SESIN FORMATIVA TUMORES GENITOURINARIOS II CNCER RENAL METASTSICO










- Slides: 48

SESIÓN FORMATIVA: TUMORES GENITOURINARIOS II CÁNCER RENAL METASTÁSICO. CÓMO INTEGRAR LOS TRATAMIENTOS DISPONIBLES EN LA SECUENCIA TERAPÉUTICA SERGIO VÁZQUEZ ESTÉVEZ Sº ONCOLOXÍA MÉDICA HOSPITAL UNIVERSITARIO LUCUS AUGUSTI. LUGO XERENCIA DE XESTIÓN INTEGRADA DE LUGO, CERVO E MONFORTE SEOM 2017. MADRID 1

2

3

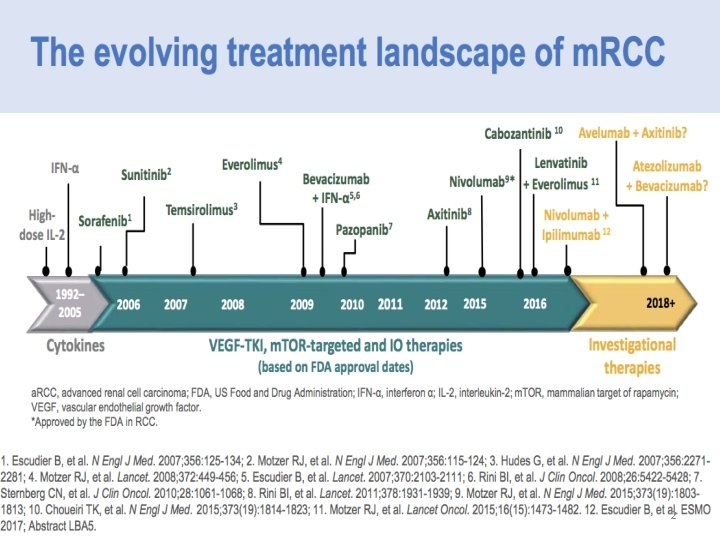
PHASE III STUDIES FIRST-LINE THERAPIES

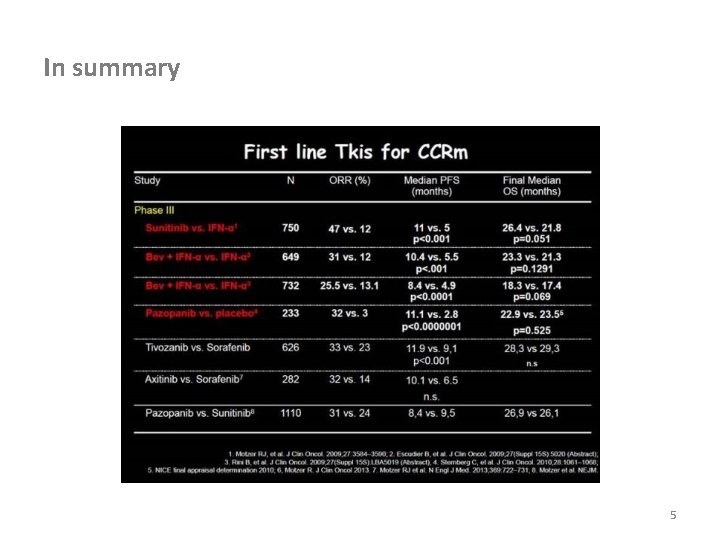
In summary 5

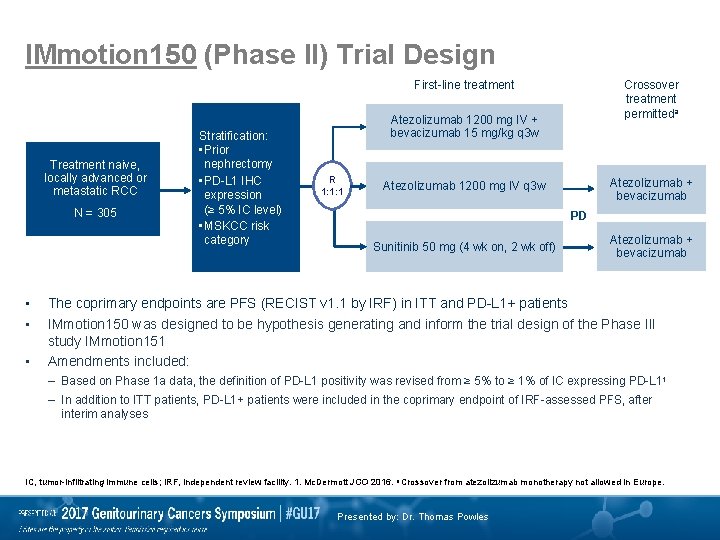
IMmotion 150 (Phase II) Trial Design First-line treatment Treatment naive, locally advanced or metastatic RCC N = 305 • • • Stratification: • Prior nephrectomy • PD-L 1 IHC expression (≥ 5% IC level) • MSKCC risk category Crossover treatment permitteda Atezolizumab 1200 mg IV + bevacizumab 15 mg/kg q 3 w R 1: 1: 1 Atezolizumab + bevacizumab Atezolizumab 1200 mg IV q 3 w PD Sunitinib 50 mg (4 wk on, 2 wk off) Atezolizumab + bevacizumab The coprimary endpoints are PFS (RECIST v 1. 1 by IRF) in ITT and PD-L 1+ patients IMmotion 150 was designed to be hypothesis generating and inform the trial design of the Phase III study IMmotion 151 Amendments included: – Based on Phase 1 a data, the definition of PD-L 1 positivity was revised from ≥ 5% to ≥ 1% of IC expressing PD-L 11 – In addition to ITT patients, PD-L 1+ patients were included in the coprimary endpoint of IRF-assessed PFS, after interim analyses IC, tumor-infiltrating immune cells; IRF, independent review facility. 1. Mc. Dermott JCO 2016. a Crossover from atezolizumab monotherapy not allowed in Europe. Presented by: Dr. Thomas Powles

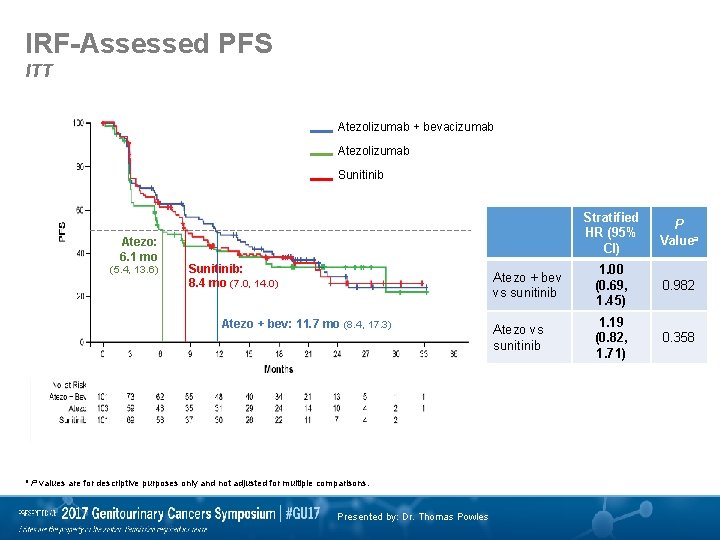
IRF-Assessed PFS ITT Atezolizumab + bevacizumab Atezolizumab Sunitinib Atezo: 6. 1 mo (5. 4, 13. 6) Sunitinib: 8. 4 mo (7. 0, 14. 0) Atezo + bev: 11. 7 mo (8. 4, 17. 3) a P values are for descriptive purposes only and not adjusted for multiple comparisons. Presented by: Dr. Thomas Powles Stratified HR (95% CI) P Valuea Atezo + bev vs sunitinib 1. 00 (0. 69, 1. 45) 0. 982 Atezo vs sunitinib 1. 19 (0. 82, 1. 71) 0. 358

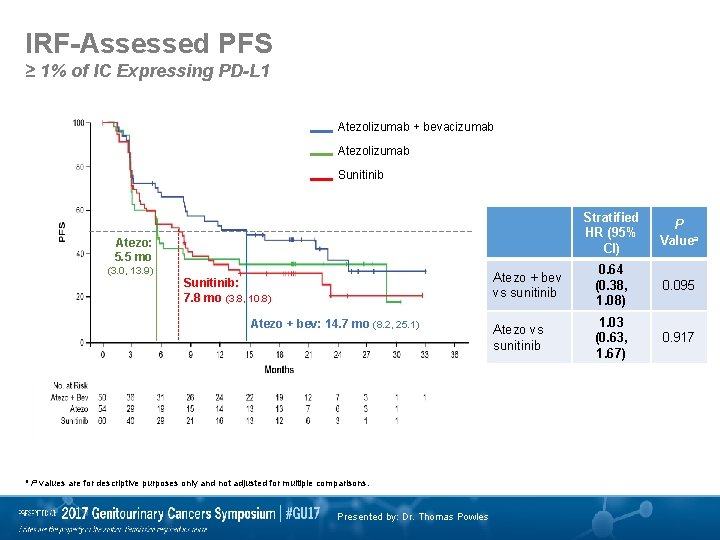
IRF-Assessed PFS ≥ 1% of IC Expressing PD-L 1 Atezolizumab + bevacizumab Atezolizumab Sunitinib Stratified HR (95% CI) P Valuea Atezo + bev vs sunitinib 0. 64 (0. 38, 1. 08) 0. 095 Atezo vs sunitinib 1. 03 (0. 63, 1. 67) 0. 917 Atezo: 5. 5 mo (3. 0, 13. 9) Sunitinib: 7. 8 mo (3. 8, 10. 8) Atezo + bev: 14. 7 mo (8. 2, 25. 1) a P values are for descriptive purposes only and not adjusted for multiple comparisons. Presented by: Dr. Thomas Powles

