Redefining remission in rheumatoid arthritis a joint ACREULAROMERACT













![PAS/RAPID 3 (2005) PAS = 1/3 [(HAQ * 3. 33) + pain + PGA] PAS/RAPID 3 (2005) PAS = 1/3 [(HAQ * 3. 33) + pain + PGA]](https://slidetodoc.com/presentation_image/4e3d25599bf6e29253686c028c5fc1b6/image-19.jpg)





















- Slides: 57

Redefining remission in rheumatoid arthritis a joint ACR/EULAR/OMERACT initiative Maarten Boers Department of Clinical Epidemiology and Biostatistics VU University Medical Center Amsterdam

Outline Background/Task Decisions and research agenda made at ACR 2007 Progress to date redefining remission in RA 2

Remission team George Wells, Ottawa Josef Smolen, Vienna Lilian van Tuyl, Amsterdam Bin Zhang, Boston Julia Funovits, Vienna ACR-EULAR ad hoc committee (40+ members) co-chairs: Maarten Boers, Amsterdam David Felson, Boston redefining remission in RA 3

Background Increasing numbers of patients reach remission Abundance of remission definitions ‘strict’ definitions: ACR, CDAI/SDAI, PAS/RAPID 3 ‘loose’ definitions: DAS, DAS 28, m. ACR, SJC 0/TJC 0/ESR 10, MDA Need for a uniform definition (RA trials, practice) redefining remission in RA 4

OMERACT Filter to select measures To be applicable in its intended setting, a measure must be truthful discriminative feasible redefining remission in RA 5

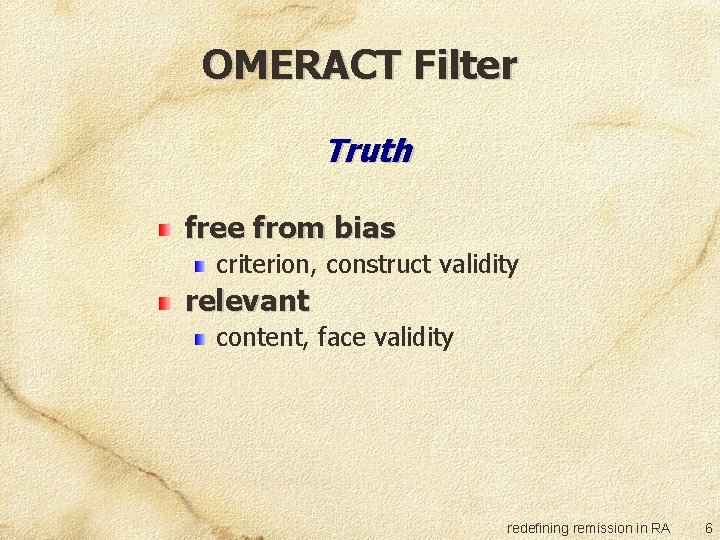
OMERACT Filter Truth free from bias criterion, construct validity relevant content, face validity redefining remission in RA 6

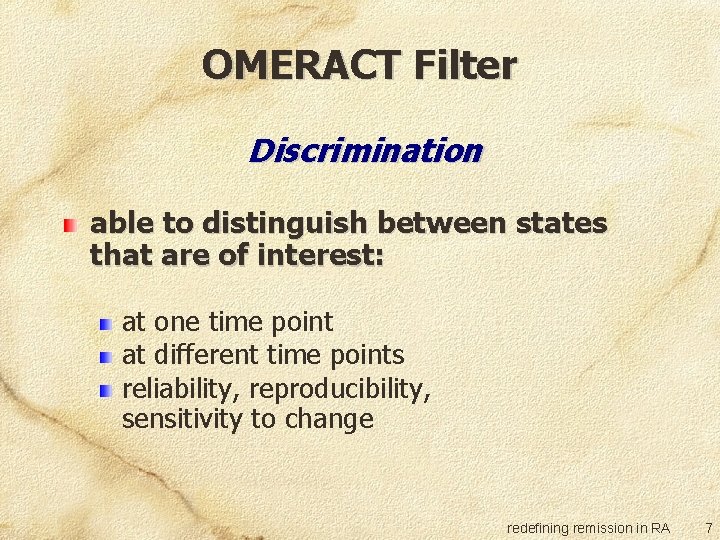
OMERACT Filter Discrimination able to distinguish between states that are of interest: at one time point at different time points reliability, reproducibility, sensitivity to change redefining remission in RA 7

