SESIN FORMATIVA DE ENSAYOS CLNICOS RETOS Y OPORTUNIDADES



















- Slides: 42

SESIÓN FORMATIVA DE ENSAYOS CLÍNICOS RETOS Y OPORTUNIDADES EN LOS ENSAYOS “FIRST IN HUMAN” Emiliano Calvo, MD Ph. D START Madrid – Centro Integral Oncológico Clara Campal Hospital Universitario HM Sanchinarro, Madrid #SEOM 2019

Disclosure Information EMILIANO CALVO 1/ Honoraria or consultation fees: Astellas, Novartis, Nanobiotix, Pfizer, Janssen-Cilag, GLG, Psi. Oxus Therapeutics, Merck, Medscape, BMS, G Pierre Fabre, Boehringer Ingelheim, Cerulean Pharma, EUSA, Gehrmann Consulting, Astra. Zeneca, Roche, Guidepoint, Servier, Celgene, Abbvie Alkermes 2/ Leadership role: Director, Clinical Research, START Madrid, Director, Clinical Research, HM Hospitals Group, Madrid 3/ Stocks or ownership: START, Oncoart Associated, International Cancer Consultants 4/ Licensing fees or royalties: None 5/ Direct research funding as project lead: Novartis, Astra. Zeneca, Beigene 6/ Institutional financial support from clinical trials: Abbvie, ACEO, Amcure, AMGEN, Astra. Zeneca, BMS, Cytomx, GSK, Genentech/Roche, H Lilly, Loxo, Nektar, Macrogenics, Menarini, Merck, Merus, Nanobiotix, Novartis, Pfizer, Pharma. Mar, Principia, PUMA, Sanofi, Taiho, Tesaro, Bei. G Incyte, Innovio, MSD, Psi. Oxus, Seattle Genetics, Mersana, GSK, Daiichi, Nektar, Astellas, ORCA, Boston Therapeutics, Dynavax, Debio. Pharm, B Regeneron, Millenium, Synthon, Spectrum, Rigontec 7/ Non-financial interests: Scientific board at Psi. Oxus 8/ Leadership in medical society: Founder and president, non-for-profit Foundation INTHEOS (Investigational Therapeutics in Oncological Scie 9/ Memberships: SEOM, EORTC, ESMO, ASCO 10/ Other relationships: HM Hospitals Group and START, Program of Early Phase Clinical Drug Development in Oncology, Employee: Medical Director, Clinical Research. Methods in Clinical Cancer Research (MCCR) Workshop, Zeist, Netherlands (Joint ECCO-AACR-EORTC-ESMO Wo Methods in Clinical Cancer, Research), Co-director. #SEOM 2019

Agenda q Unidades de ensayos clínicos “Early Phase” q Nuevos diseños de ensayos clínicos “Early Phase” q Futuro #SEOM 2019

Agenda q Unidades de ensayos clínicos “Early Phase” q Nuevos diseños de ensayos clínicos “Early Phase” q Futuro #SEOM 2019

ENSAYOS CLÍNICOS “EARLY PHASE” EN ONCOLOGÍA Phase 1 studies: “the most critical step from bench to bedside” • Not only first time in humans – Unquestionably an exciting event! • The interface between preclinical testing and the start of human exploration of a new cancer drug – Integration of preclinical pharmacokinetics, pharmacodynamics and toxicology – Starting point for rational clinical development #SEOM 2019