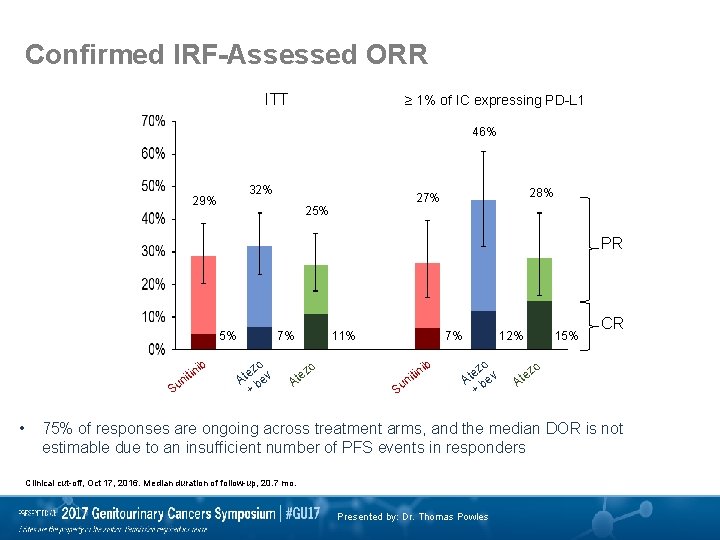
Confirmed IRF-Assessed ORR ITT ≥ 1% of IC expressing PD-L 1 46% 32% 29% 28% 27% 25% PR 5% ib itin n Su • 7% o ez v t A be + zo e At 11% 7% ib in nit Su 12% o ez v t A be + 15% z te CR o A 75% of responses are ongoing across treatment arms, and the median DOR is not estimable due to an insufficient number of PFS events in responders Clinical cut-off, Oct 17, 2016. Median duration of follow-up, 20. 7 mo. Presented by: Dr. Thomas Powles

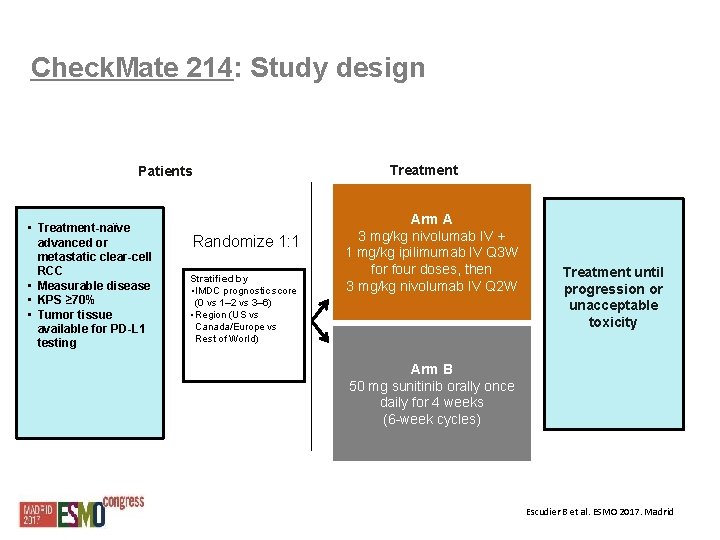
Check. Mate 214: Study design Treatment Patients • Treatment-naïve advanced or metastatic clear-cell RCC • Measurable disease • KPS ≥ 70% • Tumor tissue available for PD-L 1 testing Randomize 1: 1 Stratified by • IMDC prognostic score (0 vs 1– 2 vs 3– 6) • Region (US vs Canada/Europe vs Rest of World) Arm A 3 mg/kg nivolumab IV + 1 mg/kg ipilimumab IV Q 3 W for four doses, then 3 mg/kg nivolumab IV Q 2 W Treatment until progression or unacceptable toxicity Arm B 50 mg sunitinib orally once daily for 4 weeks (6 -week cycles) Escudier B et al. ESMO 2017. Madrid

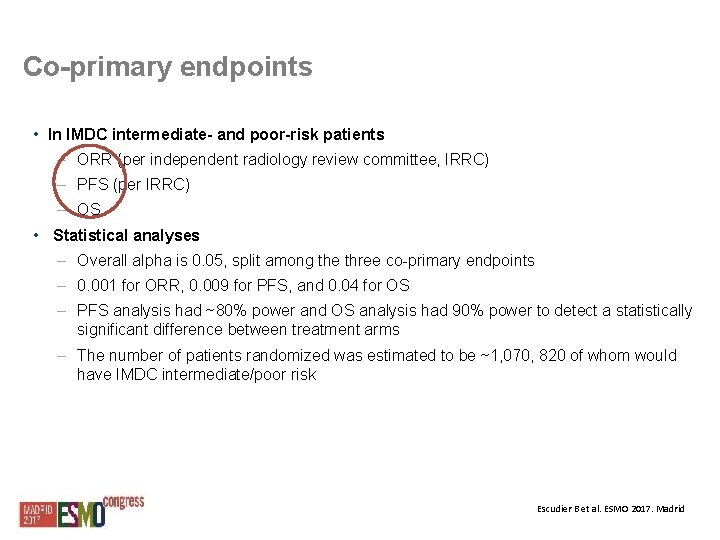
Co-primary endpoints • In IMDC intermediate- and poor-risk patients – ORR (per independent radiology review committee, IRRC) – PFS (per IRRC) – OS • Statistical analyses – Overall alpha is 0. 05, split among the three co-primary endpoints – 0. 001 for ORR, 0. 009 for PFS, and 0. 04 for OS – PFS analysis had ~80% power and OS analysis had 90% power to detect a statistically significant difference between treatment arms – The number of patients randomized was estimated to be ~1, 070, 820 of whom would have IMDC intermediate/poor risk Escudier B et al. ESMO 2017. Madrid

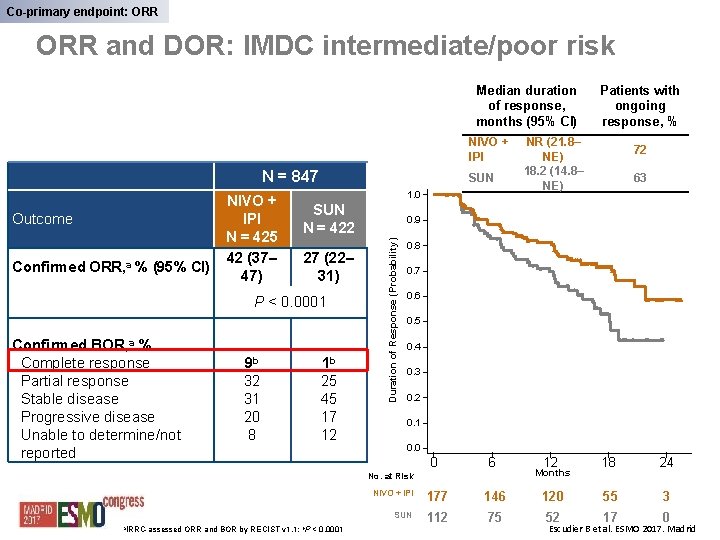
Co-primary endpoint: ORR and DOR: IMDC intermediate/poor risk Median duration of response, months (95% CI) NIVO + IPI N = 847 Confirmed ORR, a % (95% CI) 1. 0 SUN N = 422 27 (22– 31) P < 0. 0001 Confirmed BOR, a % Complete response Partial response Stable disease Progressive disease Unable to determine/not reported 9 b 1 b 32 31 20 8 25 45 17 12 < 0. 0001 72 63 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0 6 12 18 24 NIVO + IPI 177 146 120 55 3 SUN 112 75 52 17 0 No. at Risk a. IRRC-assessed ORR and BOR by RECIST v 1. 1; b. P NR (21. 8– NE) 18. 2 (14. 8– NE) 0. 9 Duration of Response (Probability) Outcome NIVO + IPI N = 425 42 (37– 47) SUN Patients with ongoing response, % Months Escudier B et al. ESMO 2017. Madrid

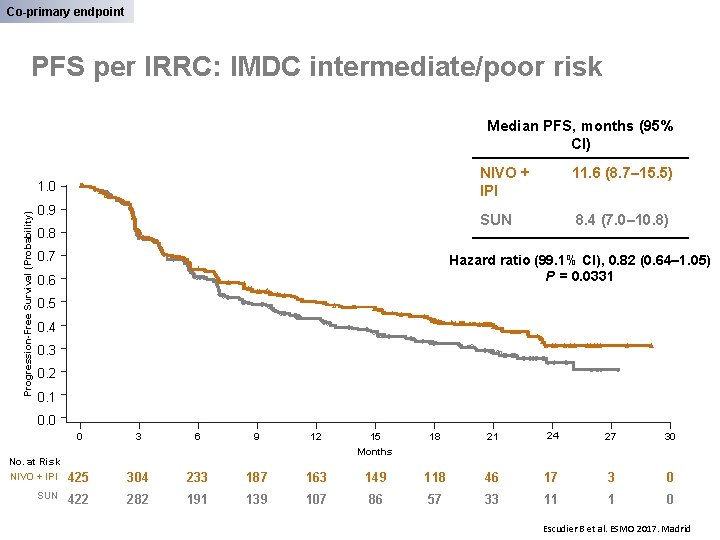
Co-primary endpoint PFS per IRRC: IMDC intermediate/poor risk Median PFS, months (95% CI) Progression-Free Survival (Probability) 1. 0 0. 9 0. 8 0. 7 NIVO + IPI 11. 6 (8. 7– 15. 5) SUN 8. 4 (7. 0– 10. 8) Hazard ratio (99. 1% CI), 0. 82 (0. 64– 1. 05) P = 0. 0331 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0 No. at Risk NIVO + IPI SUN 3 6 9 12 15 18 21 24 27 30 Months 425 304 233 187 163 149 118 46 17 3 0 422 282 191 139 107 86 57 33 11 1 0 Escudier B et al. ESMO 2017. Madrid

