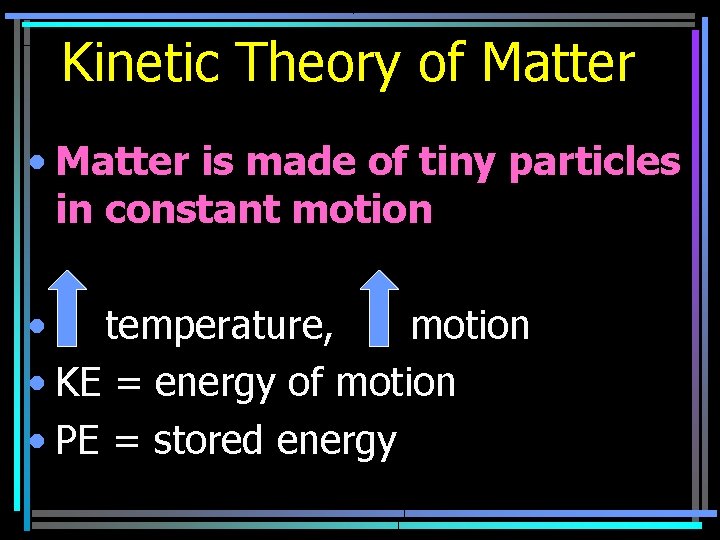
Kinetic Theory of Matter • Matter is made of tiny particles in constant motion • temperature, motion • KE = energy of motion • PE = stored energy

Kinetic Theory of Matter

Thermal Expansion • Increase temp, increase energy: particles move faster & further apart increasing the volume – Concrete slabs on bridge

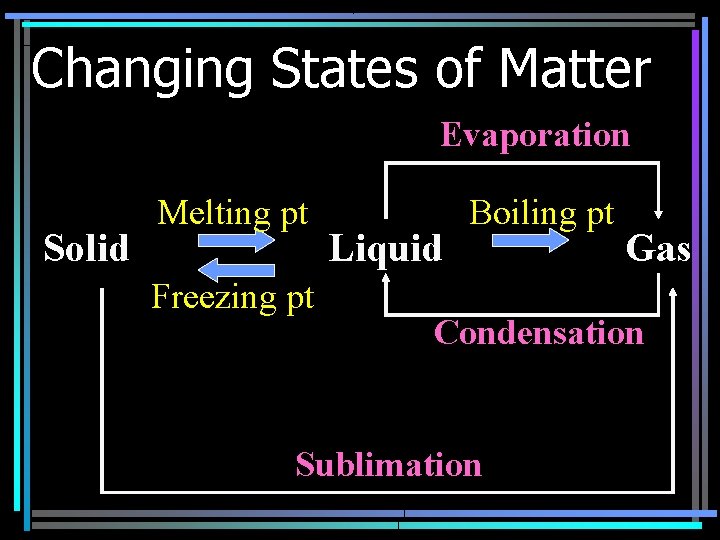
Changing States of Matter Evaporation Solid Melting pt Freezing pt Liquid Boiling pt Gas Condensation Sublimation


Heat of Fusion • Energy required for a substance to change from a solid to a liquid • Different for every substance • No temp Δ until complete – Ice to water 0°C until done – 334 k. J/kg for water

Heat of Vaporization • Energy required for a substance to change from a liquid to a gas • Different for every substance • No temp Δ until done – Water to steam 100°C – 2260 k. J/kg for water

Freezing Point Depression • Decrease the freezing point of water by dissolving particles in it – Salting icy roads & sidewalks • Ice melts as freezing pt is lowered – Ice cream makers • Ice Cream -3°C, Ice 0°C • Salt lowers freezing pt of ice so that ice cream can freeze

Boiling Point Elevation • Increase in the boiling pt of water by dissolving particles in it • Solute particles reduce how easily molecules get to surface to evaporate increasing bpt since water needs more energy

Part 2 Behavior of Gases & Fluids

• Pressure – amount of force per unit area (pascal, Pa) P = F/A • Atm. pressure @ sea level 101. 3 k. Pa – Pressure decreases w/ elevation: fewer gas particles • Gas Laws treat gases as ideal: no volume or attraction btwn molecules

Boyles Law • If you decrease the volume of a container of gas, the pressure of the gas will increase as long as temperature remains constant • Movie: Men of Honor