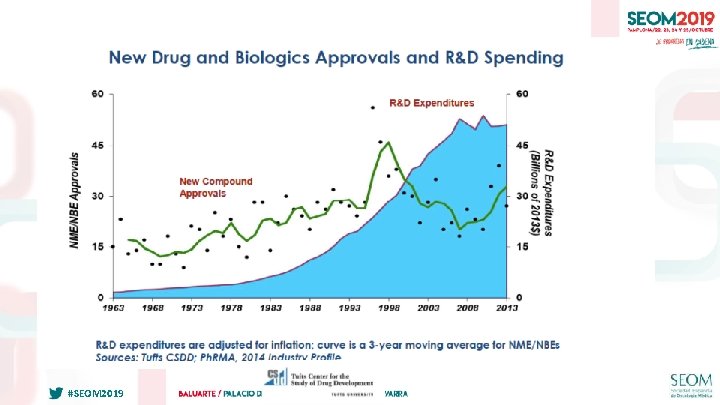
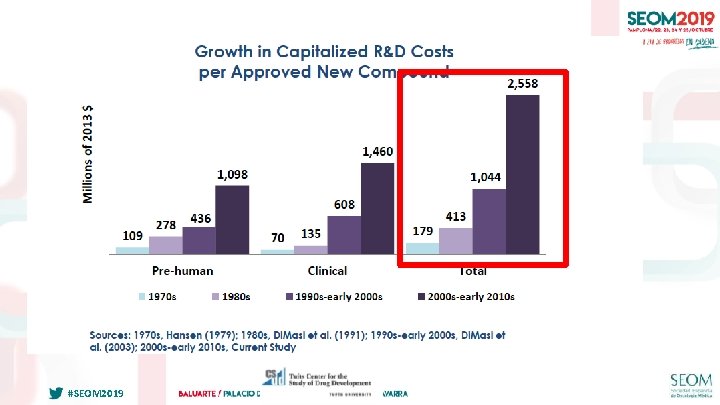
IMPORTANCIA DE LOS EECC “EARLY PHASE” EN ONCOLOGÍA #SEOM 2019

IMPORTANCIA DE LOS EECC “EARLY PHASE” EN ONCOLOGÍA #SEOM 2019

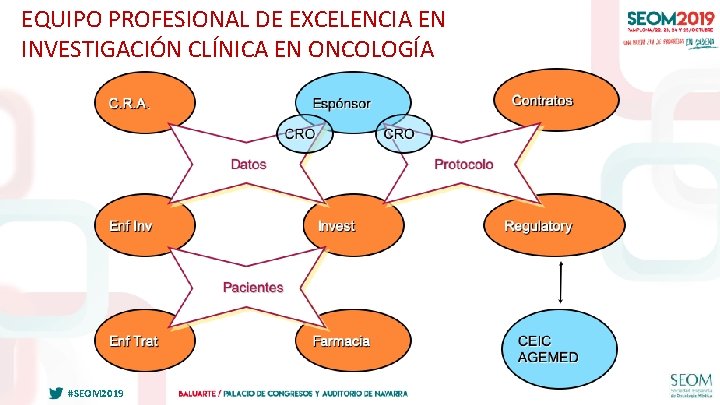
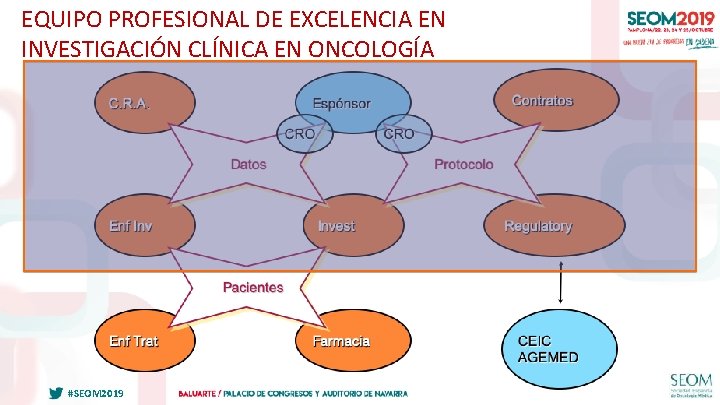
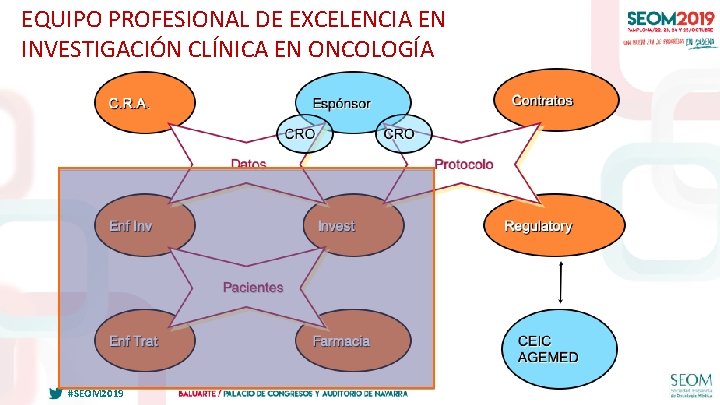
EQUIPO PROFESIONAL DE EXCELENCIA EN INVESTIGACIÓN CLÍNICA EN ONCOLOGÍA #SEOM 2019

