States of Matter What is Matter Mass is








- Slides: 16

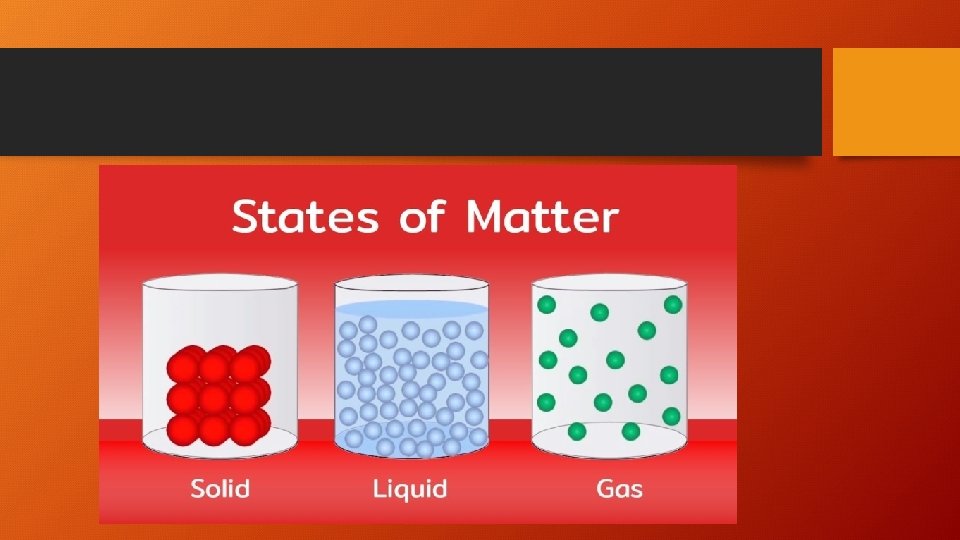
States of Matter

What is Matter? • Mass is the amount of material that makes up an object. • Volume is the amount of space that a material takes up. Anything that has both MASS and VOLUME is called MATTER!

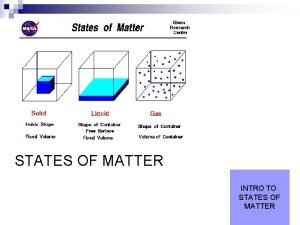
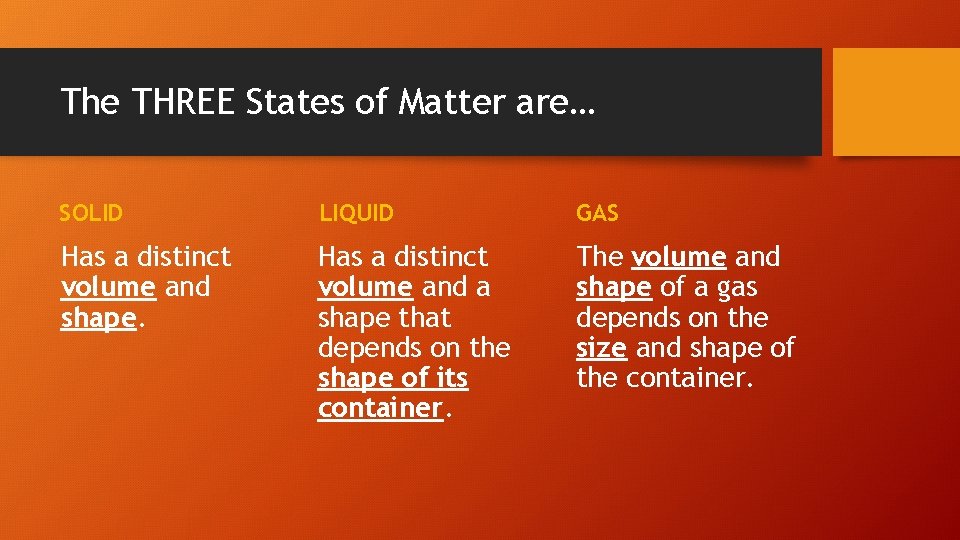
The THREE States of Matter are… SOLID LIQUID GAS Has a distinct volume and shape. Has a distinct volume and a shape that depends on the shape of its container. The volume and shape of a gas depends on the size and shape of the container.


The fourth state… • PLASMAS do not have a defined shape and volume (like a gas), but plasmas have different electrical properties than gases.