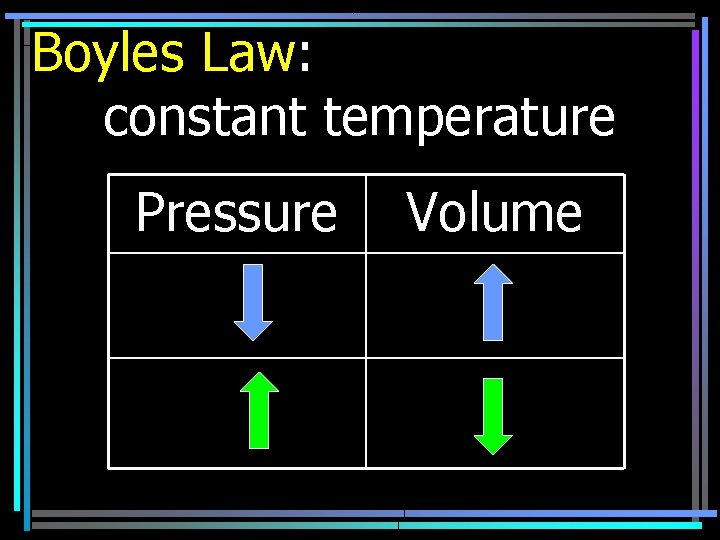
Boyles Law: constant temperature Pressure Volume

Charles Law • Volume of a gas increases as temp increases if pressure remains constant –Absolute Zero lowest possible temperature no movement of particles

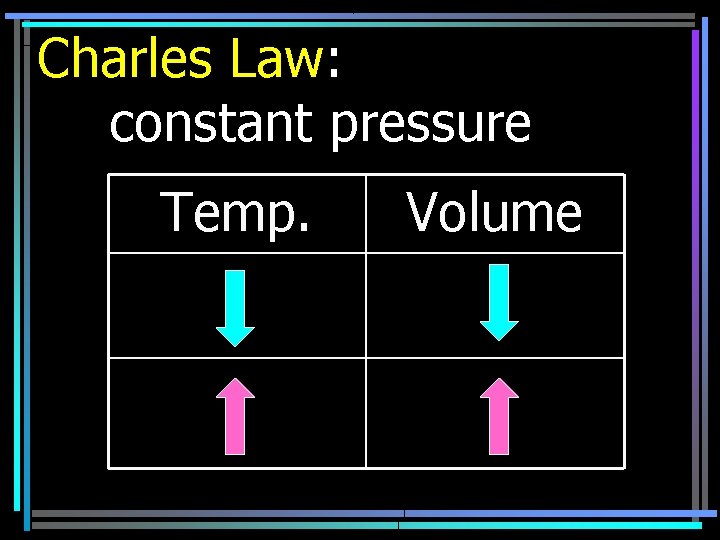
Charles Law: constant pressure Temp. Volume

Fluids • Buoyancy – the ability of a fluid (liquid or gas) to exert an upward force on an object immersed in it – Force = weight… Floats – Force < weight… Sinks – Force > weight… Rises

Archimedes’ Principle • Bouyant force on an object in a fluid equals the weight of the fluid displaced by the object

Pascal’s Principle • Pressure applied to a fluid is transmitted unchanged throughout the fluid – Ex. pistons, toothpaste –P=F/A

Bernoulli’s Principle • As the velocity of a fluid increases, the pressure on it decreases – Ex. air plane wing

Venturi Effect • A fluid flows faster when forced through narrow spaces –Ex. windy cities

 Chapter 10 review states of matter section 4
Chapter 10 review states of matter section 4 Mass to mass stoichiometry formula
Mass to mass stoichiometry formula Oxygen electrons per shell
Oxygen electrons per shell Is atomic mass and relative atomic mass the same
Is atomic mass and relative atomic mass the same Percentage composition of propane
Percentage composition of propane Inertial mass vs gravitational mass
Inertial mass vs gravitational mass Moles liters and molarity
Moles liters and molarity Molar mass unit
Molar mass unit Molar mass def
Molar mass def Molar mass to grams
Molar mass to grams Molar mass unit
Molar mass unit Mass/mass problems
Mass/mass problems Atomic
Atomic Gravitational mass vs inertial mass
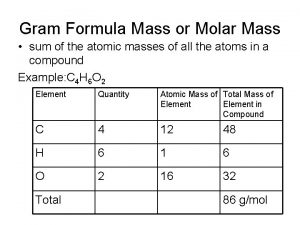
Gravitational mass vs inertial mass Gram formula mass
Gram formula mass Cl- molar mass
Cl- molar mass Pico unit
Pico unit Formula mass vs molar mass
Formula mass vs molar mass Mass to mass percent
Mass to mass percent Inertial mass vs gravitational mass
Inertial mass vs gravitational mass Co2 relative molecular mass
Co2 relative molecular mass Isotope abundance formula
Isotope abundance formula Atomic mass vs molar mass
Atomic mass vs molar mass Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Does an iron nail gain mass or lose mass when it rusts
Does an iron nail gain mass or lose mass when it rusts A car of mass 1200kg pulls a trailer of mass 400kg
A car of mass 1200kg pulls a trailer of mass 400kg Formula mass vs gram formula mass
Formula mass vs gram formula mass Formula mass vs gram formula mass
Formula mass vs gram formula mass Cold air mass overtakes warm air mass
Cold air mass overtakes warm air mass