EQUIPO PROFESIONAL DE EXCELENCIA EN INVESTIGACIÓN CLÍNICA EN ONCOLOGÍA #SEOM 2019

EQUIPO PROFESIONAL DE EXCELENCIA EN INVESTIGACIÓN CLÍNICA EN ONCOLOGÍA #SEOM 2019

#SEOM 2019

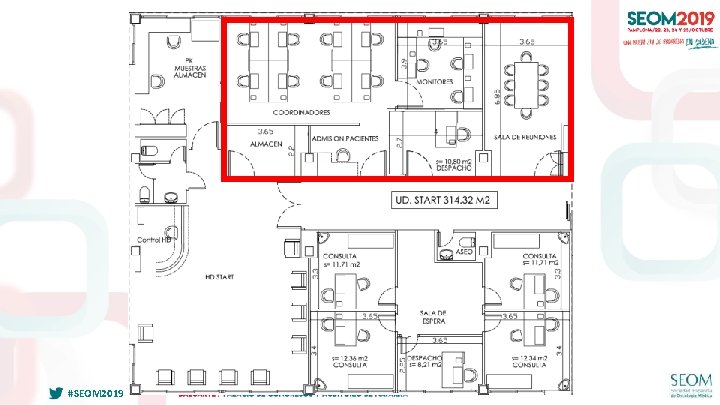
FUNCIONAMIENTO EFICIENTE UNIDADES EARLY PHASE #SEOM 2019

INTEGRACIÓN EN CENTRO ONCOLÓGICO #SEOM 2019

SINERGIAS CON OTROS PROGRAMAS “CLUSTERS” (CIOCC) #SEOM 2019

Agenda q Unidades de ensayos clínicos “Early Phase” q Nuevos diseños de ensayos clínicos “Early Phase” q Futuro #SEOM 2019

“DRIVERS” OF CHANGE • Inefficient regulatory impulse: Better drugs, faster and cheaper for our patients • Need for Early Phase trials to be more informative • Adaptation of study designs and clinical units to the type of drugs in development #SEOM 2019 16

#SEOM 2019

#SEOM 2019

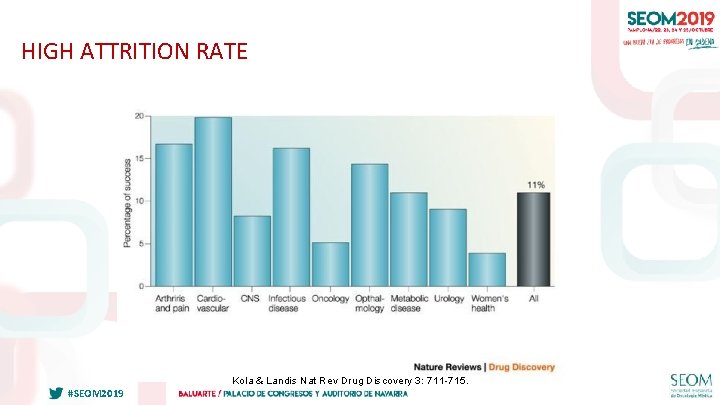
HIGH ATTRITION RATE Kola & Landis Nat Rev Drug Discovery 3: 711 -715. #SEOM 2019

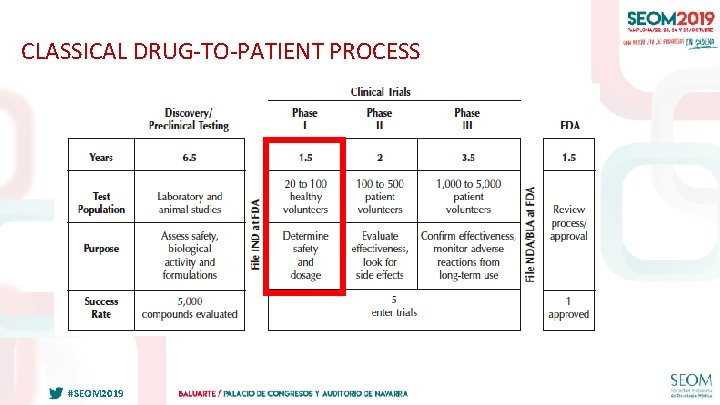
CLASSICAL DRUG-TO-PATIENT PROCESS #SEOM 2019