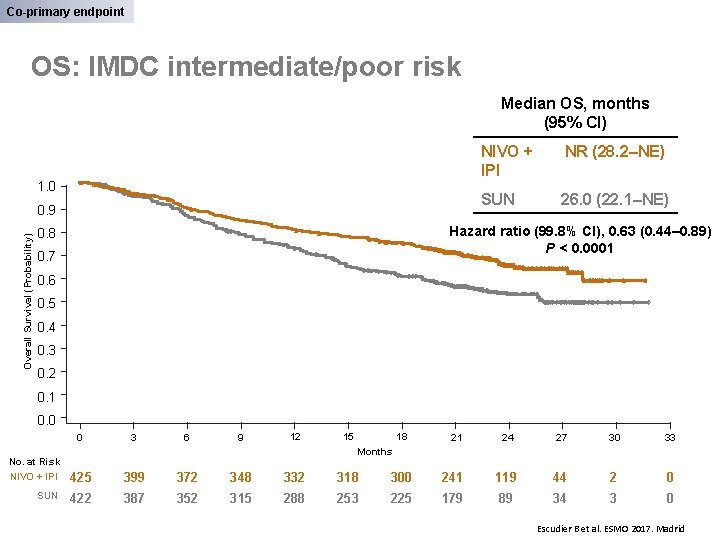
Co-primary endpoint OS: IMDC intermediate/poor risk Median OS, months (95% CI) 1. 0 Overall Survival (Probability) 0. 9 NIVO + IPI NR (28. 2–NE) SUN 26. 0 (22. 1–NE) Hazard ratio (99. 8% CI), 0. 63 (0. 44– 0. 89) P < 0. 0001 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0 3 6 9 12 15 18 21 24 27 30 33 Months No. at Risk NIVO + IPI 425 399 372 348 332 318 300 241 119 44 2 0 SUN 422 387 352 315 288 253 225 179 89 34 3 0 Escudier B et al. ESMO 2017. Madrid

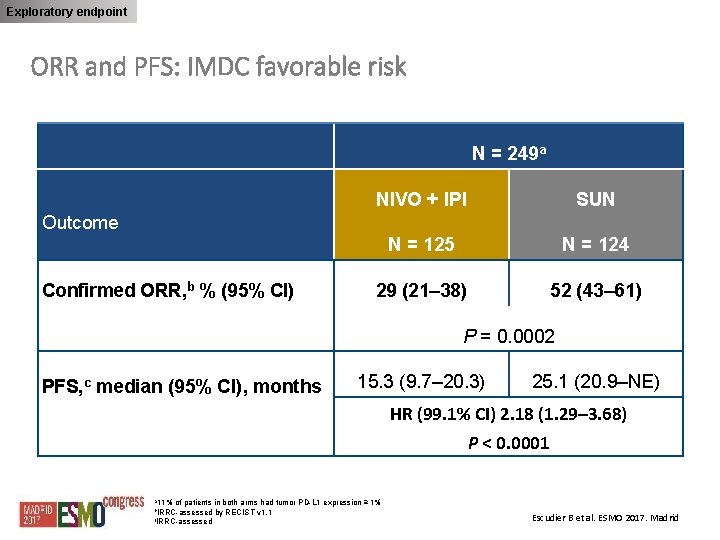
Exploratory endpoint ORR and PFS: IMDC favorable risk N = 249 a NIVO + IPI SUN N = 125 N = 124 29 (21– 38) 52 (43– 61) Outcome Confirmed ORR, b % (95% CI) P = 0. 0002 PFS, c median (95% CI), months 15. 3 (9. 7– 20. 3) 25. 1 (20. 9–NE) HR (99. 1% CI) 2. 18 (1. 29– 3. 68) P < 0. 0001 a 11% of patients in both arms had tumor PD-L 1 expression ≥ 1% b. IRRC-assessed by RECIST v 1. 1 c. IRRC-assessed Escudier B et al. ESMO 2017. Madrid

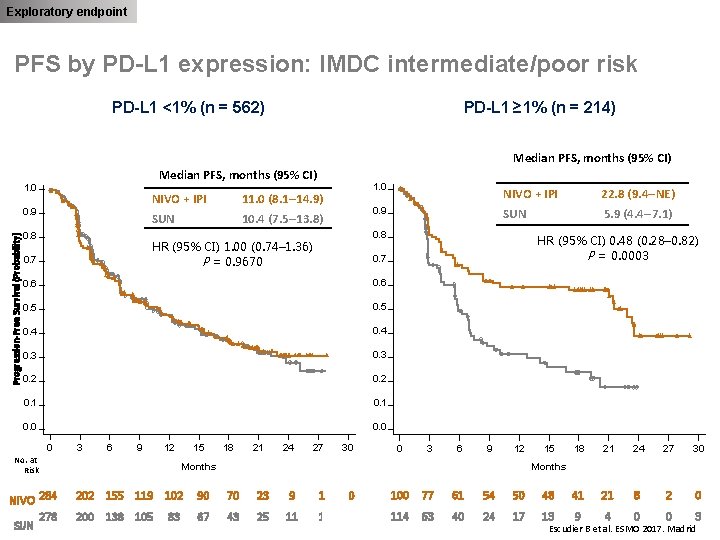
Exploratory endpoint PFS by PD-L 1 expression: IMDC intermediate/poor risk PD-L 1 <1% (n = 562) PD-L 1 ≥ 1% (n = 214) Median PFS, months (95% CI) 1. 0 Progression-Free Survival (Probability) 0. 9 0. 8 NIVO + IPI 11. 0 (8. 1– 14. 9) SUN 10. 4 (7. 5– 13. 8) NIVO + IPI 22. 8 (9. 4–NE) 0. 9 SUN 5. 9 (4. 4– 7. 1) 0. 8 HR (95% CI) 1. 00 (0. 74– 1. 36) P = 0. 9670 0. 7 1. 0 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0 3 6 9 12 No. at Risk NIVO 284 278 SUN 15 18 21 24 HR (95% CI) 0. 48 (0. 28– 0. 82) P = 0. 0003 27 30 0 3 6 9 12 Months 15 18 21 24 27 30 Months 202 155 119 102 90 70 23 9 1 0 100 77 61 54 50 48 41 21 8 2 0 200 138 105 67 43 25 11 1 0 114 63 40 24 17 13 9 4 0 0 3 83 Escudier B et al. ESMO 2017. Madrid

PHASE III STUDIES SECOND-LINE THERAPIES

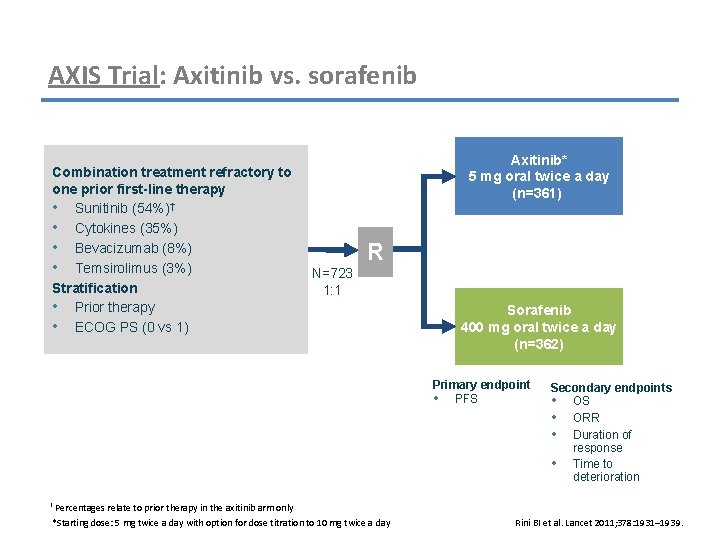
AXIS Trial: Axitinib vs. sorafenib Combination treatment refractory to one prior first-line therapy • Sunitinib (54%)† • Cytokines (35%) • Bevacizumab (8%) • Temsirolimus (3%) Stratification • Prior therapy • ECOG PS (0 vs 1) Axitinib* 5 mg oral twice a day (n=361) R N=723 1: 1 Sorafenib 400 mg oral twice a day (n=362) Primary endpoint • PFS Secondary endpoints • OS • ORR • Duration of response • Time to deterioration † Percentages relate to prior therapy in the axitinib arm only *Starting dose: 5 mg twice a day with option for dose titration to 10 mg twice a day Rini BI et al. Lancet 2011; 378: 1931– 1939.

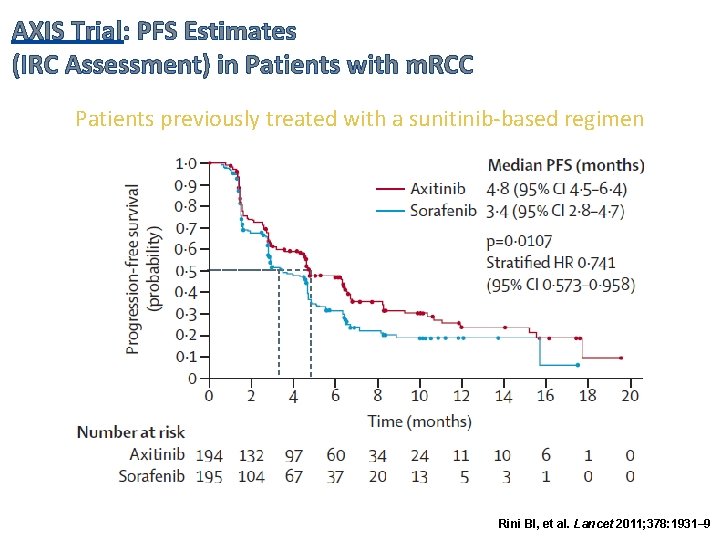
AXIS Trial: PFS Estimates (IRC Assessment) in Patients with m. RCC Patients previously treated with a sunitinib-based regimen Rini BI, et al. Lancet 2011; 378: 1931– 9