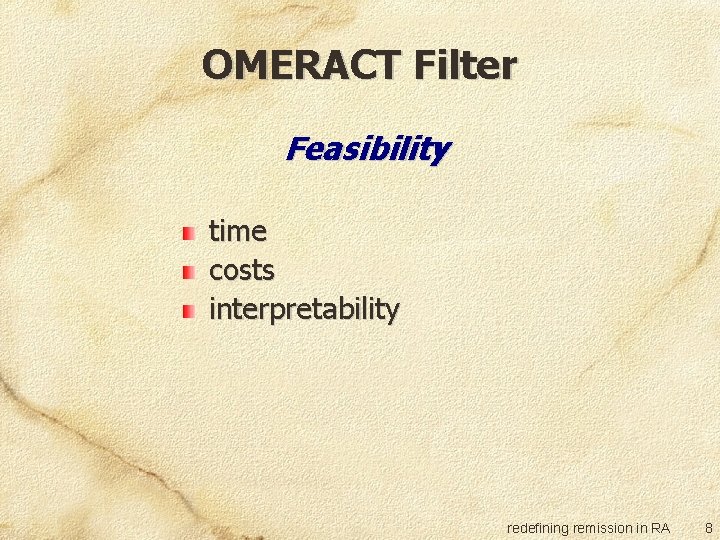
OMERACT Filter Feasibility time costs interpretability redefining remission in RA 8

Etymology Remittere (L): to send back; to decrease; to relax. . . Remission (med dictionary): An abatement or lessening of the manifestations of a disease. (Wiki): The state of absence of disease activity in patients with a chronic illness, with the possibility of return of disease activity. redefining remission in RA 9

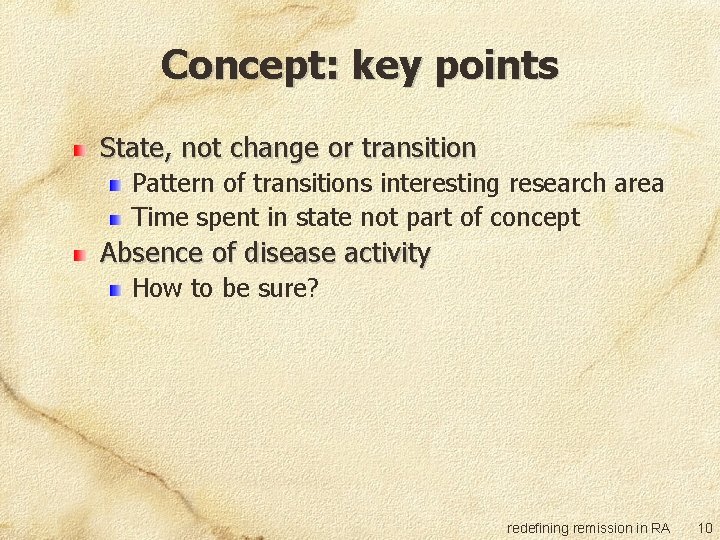
Concept: key points State, not change or transition Pattern of transitions interesting research area Time spent in state not part of concept Absence of disease activity How to be sure? redefining remission in RA 10

Concept: key points State, not change or transition Absence of disease activity Related but not identical: Cure: disease does not return Arrest: disease process is stopped Intermission: period of no activity between two periods of active disease Antithetical Relapse: return of disease activity Flare: substantial increase of disease activity redefining remission in RA 11

Current definitions: Pinals (1981) 5 or more must be fulfilled for at least 2 consecutive months: Morning stiffness not exceeding 15 minutes No fatigue No joint pain (by history) No joint tenderness or pain on motion No soft tissue swelling in joints or tendon sheaths ESR (W) <30 mm/h (f); <20 mm/h (m) redefining remission in RA 12

Pinals 3 groups classified according to the rheumatologist: complete remission, partial remission, active disease Sensitivity 72%, specificity 90% against partial remission Using 4 out of 6: sens 90%, spec 62% Read the discussion! redefining remission in RA 13

Pinals “A major obstacle to developing criteria for remission in RA is the difficulty in ascertaining the absence of inflammation by methods that are reliable and also convenient in clinical settings. . . ” redefining remission in RA 14