What happens as temperature changes? • When you add energy to matter, its temperature rises. This causes matter to expand. Expansion: An increase in the volume of something when its temperature rises. Example. Temperature of air balloon rises = volume of air increases = balloon gets bigger

• When you take energy away from matter, its temperature falls. This causes matter to contract. Contraction: Decrease in volume of something when its temperature falls. Example. Temperature of air balloon falls = volume of air decreases = balloon gets smaller

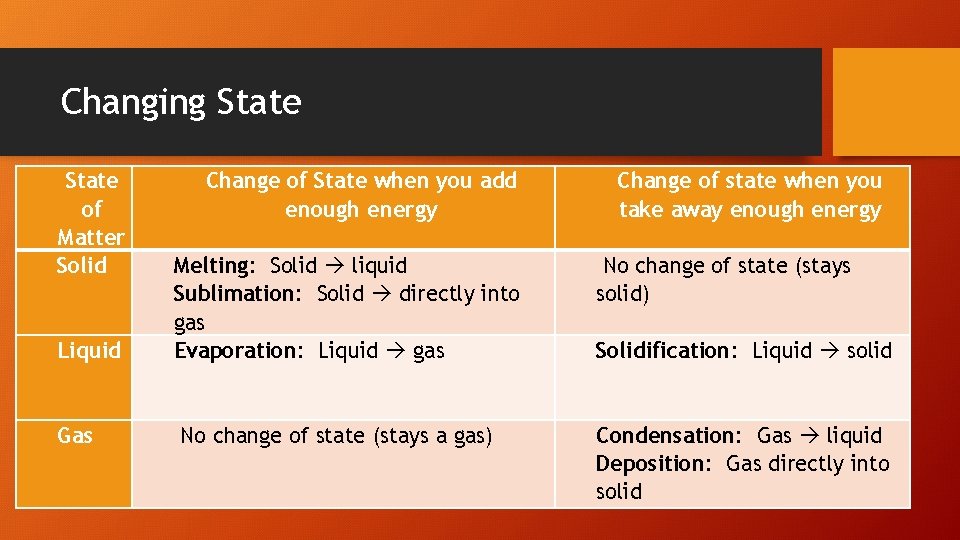
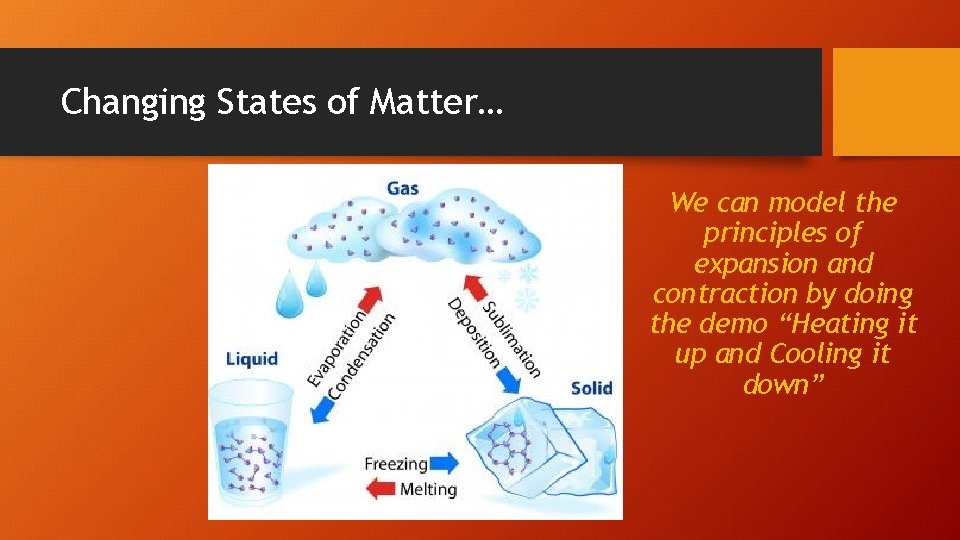
Changing State of Matter Solid Liquid Gas Change of State when you add enough energy Change of state when you take away enough energy Melting: Solid liquid Sublimation: Solid directly into gas Evaporation: Liquid gas No change of state (stays solid) No change of state (stays a gas) Condensation: Gas liquid Deposition: Gas directly into solid Solidification: Liquid solid

Changing States of Matter… We can model the principles of expansion and contraction by doing the demo “Heating it up and Cooling it down”