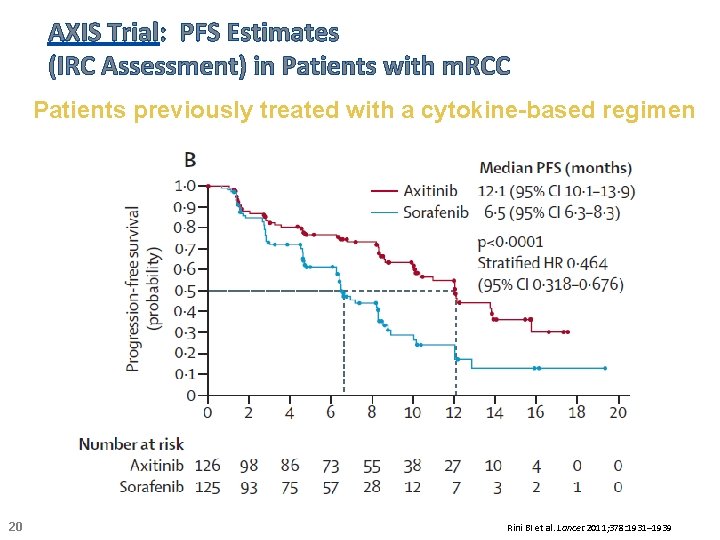
AXIS Trial: PFS Estimates (IRC Assessment) in Patients with m. RCC Patients previously treated with a cytokine-based regimen 20 Rini BI et al. Lancet 2011; 378: 1931– 1939

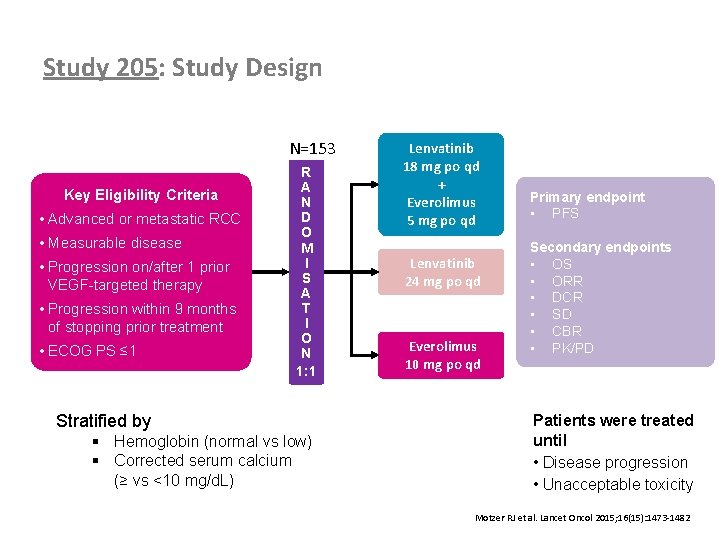
Study 205: Study Design N=153 Key Eligibility Criteria • Advanced or metastatic RCC • Measurable disease • Progression on/after 1 prior VEGF-targeted therapy • Progression within 9 months of stopping prior treatment • ECOG PS ≤ 1 Lenvatinib 18 mg po qd + Everolimus 5 mg po qd R A N D O M I S A T I O N 1: 1 Lenvatinib 24 mg po qd Everolimus 10 mg po qd Stratified by Primary endpoint • PFS Secondary endpoints • OS • ORR • DCR • SD • CBR • PK/PD Patients were treated until • Disease progression • Unacceptable toxicity § Hemoglobin (normal vs low) § Corrected serum calcium (≥ vs <10 mg/d. L) 21 Motzer RJ et al. Lancet Oncol 2015; 16(15): 1473 -1482

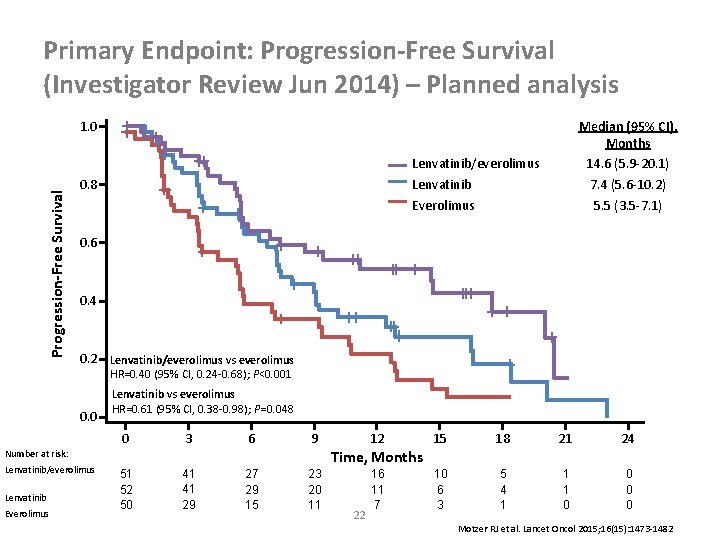
Primary Endpoint: Progression-Free Survival (Investigator Review Jun 2014) – Planned analysis Median (95% CI), Months 14. 6 (5. 9 -20. 1) 7. 4 (5. 6 -10. 2) 5. 5 (3. 5 -7. 1) Progression-Free Survival 1. 0 Lenvatinib/everolimus Lenvatinib Everolimus 0. 8 0. 6 0. 4 0. 2 Lenvatinib/everolimus vs everolimus HR=0. 40 (95% CI, 0. 24 -0. 68); P<0. 001 0. 0 Lenvatinib vs everolimus HR=0. 61 (95% CI, 0. 38 -0. 98); P=0. 048 0 3 6 9 Lenvatinib Everolimus 15 18 21 24 10 6 3 5 4 1 1 1 0 0 Time, Months Number at risk: Lenvatinib/everolimus 12 51 52 50 41 41 29 27 29 15 23 20 11 22 16 11 7 Motzer RJ et al. Lancet Oncol 2015; 16(15): 1473 -1482

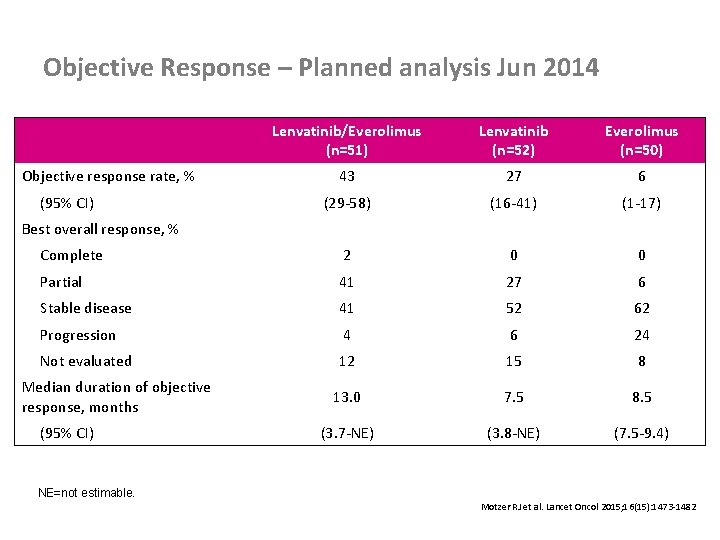
Objective Response – Planned analysis Jun 2014 Objective response rate, % (95% CI) Best overall response, % Lenvatinib/Everolimus (n=51) Lenvatinib (n=52) Everolimus (n=50) 43 27 6 (29 -58) (16 -41) (1 -17) Complete 2 0 0 Partial 41 27 6 Stable disease 41 52 62 Progression 4 6 24 Not evaluated 12 15 8 13. 0 7. 5 8. 5 (3. 7 -NE) (3. 8 -NE) (7. 5 -9. 4) Median duration of objective response, months (95% CI) NE=not estimable. 23 Motzer RJ et al. Lancet Oncol 2015; 16(15): 1473 -1482

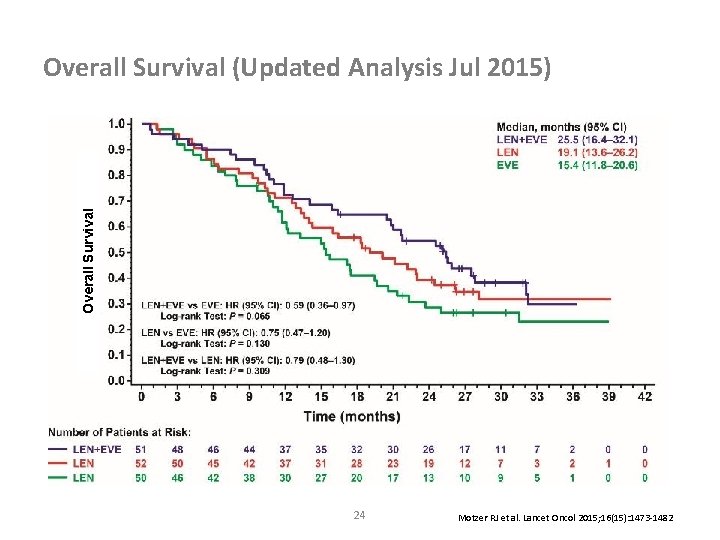
Overall Survival (Updated Analysis Jul 2015) 24 Motzer RJ et al. Lancet Oncol 2015; 16(15): 1473 -1482