Pinals “A major obstacle. . . ” “Substantial variation appears to exist in the concept of remission within the group of participating rheumatologists. . . ” redefining remission in RA 15

DAS/DAS 28 DAS: Ritchie joint index and 44 swollen joint ct DAS 28: 28 tender & swollen joint count Both use a ‘general health’ VAS (0 -100) DAS 28 = 0. 56 sqrt (TJC) + 0. 28 sqrt (SJC) + 0. 70 ln (ESR) + 0. 014 GH. DAS 28 remission: 2. 6 DAS remission: 1. 6 redefining remission in RA 16

DAS 28 remission (1996) Validation against ARA criteria in Nijmegen obs. data, moderately active disease ‘m. ACR’: Fatigue not assessed Remission defined as 4 out of 5 remaining criteria 3 months instead of 2 months period; single visit data used Sens and Spec against m. ACR 87% redefining remission in RA 17

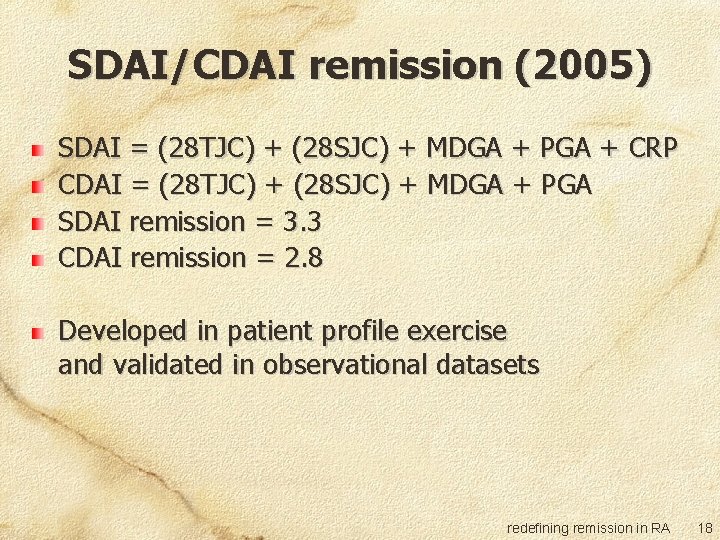
SDAI/CDAI remission (2005) SDAI = (28 TJC) + (28 SJC) + MDGA + PGA + CRP CDAI = (28 TJC) + (28 SJC) + MDGA + PGA SDAI remission = 3. 3 CDAI remission = 2. 8 Developed in patient profile exercise and validated in observational datasets redefining remission in RA 18
![PASRAPID 3 2005 PAS 13 HAQ 3 33 pain PGA PAS/RAPID 3 (2005) PAS = 1/3 [(HAQ * 3. 33) + pain + PGA]](https://slidetodoc.com/presentation_image/4e3d25599bf6e29253686c028c5fc1b6/image-19.jpg)
PAS/RAPID 3 (2005) PAS = 1/3 [(HAQ * 3. 33) + pain + PGA] PAS remission: 1. 25 (judgment) RAPID 3 = 1/3 [(HAQ * 3. 33) + pain + PGH] RAPID 3 remission: 1. 0 (judgment) redefining remission in RA 19

MDA (minimal disease activity; 2003) State of disease activity deemed a useful target of treatment by patient and physician, given current treatment possibilities and limitations. Derived from profile exercises at OMERACT Initial ‘remission’ decision node: TJC = 0, SJC = 0, ESR = 10 or less redefining remission in RA 20

MDA (minimal disease activity) Initial ‘remission’ decision node: TJC = 0, SJC = 0, ESR = 10 or less. If not in remission, choose system: DAS 2. 85 or less 5 out of 7 core set criteria Pain 2 SJC 1 TJC 1 HAQ 0. 5 MDGA 1. 5 PGA 2 ESR 20 redefining remission in RA 21