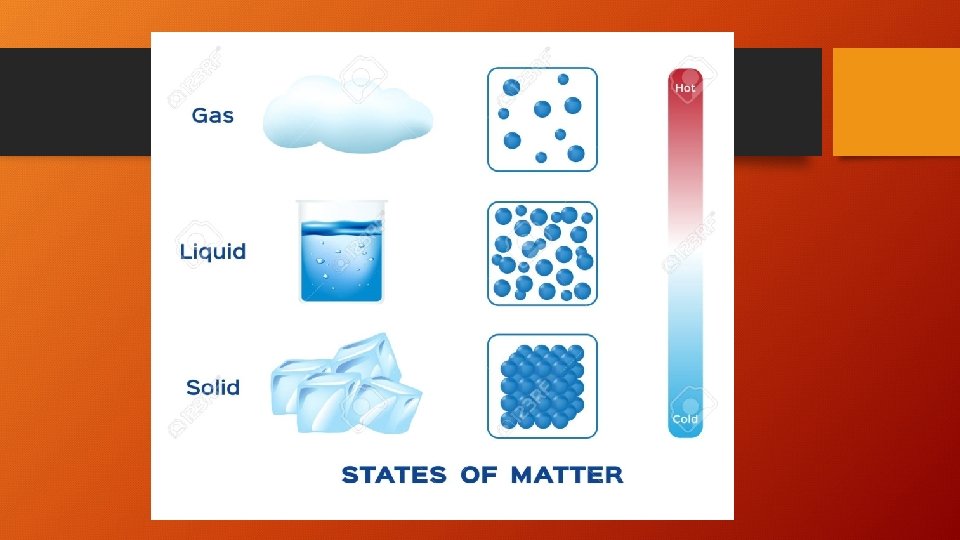
Kinetic Molecular Theory Matter is made up of tiny particles. These have KINETIC energy… This means they are always moving! • Particles of a solid are packed close together. They are so close, they CAN`T move freely, they can only vibrate. • Particles of a liquid are spaced farther apart. They can slide past each other. • Particles of gas are spaced very far apart. They move around quickly.

• When energy is ADDED to particles, they move faster. They move farther apart and the matter EXPANDS. • When energy is REMOVED from particles, they move more slowly. This brings them closer together and the matter CONTRACTS.


Diffusion Demo

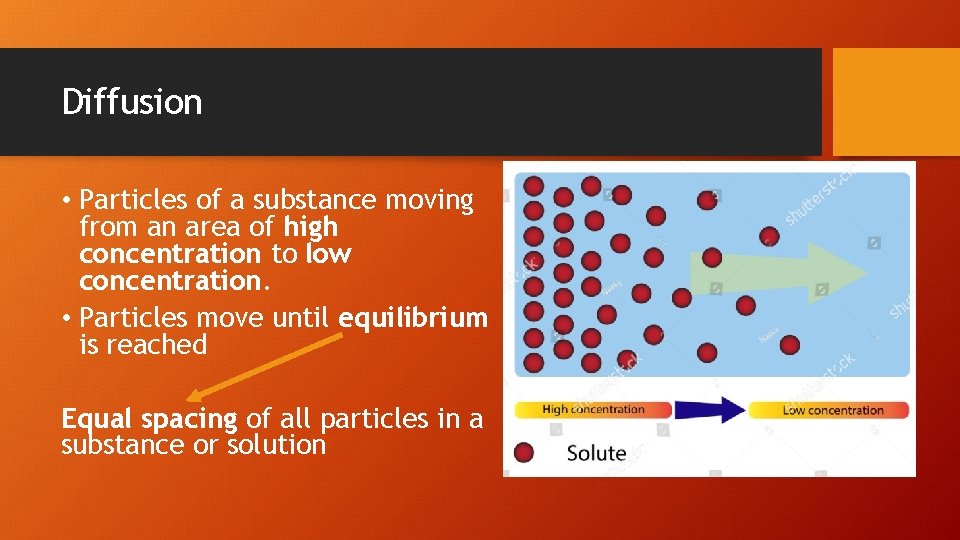
Diffusion • Particles of a substance moving from an area of high concentration to low concentration. • Particles move until equilibrium is reached Equal spacing of all particles in a substance or solution