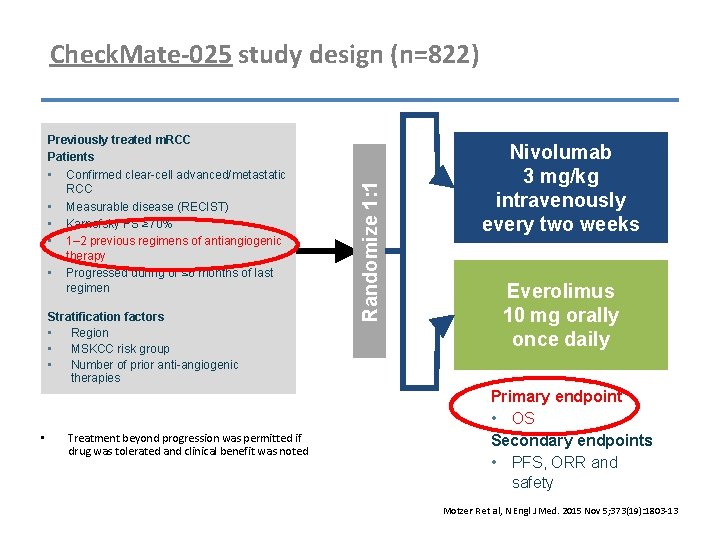
Previously treated m. RCC Patients • Confirmed clear-cell advanced/metastatic RCC • Measurable disease (RECIST) • Karnofsky PS ≥ 70% • 1– 2 previous regimens of antiangiogenic therapy • Progressed during or ≤ 6 months of last regimen Stratification factors • Region • MSKCC risk group • Number of prior anti-angiogenic therapies • Treatment beyond progression was permitted if drug was tolerated and clinical benefit was noted Randomize 1: 1 Check. Mate-025 study design (n=822) Nivolumab 3 mg/kg intravenously every two weeks Everolimus 10 mg orally once daily Primary endpoint • OS Secondary endpoints • PFS, ORR and safety Motzer R et al, N Engl J Med. 2015 Nov 5; 373(19): 1803 -13

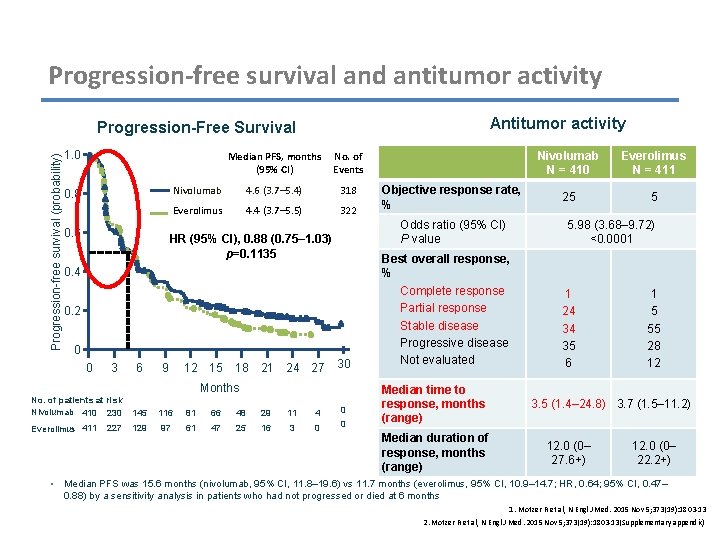
Progression-free survival and antitumor activity Antitumor activity Progression-free survival (probability) Progression-Free Survival 1. 0 Median PFS, months No. of (95% CI) Events 0. 8 0. 6 Nivolumab 4. 6 (3. 7– 5. 4) 318 Everolimus 4. 4 (3. 7– 5. 5) 322 0. 4 0. 2 0 3 6 9 12 15 18 21 24 27 30 Months No. of patients at risk Nivolumab 410 230 145 116 81 66 48 29 11 4 0 Everolimus 411 129 97 61 47 25 16 3 0 0 • 227 Objective response rate, % Odds ratio (95% CI) P value HR (95% CI), 0. 88 (0. 75– 1. 03) p=0. 1135 0 Best overall response, % Complete response Partial response Stable disease Progressive disease Not evaluated Median time to response, months (range) Median duration of response, months (range) Nivolumab N = 410 Everolimus N = 411 25 5 5. 98 (3. 68– 9. 72) <0. 0001 1 24 34 35 6 1 5 55 28 12 3. 5 (1. 4– 24. 8) 3. 7 (1. 5– 11. 2) 12. 0 (0– 27. 6+) 12. 0 (0– 22. 2+) Median PFS was 15. 6 months (nivolumab, 95% CI, 11. 8– 19. 6) vs 11. 7 months (everolimus, 95% CI, 10. 9– 14. 7; HR, 0. 64; 95% CI, 0. 47– 0. 88) by a sensitivity analysis in patients who had not progressed or died at 6 months 1. Motzer R et al, N Engl J Med. 2015 Nov 5; 373(19): 1803 -13 2. Motzer R et al, N Engl J Med. 2015 Nov 5; 373(19): 1803 -13(Supplementary appendix)

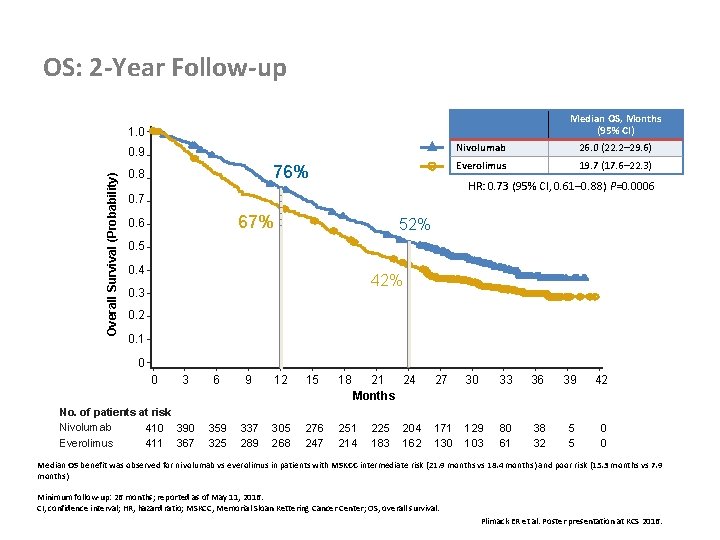
OS: 2 -Year Follow-up Median OS, Months (95% CI) 1. 0 Overall Survival (Probability) 0. 9 76% 0. 8 26. 0 (22. 2– 29. 6) Everolimus 19. 7 (17. 6– 22. 3) HR: 0. 73 (95% CI, 0. 61– 0. 88) P=0. 0006 0. 7 67% 0. 6 Nivolumab 52% 0. 5 0. 4 42% 0. 3 0. 2 0. 1 0 0 3 No. of patients at risk Nivolumab 410 390 Everolimus 411 367 6 9 12 15 18 21 24 Months 359 325 337 289 305 268 276 247 251 214 225 183 204 162 27 30 33 36 39 42 171 130 129 103 80 61 38 32 5 5 0 0 Median OS benefit was observed for nivolumab vs everolimus in patients with MSKCC intermediate risk (21. 9 months vs 18. 4 months) and poor risk (15. 3 months vs 7. 9 months) Minimum follow-up: 26 months; reported as of May 11, 2016. CI, confidence interval; HR, hazard ratio; MSKCC, Memorial Sloan Kettering Cancer Center; OS, overall survival. Plimack ER et al. Poster presentation at KCS 2016.

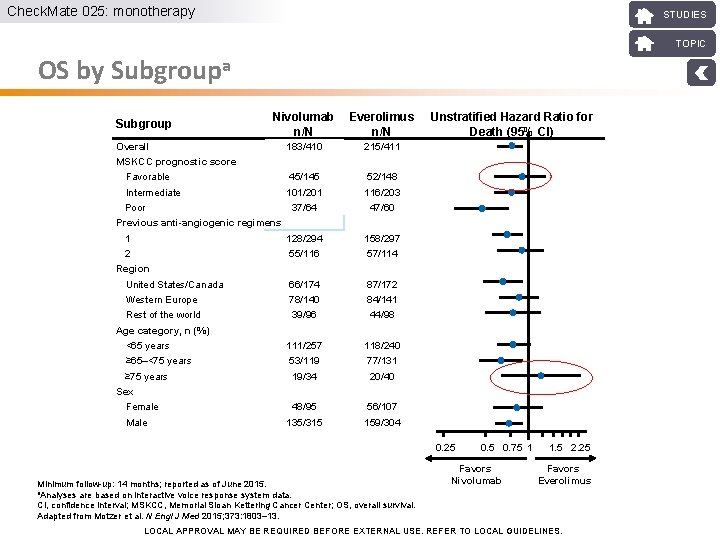
Check. Mate 025: monotherapy STUDIES TOPIC OS by Subgroupa Subgroup Nivolumab n/N Everolimus n/N Unstratified Hazard Ratio for Death (95% CI) 183/410 215/411 Overall MSKCC prognostic score Favorable 45/145 52/148 Intermediate 101/201 116/203 37/64 47/60 Poor Previous anti-angiogenic regimens 1 128/294 158/297 2 55/116 57/114 United States/Canada 66/174 87/172 Western Europe 78/140 84/141 Rest of the world 39/96 44/98 Region Age category, n (%) <65 years 111/257 118/240 ≥ 65–<75 years 53/119 77/131 ≥ 75 years 19/34 20/40 Sex Female Male 48/95 56/107 135/315 159/304 0. 25 Minimum follow-up: 14 months; reported as of June 2015. a. Analyses are based on interactive voice response system data. CI, confidence interval; MSKCC, Memorial Sloan Kettering Cancer Center; OS, overall survival. Adapted from Motzer et al. N Engl J Med 2015; 373: 1803– 13. 0. 5 0. 75 1 Favors Nivolumab 1. 5 2. 25 Favors Everolimus LOCAL APPROVAL MAY BE REQUIRED BEFORE EXTERNAL USE. REFER TO LOCAL GUIDELINES.