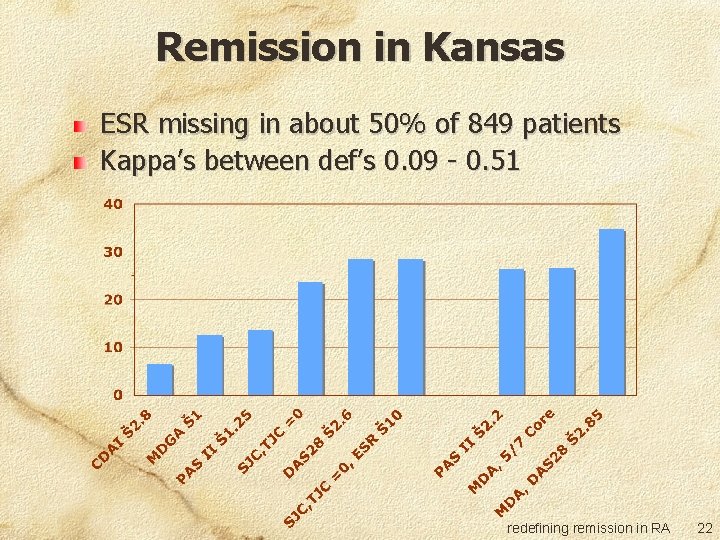
Remission in Kansas ESR missing in about 50% of 849 patients Kappa’s between def’s 0. 09 - 0. 51 redefining remission in RA 22

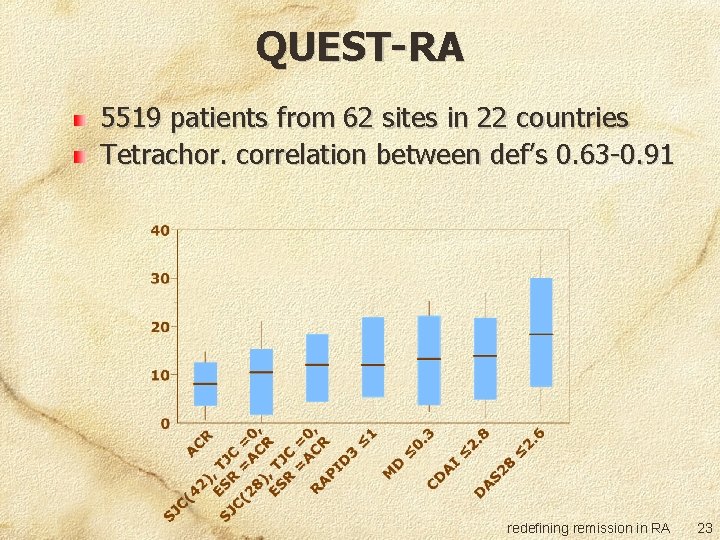
QUEST-RA 5519 patients from 62 sites in 22 countries Tetrachor. correlation between def’s 0. 63 -0. 91 redefining remission in RA 23

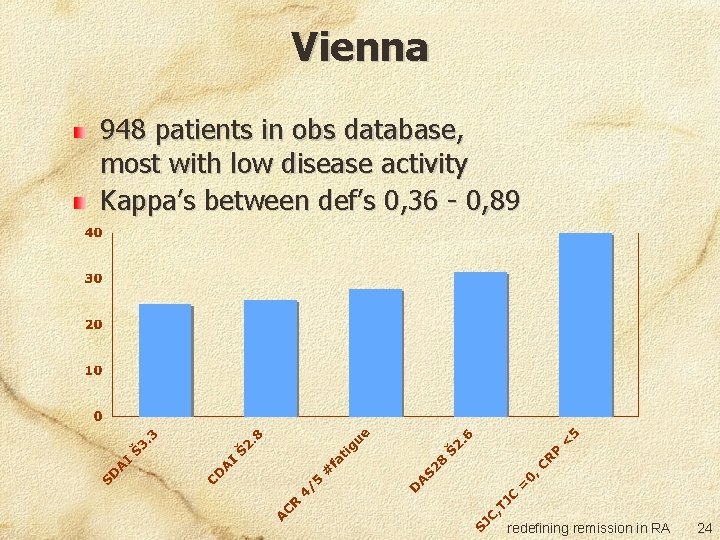
Vienna 948 patients in obs database, most with low disease activity Kappa’s between def’s 0, 36 - 0, 89 redefining remission in RA 24

Background: Conclusion ACR, CDAI/SDAI, PAS/RAPID 3 ‘strict’ Applying the 2 month duration requirement in the ACR criteria probably decreases prevalence by at least 50% m. ACR and SJC 0/TJC 0/ESR 10 ‘lax’ These and DAS 28 remission criterion resemble DAS 28 MDA redefining remission in RA 25