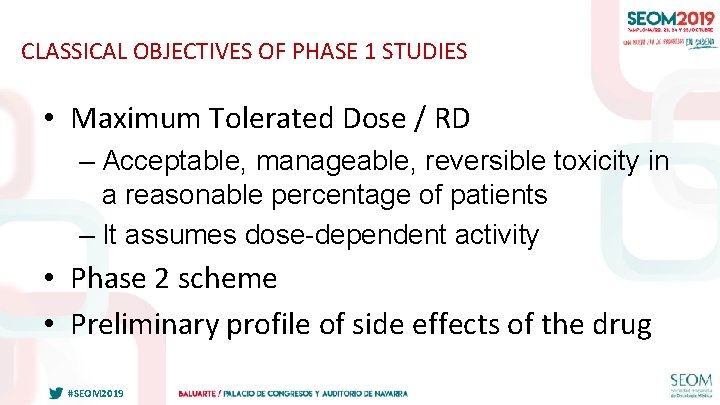
CLASSICAL OBJECTIVES OF PHASE 1 STUDIES • Maximum Tolerated Dose / RD – Acceptable, manageable, reversible toxicity in a reasonable percentage of patients – It assumes dose-dependent activity • Phase 2 scheme • Preliminary profile of side effects of the drug #SEOM 2019

Unmet need in Clinical Drug Development: Transform Early Phase Clinical Trials to become more informative #SEOM 2019

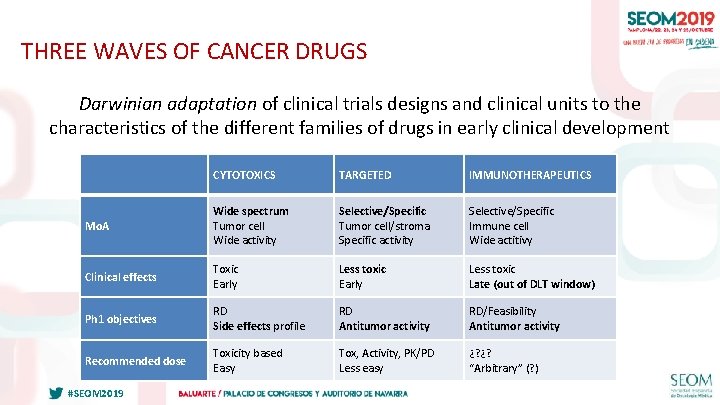
THREE WAVES OF CANCER DRUGS Darwinian adaptation of clinical trials designs and clinical units to the characteristics of the different families of drugs in early clinical development CYTOTOXICS TARGETED IMMUNOTHERAPEUTICS Mo. A Wide spectrum Tumor cell Wide activity Selective/Specific Tumor cell/stroma Specific activity Selective/Specific Immune cell Wide actitivy Clinical effects Toxic Early Less toxic Late (out of DLT window) Ph 1 objectives RD Side effects profile RD Antitumor activity RD/Feasibility Antitumor activity Recommended dose Toxicity based Easy Tox, Activity, PK/PD Less easy ¿? ¿? “Arbitrary” (? ) #SEOM 2019 23

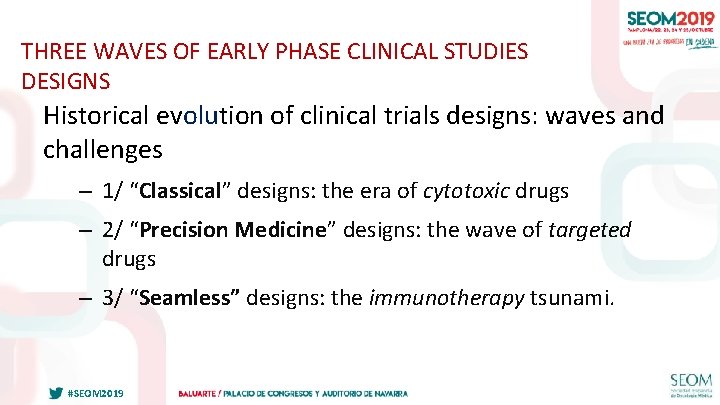
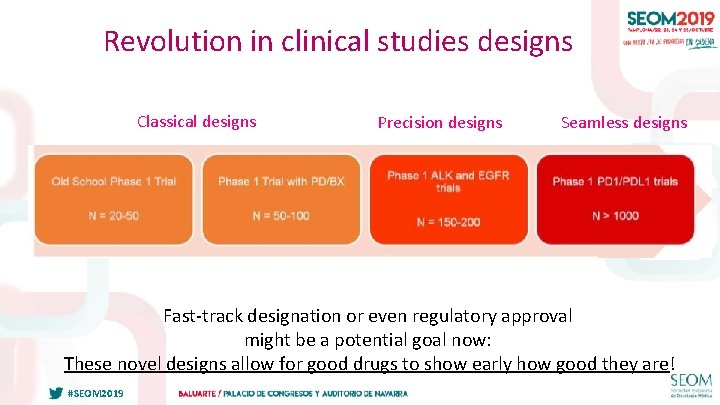
THREE WAVES OF EARLY PHASE CLINICAL STUDIES DESIGNS Historical evolution of clinical trials designs: waves and challenges – 1/ “Classical” designs: the era of cytotoxic drugs – 2/ “Precision Medicine” designs: the wave of targeted drugs – 3/ “Seamless” designs: the immunotherapy tsunami. #SEOM 2019 24