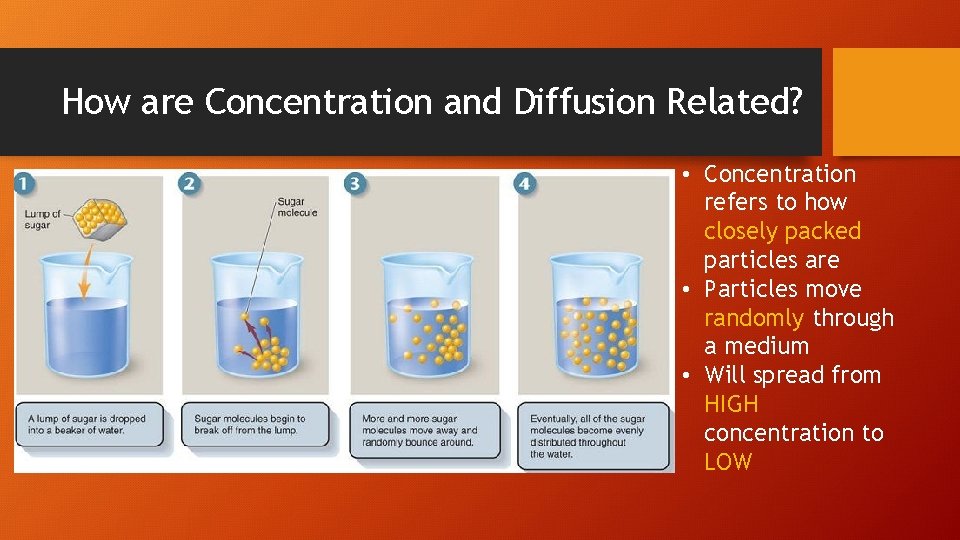
How are Concentration and Diffusion Related? • Concentration refers to how closely packed particles are • Particles move randomly through a medium • Will spread from HIGH concentration to LOW

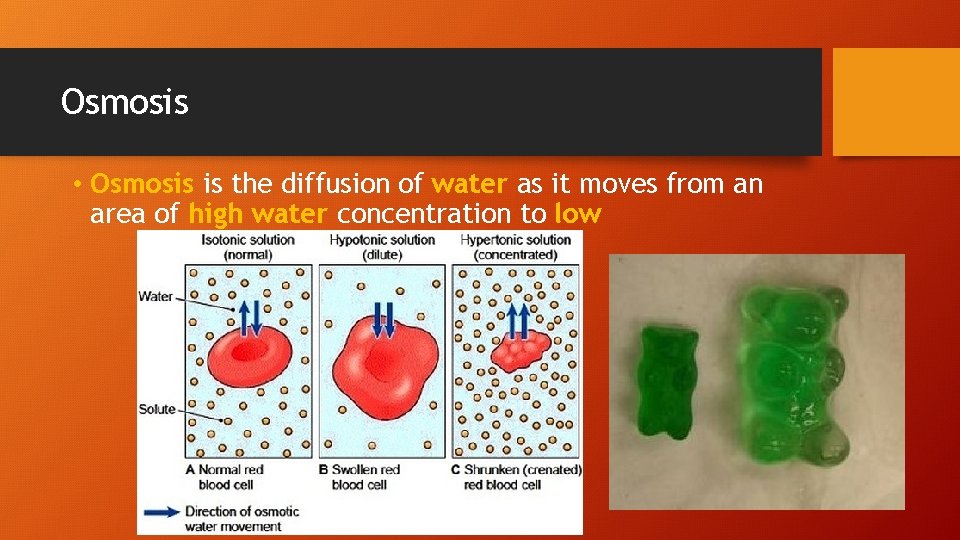
Osmosis • Osmosis is the diffusion of water as it moves from an area of high water concentration to low
 Southern states
Southern states Tyranny
Tyranny What were the 11 free states
What were the 11 free states Four phases of matter
Four phases of matter Phases of matter concept map
Phases of matter concept map States of matter foldable
States of matter foldable Four phase changes
Four phase changes State of matter in chemical equations
State of matter in chemical equations Changing states of matter
Changing states of matter States of matter solid liquid gas
States of matter solid liquid gas States of matter graph
States of matter graph Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids The kinetic theory of matter states that
The kinetic theory of matter states that States of matter objectives
States of matter objectives Whats the four states of matter
Whats the four states of matter Four states of matter
Four states of matter Thermal energy in states of matter
Thermal energy in states of matter