Task A joint ACR / EULAR / OMERACT initiative to: Study current remission definitions Explore theoretical concept of remission Re-define remission in RA redefining remission in RA 26

Decisions made at ACR 2007* Conceptual issues: A strict definition: no clinical disease lack of damage progression over time Not in the definition: Long term outcomes (phys. function, damage): used to determine validity of a new definition Therapy *Van Tuyl, LHD et al. Arthritis Rheum (AC&R) 2009; 61: 704 -10. redefining remission in RA 27

Decisions made at ACR 2007 (2) Measurement issues Definition should include as a minimum: Tender joint count (full joint count preferred) Swollen joint count (idem) An acute phase reactant Definition should not include: Duration of remission redefining remission in RA 28

Decisions made at ACR 2007 (3) Potential setting and use A remission definition for practice settings is needed and part of the task Trial and practice definitions should be closely linked redefining remission in RA 29

Research agenda – ACR 2007 Conceptual issues: Assessment of reliability/reproducibility of the remission definition Predictive validity of candidate definition against X-rays and physical function Relationship between remission and MDA and longer term outcome (function, disability) The role of new imaging (eg. US and MRI) in the definition, measurement, assessment and monitoring of the remission redefining remission in RA 30

Research agenda – ACR 2007 (2) Measurement issues What disease activity measures to include? Exact question in physician and patient globals? What about between-physician variability? Do we need 28 joints or more? Should we give priority to specific joints? Should we ask patients directly if they feel they are in remission? For patients in remission at one time point, what is the likelihood to be in remission at adjacent time points? redefining remission in RA 31

Research agenda – ACR 2007 (3) Potential settings and uses Are there equivalent measures, easier to use in practice, which give the same information? Can the practice setting definition include fewer measures whilst retaining a strong resemblance to the trial definition? redefining remission in RA 32

Progress to date Challenges* No good previous example on how to do this. . . Initial delays caused by difficulties in obtaining datasets Trial datasets only contain information on the core set No observational datasets in current exercise Systematic review of evidence for validity of current definitions Formulation of candidate definitions Cutpoints chosen from survey by Aletaha Sparse and comprehensive combinations of core measures redefining remission in RA 33

Progress to date Challenges* Systematic review of evidence for validity of current definitions Formulation of candidate definitions Validation: How well does presence of remission by this candidate definition predict a good outcome? stability in damage stability in physical function Further validation: analyses in subsets of patients with a poor prognosis good outcome defined as stability in BOTH damage and function redefining remission in RA 34

Progress to date Challenges* Systematic review of evidence for validity of current definitions Formulation of candidate definitions Validation: Further validation: Committee survey on acceptable levels of residual activity in measures Determination of residual disease activity in candidate definitions This Saturday: selection of short list/provisional def. redefining remission in RA 35

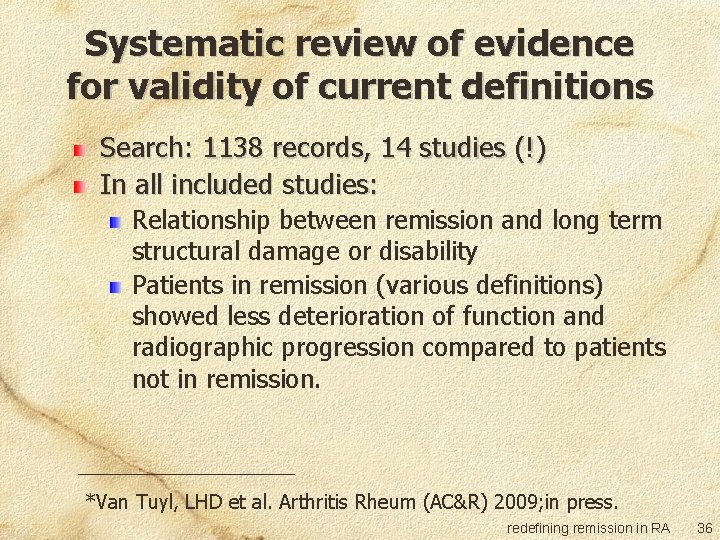
Systematic review of evidence for validity of current definitions Search: 1138 records, 14 studies (!) In all included studies: Relationship between remission and long term structural damage or disability Patients in remission (various definitions) showed less deterioration of function and radiographic progression compared to patients not in remission. *Van Tuyl, LHD et al. Arthritis Rheum (AC&R) 2009; in press. redefining remission in RA 36