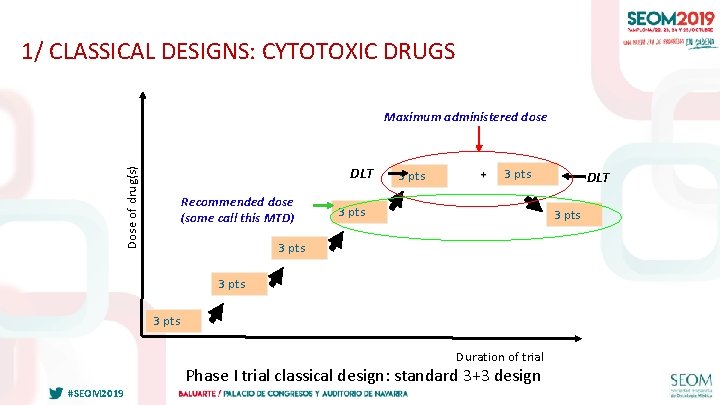
1/ CLASSICAL DESIGNS: CYTOTOXIC DRUGS Maximum administered dose Dose of drug(s) DLT Recommended dose (some call this MTD) 3 pts + 3 pts 3 pts Duration of trial #SEOM 2019 DLT Phase I trial classical design: standard 3+3 design

1/ CLASSICAL DESIGNS: CYTOTOXIC DRUGS “Pharmacological Audit Trail” #SEOM 2019 Workman P. Nature Chemical Biology 12, 689 -700, 2006

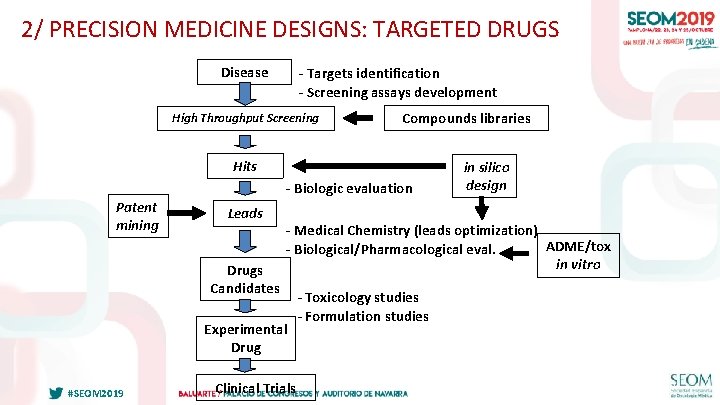
2/ PRECISION MEDICINE DESIGNS: TARGETED DRUGS Disease - Targets identification - Screening assays development High Throughput Screening Compounds libraries Hits - Biologic evaluation Patent mining Leads Drugs Candidates - Medical Chemistry (leads optimization) ADME/tox - Biological/Pharmacological eval. in vitro Experimental Drug #SEOM 2019 in silico design Clinical Trials - Toxicology studies - Formulation studies 27