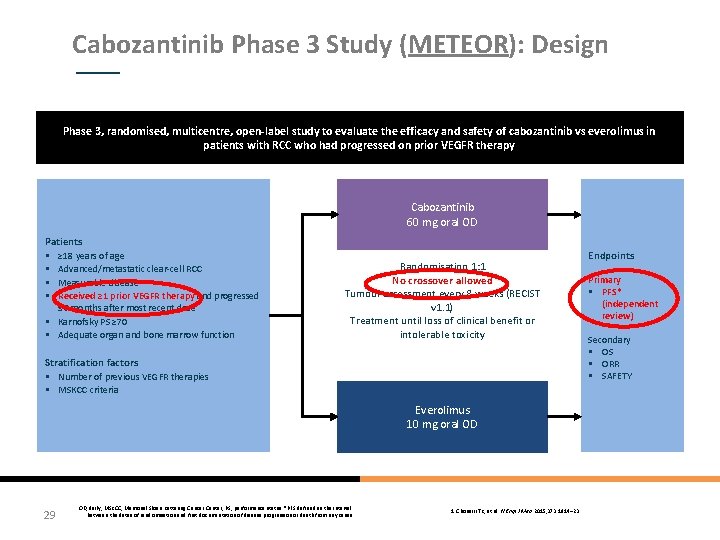
Cabozantinib Phase 3 Study (METEOR): Design Phase 3, randomised, multicentre, open-label study to evaluate the efficacy and safety of cabozantinib vs everolimus in patients with RCC who had progressed on prior VEGFR therapy Cabozantinib 60 mg oral OD Patients § ≥ 18 years of age § Advanced/metastatic clear-cell RCC § Measurable disease § Received ≥ 1 prior VEGFR therapy and progressed ≤ 6 months after most recent dose § Karnofsky PS ≥ 70 § Adequate organ and bone marrow function Randomisation 1: 1 No crossover allowed Tumour assessment every 8 weeks (RECIST v 1. 1) Treatment until loss of clinical benefit or intolerable toxicity Stratification factors § Number of previous VEGFR therapies § MSKCC criteria Everolimus 10 mg oral OD 29 OD, daily; MSKCC, Memorial Sloan Kettering Cancer Center; PS, performance status. *PFS defined as the interval between the dates of randomisation and first documentation of disease progression or death from any cause 1. Choueiri TK, et al. N Engl J Med 2015; 373: 1814– 23 Endpoints Primary § PFS* (independent review) Secondary § OS § ORR § SAFETY

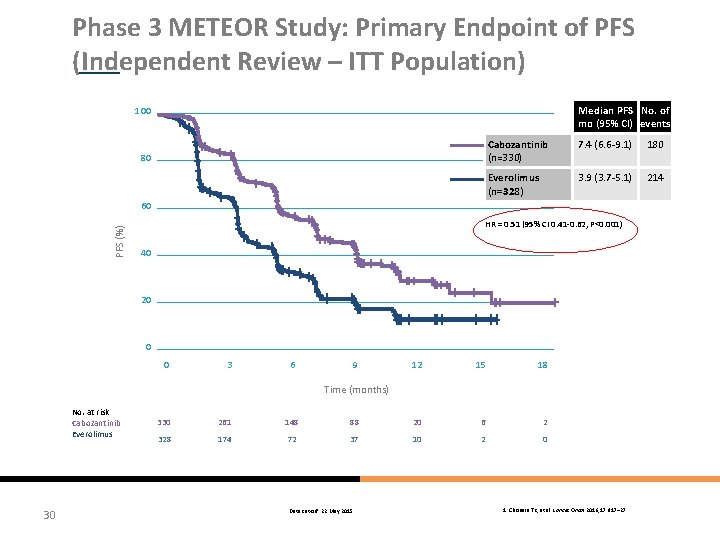
Phase 3 METEOR Study: Primary Endpoint of PFS (Independent Review – ITT Population) Median PFS No. of mo (95% CI) events 100 80 Cabozantinib (n=330) 7. 4 (6. 6 -9. 1) 180 Everolimus (n=328) 3. 9 (3. 7 -5. 1) 214 PFS (%) 60 HR = 0. 51 (95% CI 0. 41 -0. 62, P<0. 001) 40 20 0 0 3 6 9 12 15 18 Time (months) No. at risk Cabozantinib Everolimus 30 330 261 148 88 20 6 2 328 174 72 37 10 2 0 Data cut-off: 22 May 2015 1. Choueiri TK, et al. Lancet Oncol 2016; 17: 917– 27

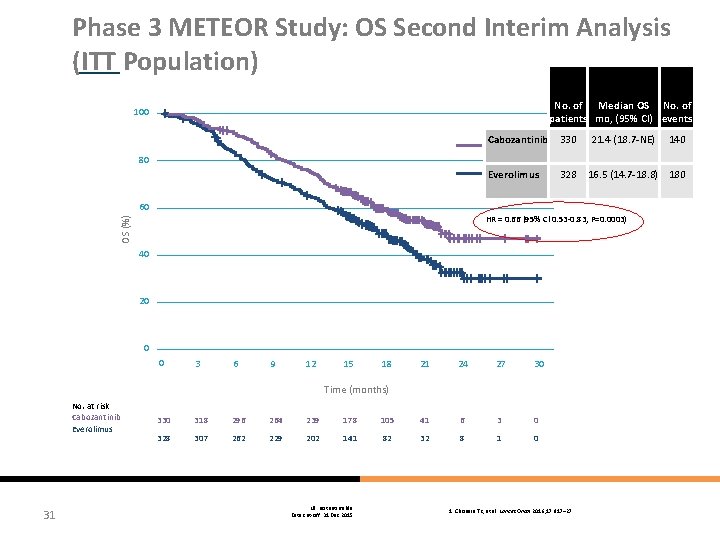
Phase 3 METEOR Study: OS Second Interim Analysis (ITT Population) No. of Median OS No. of patients mo, (95% CI) events 100 Cabozantinib 330 Everolimus 328 21. 4 (18. 7 -NE) 140 80 60 OS (%) HR = 0. 66 (95% Cl 0. 53 -0. 83, P=0. 0003) 40 20 0 0 3 6 9 12 15 18 21 24 27 30 Time (months) No. at risk Cabozantinib Everolimus 31 16. 5 (14. 7 -18. 8) 180 330 318 296 264 239 178 105 41 6 3 0 328 307 262 229 202 141 82 32 8 1 0 NE: not estimable Data cut-off: 31 Dec 2015 1. Choueiri TK, et al. Lancet Oncol 2016; 17: 917– 27

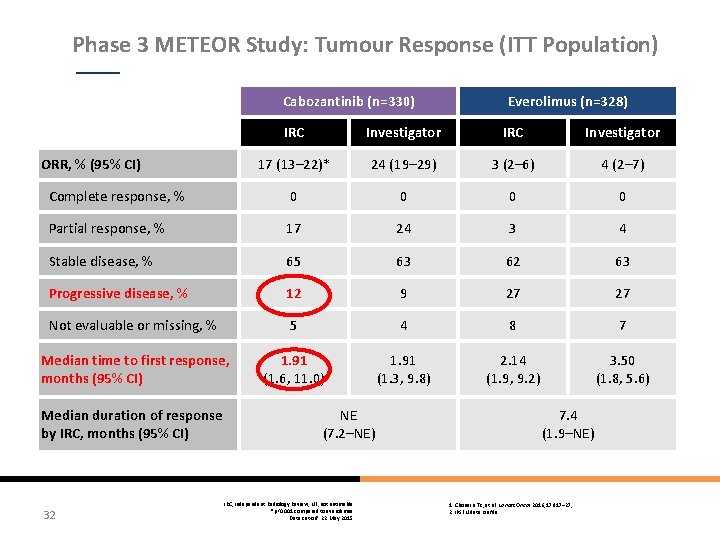
Phase 3 METEOR Study: Tumour Response (ITT Population) Cabozantinib (n=330) Everolimus (n=328) IRC Investigator 17 (13– 22)* 24 (19– 29) 3 (2– 6) 4 (2– 7) Complete response, % 0 0 Partial response, % 17 24 3 4 Stable disease, % 65 63 62 63 Progressive disease, % 12 9 27 27 Not evaluable or missing, % 5 4 8 7 Median time to first response, months (95% CI) 1. 91 (1. 6, 11. 0) 1. 91 (1. 3, 9. 8) 2. 14 (1. 9, 9. 2) 3. 50 (1. 8, 5. 6) ORR, % (95% CI) Median duration of response by IRC, months (95% CI) 32 NE (7. 2–NE) IRC, Independent Radiology Review; NE, not estimable *p<0. 001 compared to everolimus Data cut-off: 22 May 2015 7. 4 (1. 9–NE) 1. Choueiri TK, et al. Lancet Oncol 2016; 17: 917– 27; 2. IPSEN data on file

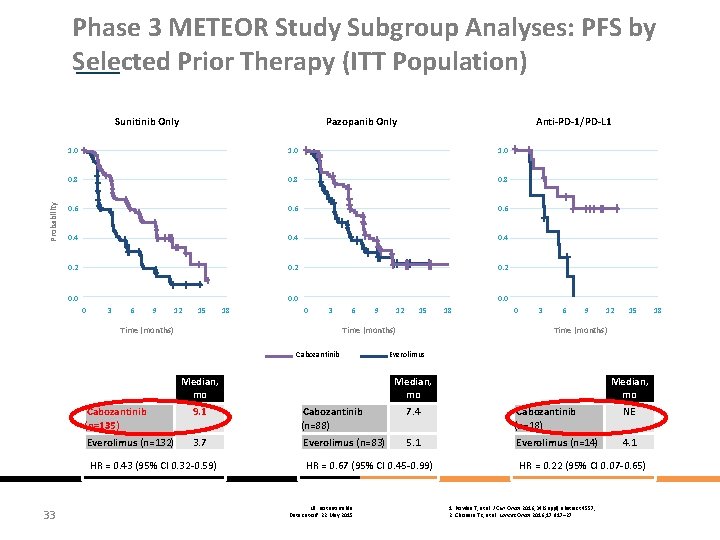
Phase 3 METEOR Study Subgroup Analyses: PFS by Selected Prior Therapy (ITT Population) Probability Sunitinib Only Pazopanib Only Anti-PD-1/PD-L 1 1. 0 0. 8 0. 6 0. 4 0. 2 0. 0 0 3 6 9 12 15 Time (months) 18 0. 0 0 3 6 9 12 15 18 0 3 6 9 12 15 Time (months) Cabozantinib Everolimus Median, mo Cabozantinib (n=135) 9. 1 Cabozantinib (n=88) 7. 4 Cabozantinib (n=18) NE Everolimus (n=132) 3. 7 Everolimus (n=83) 5. 1 Everolimus (n=14) 4. 1 HR = 0. 43 (95% Cl 0. 32 -0. 59) 33 Median, mo HR = 0. 67 (95% Cl 0. 45 -0. 99) NE: not estimable Data cut-off: 22 May 2015 HR = 0. 22 (95% Cl 0. 07 -0. 65) 1. Powles T, et al. J Clin Oncol 2016; 34(Suppl): abstract 4557; 2. Choueiri TK, et al. Lancet Oncol 2016; 17: 917– 27 18