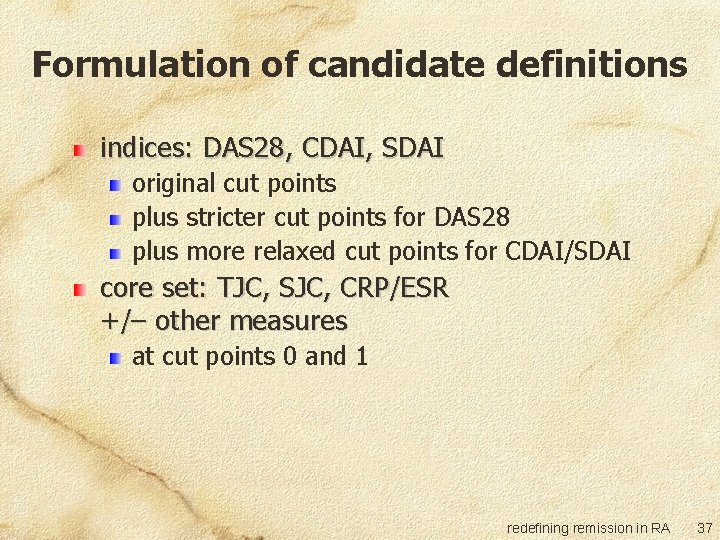
Formulation of candidate definitions indices: DAS 28, CDAI, SDAI original cut points plus stricter cut points for DAS 28 plus more relaxed cut points for CDAI/SDAI core set: TJC, SJC, CRP/ESR +/– other measures at cut points 0 and 1 redefining remission in RA 37

Validation: Gold standard outcome between y 1 and y 2: no damage progression (Svd. H =< 0) HAQ good (=<0. 5) and no deterioration (=<0) Does presence of remission by definition # at 6 months lead to increased prevalence of the gold standard outcome? Answer: yes, better for HAQ than damage, but no choice between definitions possible redefining remission in RA 38

Challenges Lack of damage progression frequently seen in patients not in remission, and even more so in intensive treatment & biological trials. . . Normal HAQ difficult to attain in longstanding disease (irreversibility and comorbidity) redefining remission in RA 39

Datasets Randomized controlled trials ASPIRE, ERA, PREMIER, TEMPO (MTX, biologicals; 1 -2 years) Extension trials PREMIER (5 years; no treatment assignment, from year 3 onward all patients received adalimumab) COBRA redefining remission in RA 40

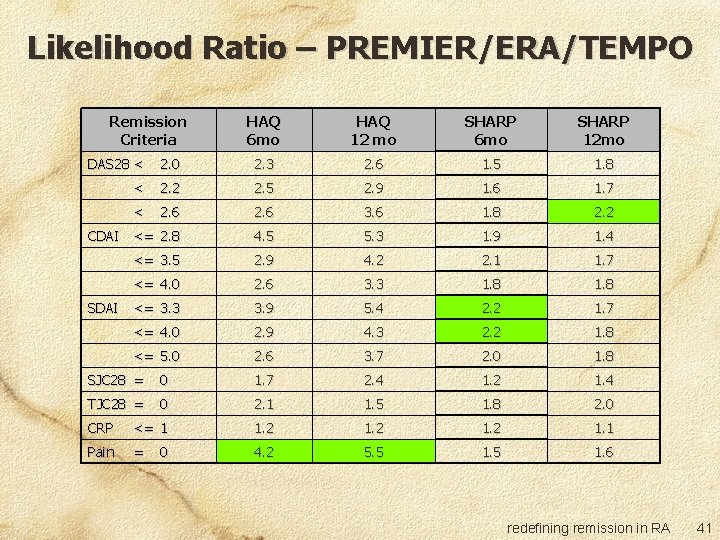
Likelihood Ratio – PREMIER/ERA/TEMPO Remission Criteria HAQ 6 mo HAQ 12 mo SHARP 6 mo SHARP 12 mo DAS 28 < 2. 0 2. 3 2. 6 1. 5 1. 8 < 2. 2 2. 5 2. 9 1. 6 1. 7 < 2. 6 3. 6 1. 8 2. 2 CDAI <= 2. 8 4. 5 5. 3 1. 9 1. 4 <= 3. 5 2. 9 4. 2 2. 1 1. 7 <= 4. 0 2. 6 3. 3 1. 8 SDAI <= 3. 3 3. 9 5. 4 2. 2 1. 7 <= 4. 0 2. 9 4. 3 2. 2 1. 8 <= 5. 0 2. 6 3. 7 2. 0 1. 8 SJC 28 = 0 1. 7 2. 4 1. 2 1. 4 TJC 28 = 0 2. 1 1. 5 1. 8 2. 0 CRP <= 1 1. 2 1. 1 Pain = 4. 2 5. 5 1. 6 0 redefining remission in RA 41