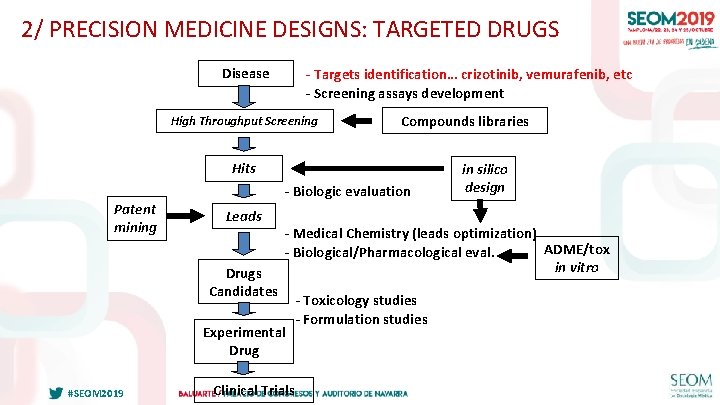
2/ PRECISION MEDICINE DESIGNS: TARGETED DRUGS Disease - Targets identification… crizotinib, vemurafenib, etc - Screening assays development High Throughput Screening Compounds libraries Hits - Biologic evaluation Patent mining Leads Drugs Candidates - Medical Chemistry (leads optimization) ADME/tox - Biological/Pharmacological eval. in vitro Experimental Drug #SEOM 2019 in silico design Clinical Trials - Toxicology studies - Formulation studies 28

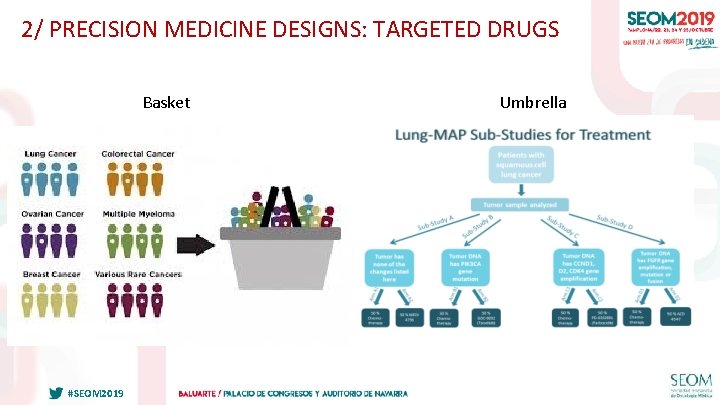
2/ PRECISION MEDICINE DESIGNS: TARGETED DRUGS Basket #SEOM 2019 Umbrella

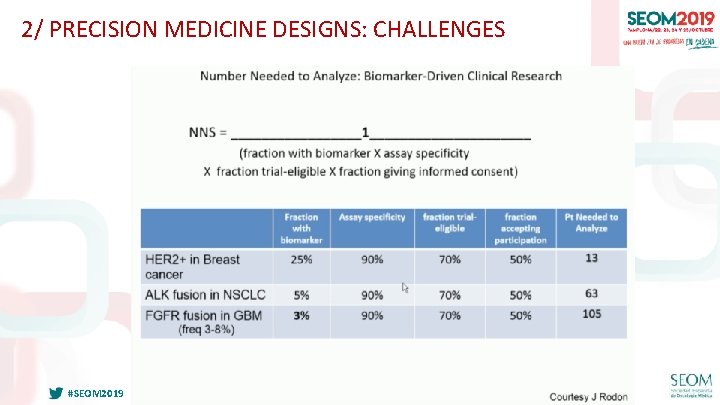
2/ PRECISION MEDICINE DESIGNS: CHALLENGES #SEOM 2019

2/ PRECISION MEDICINE DESIGNS: CHALLENGES • Rapid evolution of knowledge about targets and drugs – Agents found not to be effective against target – Evaluation of the wrong molecular aberration (MET amp vs exon 14 skipping mut) – Variants of unknown significance – Agent found to be efficacious… is there a rationale for continuing study? • It requires flexible, adaptive design and only a few arms may ultimately be successful -> what to do next? • Tumor heterogeneity • Who pays for molecular testing platform? • Non-biopsiable disease #SEOM 2019

2/ PRECISION MEDICINE DESIGNS • They are here to stay!!: – Accessible holistic molecular screening – Enthusiasm of patients and physicians for molecular screening – Liquid biopsies #SEOM 2019