REAL WORLD EVIDENCE


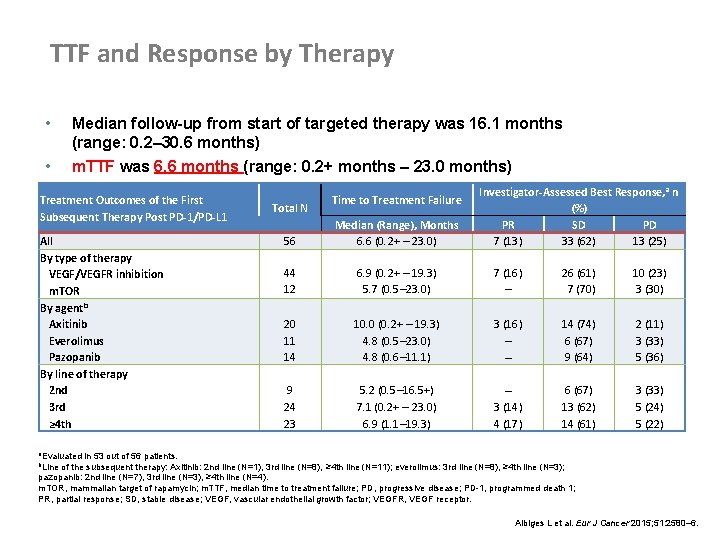
TTF and Response by Therapy • • Median follow-up from start of targeted therapy was 16. 1 months (range: 0. 2– 30. 6 months) m. TTF was 6. 6 months (range: 0. 2+ months – 23. 0 months) Treatment Outcomes of the First Subsequent Therapy Post PD-1/PD-L 1 All By type of therapy VEGF/VEGFR inhibition m. TOR By agentb Axitinib Everolimus Pazopanib By line of therapy 2 nd 3 rd ≥ 4 th Total N Time to Treatment Failure Investigator-Assessed Best Response, a n (%) PR SD PD 7 (13) 33 (62) 13 (25) 56 Median (Range), Months 6. 6 (0. 2+ – 23. 0) 44 12 6. 9 (0. 2+ – 19. 3) 5. 7 (0. 5– 23. 0) 7 (16) – 26 (61) 7 (70) 10 (23) 3 (30) 20 11 14 10. 0 (0. 2+ – 19. 3) 4. 8 (0. 5– 23. 0) 4. 8 (0. 6– 11. 1) 3 (16) – – 14 (74) 6 (67) 9 (64) 2 (11) 3 (33) 5 (36) 9 24 23 5. 2 (0. 5– 16. 5+) 7. 1 (0. 2+ – 23. 0) 6. 9 (1. 1– 19. 3) – 3 (14) 4 (17) 6 (67) 13 (62) 14 (61) 3 (33) 5 (24) 5 (22) a. Evaluated in 53 out of 56 patients. b. Line of the subsequent therapy: Axitinib: 2 nd line (N=1), 3 rd line (N=8), ≥ 4 th line (N=11); everolimus: 3 rd line (N=8), ≥ 4 th line (N=3); pazopanib: 2 nd line (N=7), 3 rd line (N=3), ≥ 4 th line (N=4). m. TOR, mammalian target of rapamycin; m. TTF, median time to treatment failure; PD, progressive disease; PD-1, programmed death 1; PR, partial response; SD, stable disease; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor. Albiges L et al. Eur J Cancer 2015; 51: 2580– 6.

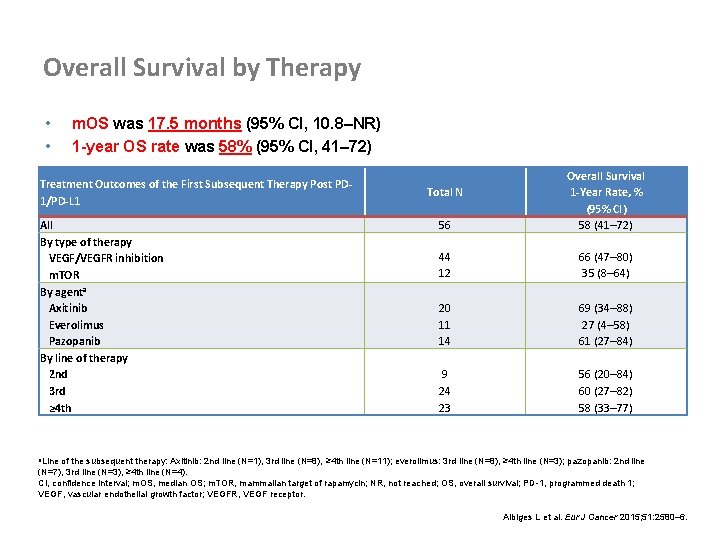
Overall Survival by Therapy • • m. OS was 17. 5 months (95% CI, 10. 8–NR) 1 -year OS rate was 58% (95% CI, 41– 72) Treatment Outcomes of the First Subsequent Therapy Post PD 1/PD-L 1 All By type of therapy VEGF/VEGFR inhibition m. TOR By agenta Axitinib Everolimus Pazopanib By line of therapy 2 nd 3 rd ≥ 4 th 56 Overall Survival 1 -Year Rate, % (95% CI) 58 (41– 72) 44 12 66 (47– 80) 35 (8– 64) 20 11 14 69 (34– 88) 27 (4– 58) 61 (27– 84) 9 24 23 56 (20– 84) 60 (27– 82) 58 (33– 77) Total N a. Line of the subsequent therapy: Axitinib: 2 nd line (N=1), 3 rd line (N=8), ≥ 4 th line (N=11); everolimus: 3 rd line (N=8), ≥ 4 th line (N=3); pazopanib: 2 nd line (N=7), 3 rd line (N=3), ≥ 4 th line (N=4). CI, confidence interval; m. OS, median OS; m. TOR, mammalian target of rapamycin; NR, not reached; OS, overall survival; PD-1, programmed death 1; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor. Albiges L et al. Eur J Cancer 2015; 51: 2580– 6.


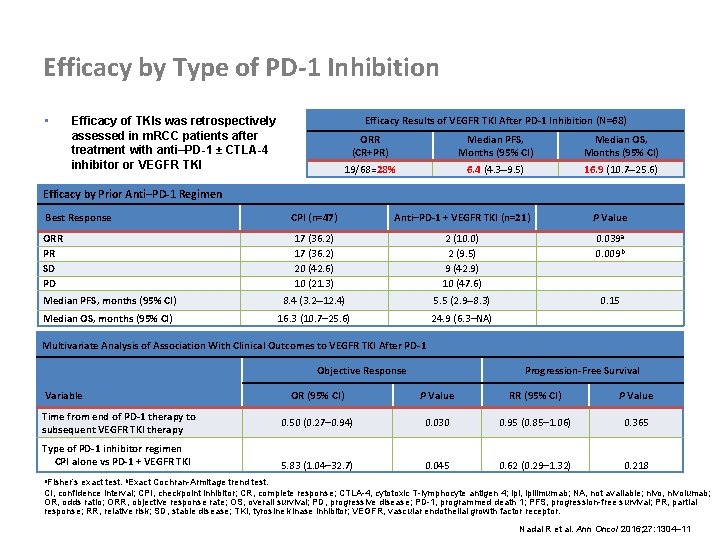
Efficacy by Type of PD-1 Inhibition • Efficacy Results of VEGFR TKI After PD-1 Inhibition (N=68) Efficacy of TKIs was retrospectively assessed in m. RCC patients after treatment with anti–PD-1 ± CTLA-4 inhibitor or VEGFR TKI ORR (CR+PR) Median PFS, Months (95% CI) Median OS, Months (95% CI) 19/68=28% 6. 4 (4. 3– 9. 5) 16. 9 (10. 7– 25. 6) Efficacy by Prior Anti–PD-1 Regimen Best Response CPI (n=47) Anti–PD-1 + VEGFR TKI (n=21) P Value ORR PR SD PD 17 (36. 2) 20 (42. 6) 10 (21. 3) 2 (10. 0) 2 (9. 5) 9 (42. 9) 10 (47. 6) 0. 039 a 0. 009 b Median PFS, months (95% CI) 8. 4 (3. 2– 12. 4) 5. 5 (2. 9– 8. 3) 0. 15 Median OS, months (95% CI) 16. 3 (10. 7– 25. 6) 24. 9 (6. 3–NA) Multivariate Analysis of Association With Clinical Outcomes to VEGFR TKI After PD-1 Objective Response Variable Time from end of PD-1 therapy to subsequent VEGFR TKI therapy Type of PD-1 inhibitor regimen CPI alone vs PD-1 + VEGFR TKI Progression-Free Survival OR (95% CI) P Value RR (95% CI) P Value 0. 50 (0. 27– 0. 94) 0. 030 0. 95 (0. 85– 1. 06) 0. 365 5. 83 (1. 04– 32. 7) 0. 045 0. 62 (0. 29– 1. 32) 0. 218 a. Fisher’s exact test. b. Exact Cochran-Armitage trend test. CI, confidence interval; CPI, checkpoint inhibitor; CR, complete response; CTLA-4, cytotoxic T-lymphocyte antigen 4; ipi, ipilimumab; NA, not available; nivo, nivolumab; OR, odds ratio; ORR, objective response rate; OS, overall survival; PD, progressive disease; PD-1, programmed death 1; PFS, progression-free survival; PR, partial response; RR, relative risk; SD, stable disease; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor. Nadal R et al. Ann Oncol 2016; 27: 1304– 11