Residual disease activity 1 core set measure: 30 -40 (CRP 60 -70) 2 measures: 20 -30 3 measures: 15 -20 4 measures: 10 -15 5 measures: 8 -12 6 measures: <10 redefining remission in RA 42

Further validation: Repeat exercise in poor prognosis patients RF/a. CCP+, damage at baseline Repeat exercise in MTX treated patients Redefine outcome: no damage progression AND HAQ good & stable Does presence of remission by definition # at 6 months lead to increased prevalence of the gold standard outcome? Answer: yes, better for HAQ than damage, but no choice between definitions possible redefining remission in RA 43

Face validity Describe the residual disease activity that each definition allows in term of: Swollen joint count Tender joint count ESR / CRP Physician global assessment Patient global assessment Pain redefining remission in RA 44

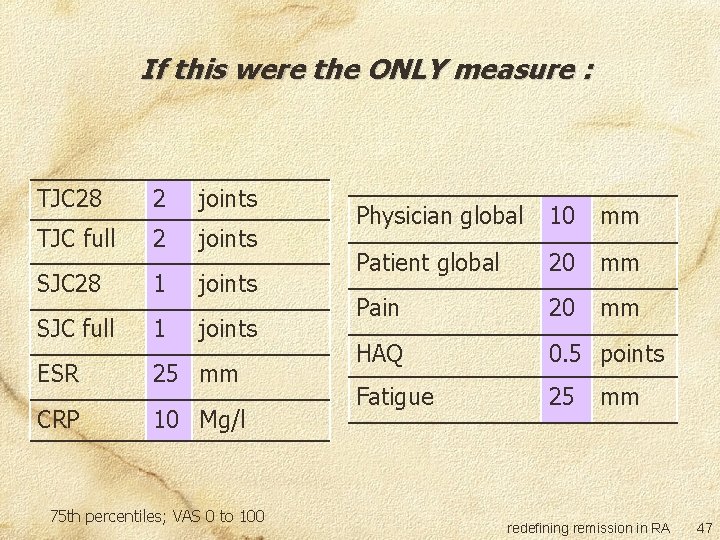
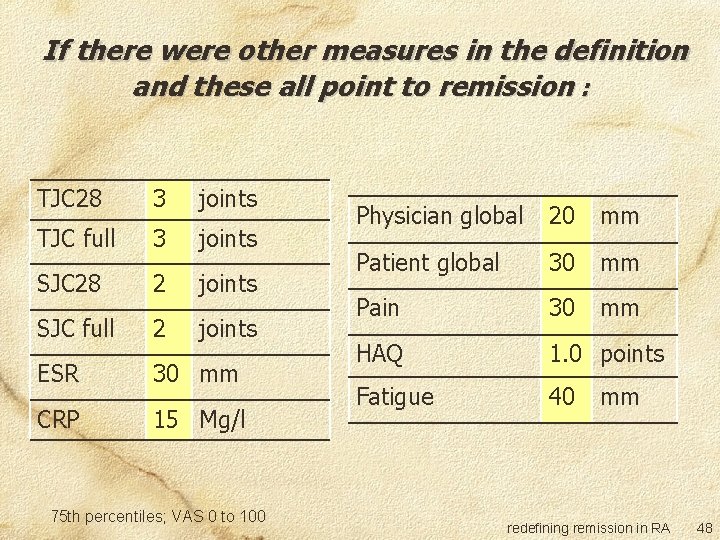
Committee survey Two questions: If this were the ONLY measure to define remission, what is the maximum level of disease activity you are willing to accept? If there were other measures in the definition and these all point to remission, what is the maximum level of disease activity you are willing to accept in this measure? redefining remission in RA 45