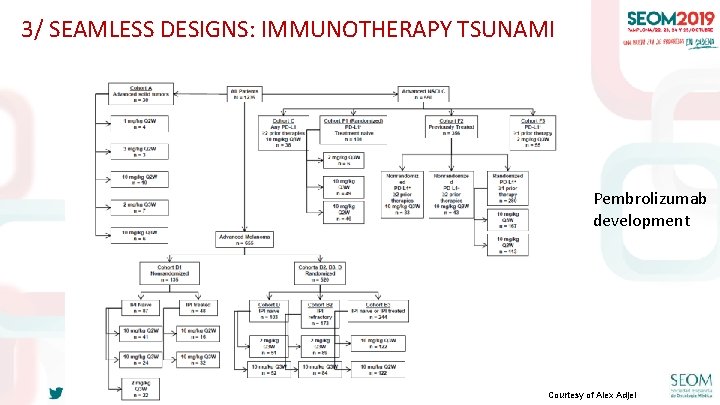
3/ SEAMLESS DESIGNS: IMMUNOTHERAPY TSUNAMI Pembrolizumab development #SEOM 2019 Courtesy of Alex Adjei

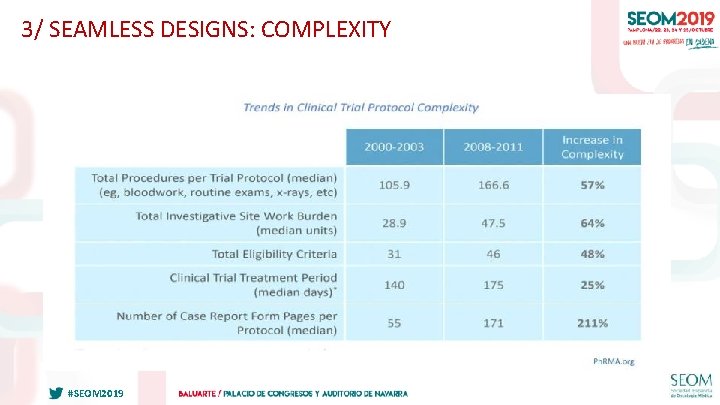
3/ SEAMLESS DESIGNS: COMPLEXITY Slide 5 #SEOM 2019

3/ SEAMLESS DESIGNS: CHALLENGES • Challenging intellectual complexity – Slots updates, dynamic selection criteria, dose/escalation mistakes • Trials never seem to close to enrollment – Higher workload (PIs, RNs…) per study – Increasing number of amendments (reconsents, training) • Competitive/challenging slots: many arms, few slots, many sites #SEOM 2019

3/ SEAMLESS DESIGNS: IMMUNOTHERAPY TSUNAMI • New endpoints in Early Phase: costs, PROs, efficacy… • Re-building of Clinical Trials programs – Sophisticated low-volume “three-star Michelin” plus very efficient high-volume “Mc. Donalds franchise” in same restaurant! – Different tumor type populations • Knowledge and expertise needed • Synergy early/late phase programs #SEOM 2019

Agenda q Unidades de ensayos clínicos “Early Phase” q Nuevos diseños de ensayos clínicos “Early Phase” q Futuro #SEOM 2019

Revolution in clinical studies designs Classical designs Precision designs Seamless designs Slide 4 Fast-track designation or even regulatory approval might be a potential goal now: These novel designs allow for good drugs to show early how good they are! #SEOM 2019

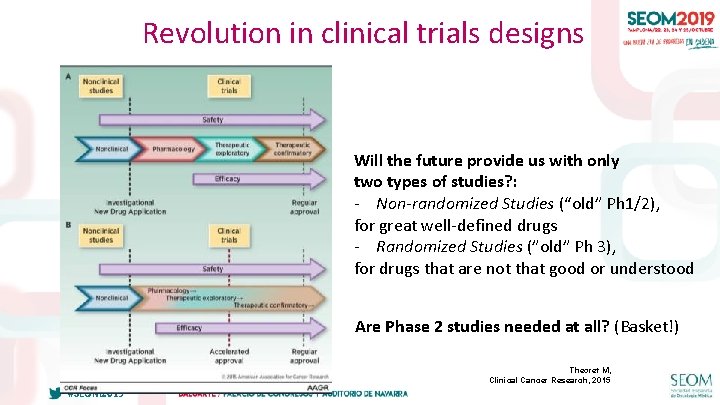
Revolution in clinical trials designs Will the future provide us with only two types of studies? : - Non-randomized Studies (“old” Ph 1/2), for great well-defined drugs - Randomized Studies (”old” Ph 3), for drugs that are not that good or understood Are Phase 2 studies needed at all? (Basket!) Theoret M, Clinical Cancer Research, 2015 #SEOM 2019