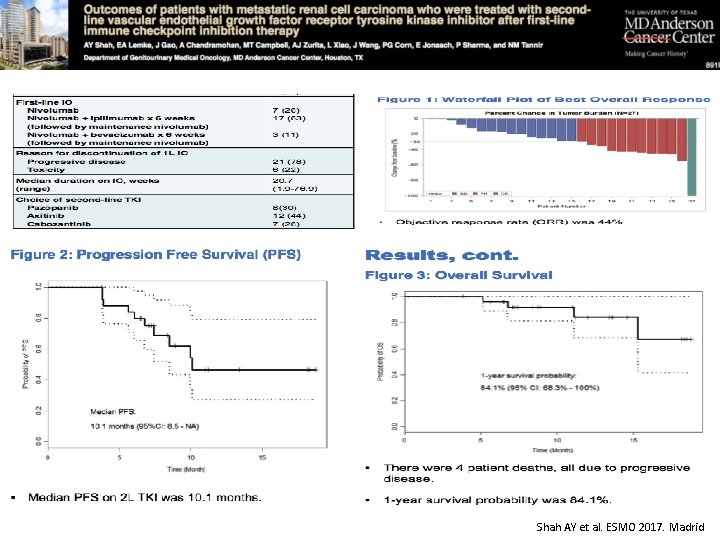
Shah AY et al. ESMO 2017. Madrid

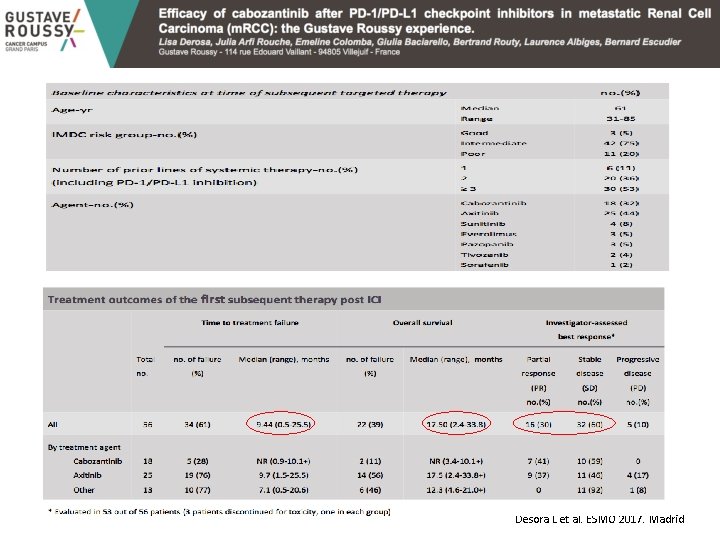
Desora L et al. ESMO 2017. Madrid

WHAT ABOUT THE FUTURE…? 42

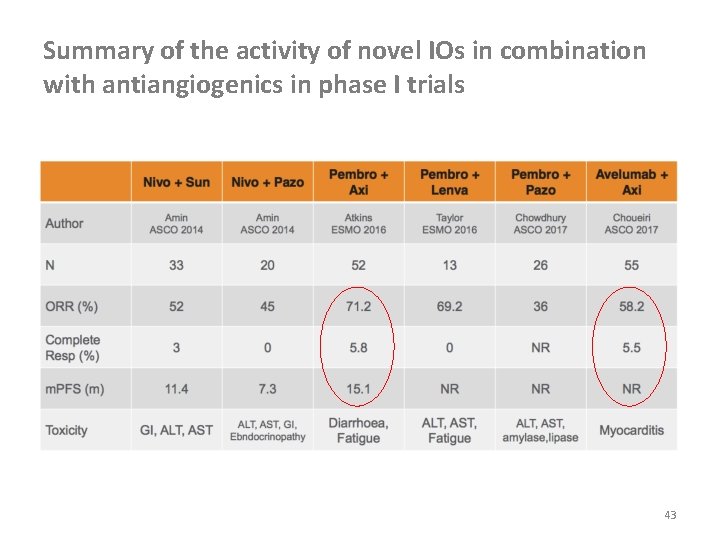
Summary of the activity of novel IOs in combination with antiangiogenics in phase I trials 43

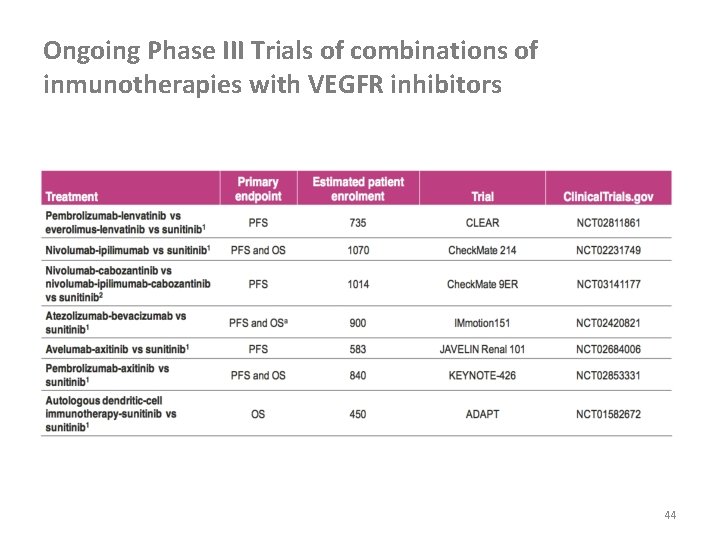
Ongoing Phase III Trials of combinations of inmunotherapies with VEGFR inhibitors 44

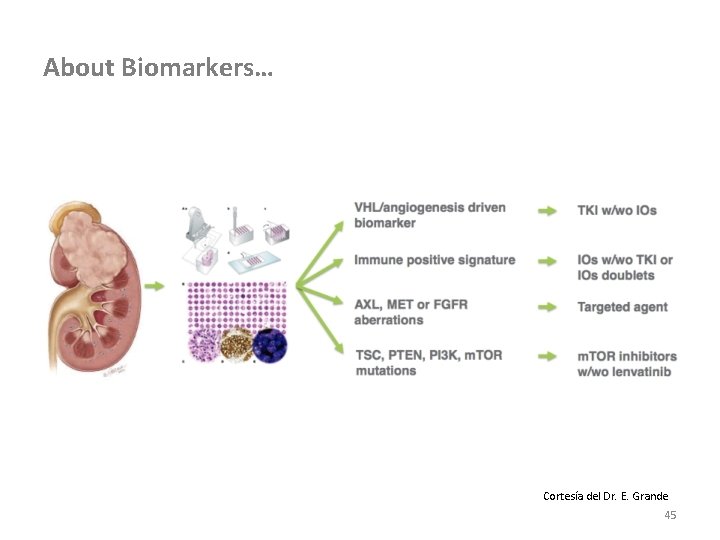
About Biomarkers… Cortesía del Dr. E. Grande 45

TAKE HOME MESSAGES 46

• 1ª línea: ü CCRm de intermedio y mal pronóstico que cumplan criterios para IO: Nivolumab + Ipilimumab es el nuevo SOC ü CCRm de buen pronóstico y en los pacientes que no cumplan criterios para IO: Anti. VEGF • 2ª línea: ü IO en 1ª: anti-VEGF ü Anti-VEGF en 1ª: nivolumab, cabozantinib, axitinib, lenvatinib + everolimus… • No existe evidencia suficiente para definir un perfil clínico de paciente para cada terapia • Necesidad de biomarcadores y un buen diseño de los estudios • Futuro: combinaciones de IO + ITK o de otras IO + IO 47

GRACIAS 48
 Res extra commercium
Res extra commercium Ira pré renal renal e pós renal
Ira pré renal renal e pós renal Cncer
Cncer Investigacionales
Investigacionales Renal corpuscle
Renal corpuscle Tumores benignos de esofago
Tumores benignos de esofago Genes supresores de tumores
Genes supresores de tumores Genes supresores de tumores
Genes supresores de tumores Tumores de celulas germinativas
Tumores de celulas germinativas Tumores de fosa anterior
Tumores de fosa anterior Triada mackler
Triada mackler Tumores pulmonares benignos
Tumores pulmonares benignos Telangiactasia
Telangiactasia Iniciar sesin
Iniciar sesin Kulağın algıladığı titreşime ne denir
Kulağın algıladığı titreşime ne denir Desibel sirop
Desibel sirop Ses nedir
Ses nedir Dr sesin
Dr sesin Como redactar un tweet
Como redactar un tweet Sesin 4
Sesin 4 Iniciar sesin
Iniciar sesin Siglas ejemplos
Siglas ejemplos Carlos sesin
Carlos sesin Partes de una rubrica
Partes de una rubrica Ventajas de la evaluacion formativa
Ventajas de la evaluacion formativa Agente evaluador interno
Agente evaluador interno What is formative assessment
What is formative assessment Organizador grafico de evaluacion formativa
Organizador grafico de evaluacion formativa Avaliação somativa
Avaliação somativa Avaluació formativa i formadora diferencia
Avaluació formativa i formadora diferencia Avaliação formativa alternativa
Avaliação formativa alternativa Validitatea instrumentelor de evaluare
Validitatea instrumentelor de evaluare Textos persuasivos periodisticos
Textos persuasivos periodisticos Ambiguo significado
Ambiguo significado Impresa formativa simulata esempio
Impresa formativa simulata esempio Que es una secuencia formativa
Que es una secuencia formativa Istituto comprensivo don milani caltanissetta
Istituto comprensivo don milani caltanissetta Cretera
Cretera Strumenti per la valutazione formativa
Strumenti per la valutazione formativa Valutazione formativa esempi
Valutazione formativa esempi Funções da avaliação diagnóstica, formativa e somativa
Funções da avaliação diagnóstica, formativa e somativa Secuencia formativa ejemplo
Secuencia formativa ejemplo Formative feedback ideas
Formative feedback ideas Evaluación sumativa
Evaluación sumativa Estrategias de evaluación formativa
Estrategias de evaluación formativa Arteria renal
Arteria renal Renal
Renal Uremia pathogenesis
Uremia pathogenesis Renal blood flow
Renal blood flow