REGULATORY IMPULSE, BUT NOT ENOUGH • • • #SEOM 2019 Regulatory agencies lately allow for quicker access of patients to innovative drugs – Outstanding signal of activity in Ph 1/2 might be enough for breakthrough designation or conditional approvals – Increasing number of approved novel drugs to compete against each others However, the real medical value is to be confirmed with randomized studies after fast-track conditional approval – Is it ethical? How to do it? Still, faster approval does not bring lower prices and wider access to drugs – Fixed high prices – Disconnection between value and cost

REGULATORY IMPULSE, BUT NOT ENOUGH Drug Access market: - Highly interventional - We need a libertarian open-market revolution 100 million Euros Price and Value 10 Euros “Todo necio confunde valor y precio” (Antonio Machado) #SEOM 2019

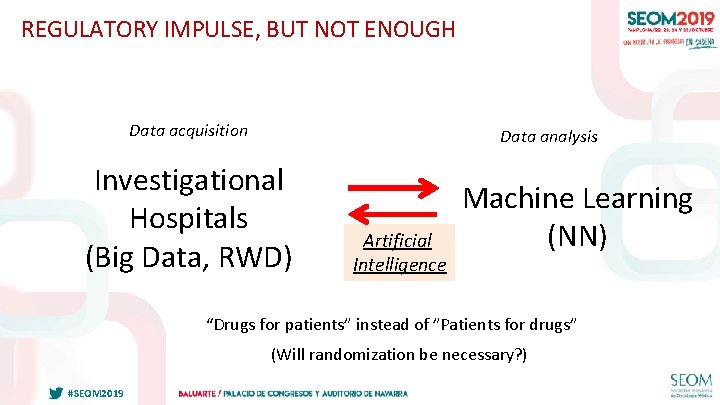
REGULATORY IMPULSE, BUT NOT ENOUGH Data acquisition Data analysis Investigational Hospitals (Big Data, RWD) Artificial Intelligence Machine Learning (NN) “Drugs for patients” instead of ”Patients for drugs” (Will randomization be necessary? ) #SEOM 2019
 Nuevos retos nuevas oportunidades
Nuevos retos nuevas oportunidades Desobel sirop
Desobel sirop Soundakustik
Soundakustik Sesin psikoakustik özellikleri
Sesin psikoakustik özellikleri Dr sesin
Dr sesin Sesin 4
Sesin 4 Como cambiar el arroba en twitter
Como cambiar el arroba en twitter Iniciar sesin
Iniciar sesin Convenciones institucionales
Convenciones institucionales Carlos sesin
Carlos sesin Iniciar sesin
Iniciar sesin Caldeiras aquatubulares de tubos retos
Caldeiras aquatubulares de tubos retos Pronome de vitória amava o sol
Pronome de vitória amava o sol Pronomes pessoais do caso retos
Pronomes pessoais do caso retos Retos de la lectura digital en mexico
Retos de la lectura digital en mexico O que e pronomes
O que e pronomes Que é um pronome
Que é um pronome Entre eu ou entre mim
Entre eu ou entre mim Alberto aibar
Alberto aibar Los retos escolares en la era digital
Los retos escolares en la era digital Keyexelate
Keyexelate Retos de convivencia
Retos de convivencia Naturaleza y retos de las escuelas normales
Naturaleza y retos de las escuelas normales Pronomes pessoais retos
Pronomes pessoais retos Retos y limites de las autoridades
Retos y limites de las autoridades Retos primarios y secundarios
Retos primarios y secundarios Ifce um robo caminhando
Ifce um robo caminhando No hay segundas oportunidades para una primera impresión
No hay segundas oportunidades para una primera impresión Cada dia senhor surgem oportunidades
Cada dia senhor surgem oportunidades Oportunidades diferenciadas
Oportunidades diferenciadas Esquema sqa
Esquema sqa Oportunidades valencia
Oportunidades valencia Foda n
Foda n Oportunidades de exportacion hortalizas
Oportunidades de exportacion hortalizas Matriz de riesgos y oportunidades iso 14001
Matriz de riesgos y oportunidades iso 14001 Dinamica sobre oportunidades
Dinamica sobre oportunidades Dios nos da nuevas oportunidades
Dios nos da nuevas oportunidades Oportunidades no paraguai
Oportunidades no paraguai Matriz de oportunidades
Matriz de oportunidades Que es un ensayo literario
Que es un ensayo literario Ensayos destructivos definicion
Ensayos destructivos definicion Ideal portaliano
Ideal portaliano Ensayo en formato mla
Ensayo en formato mla