Methods 25 respondents VAS scales from 0 to 100 75 th percentiles redefining remission in RA 46

If this were the ONLY measure : TJC 28 2 joints TJC full 2 joints SJC 28 1 joints SJC full 1 joints ESR 25 mm CRP 10 Mg/l 75 th percentiles; VAS 0 to 100 Physician global 10 mm Patient global 20 mm Pain 20 mm HAQ 0. 5 points Fatigue 25 mm redefining remission in RA 47

If there were other measures in the definition and these all point to remission : TJC 28 3 joints TJC full 3 joints SJC 28 2 joints SJC full 2 joints ESR 30 mm CRP 15 Mg/l 75 th percentiles; VAS 0 to 100 Physician global 20 mm Patient global 30 mm Pain 30 mm HAQ 1. 0 points Fatigue 40 mm redefining remission in RA 48

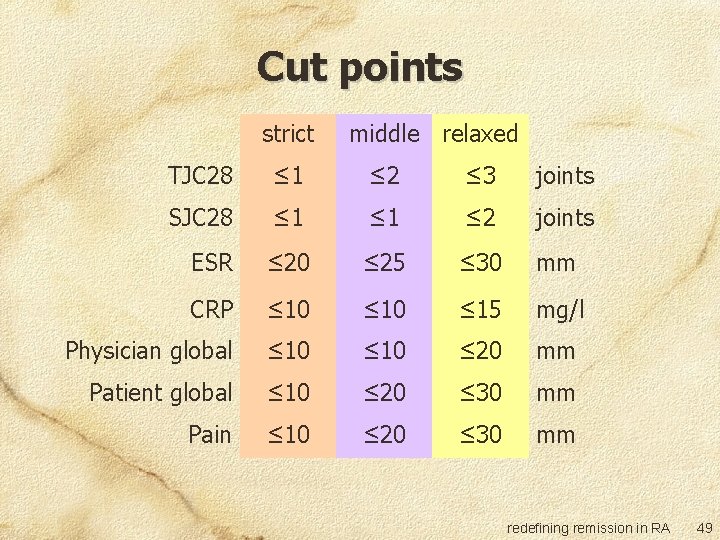
Cut points strict middle relaxed TJC 28 ≤ 1 ≤ 2 ≤ 3 joints SJC 28 ≤ 1 ≤ 2 joints ESR ≤ 20 ≤ 25 ≤ 30 mm CRP ≤ 10 ≤ 15 mg/l Physician global ≤ 10 ≤ 20 mm Patient global ≤ 10 ≤ 20 ≤ 30 mm Pain ≤ 10 ≤ 20 ≤ 30 mm redefining remission in RA 49

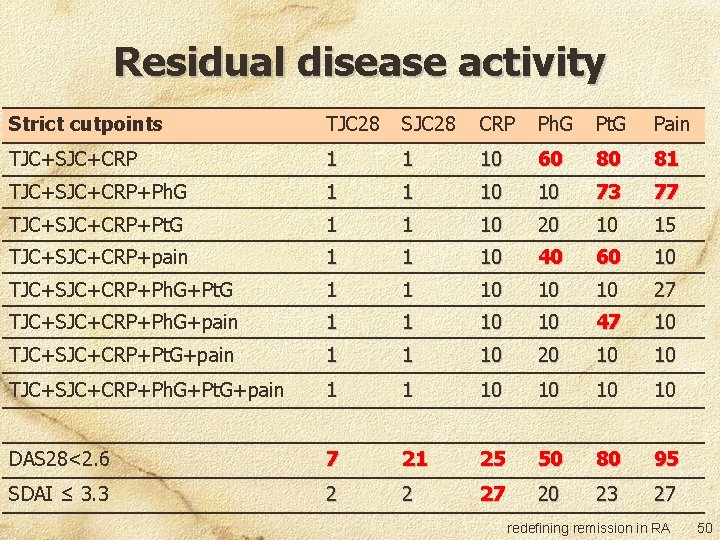
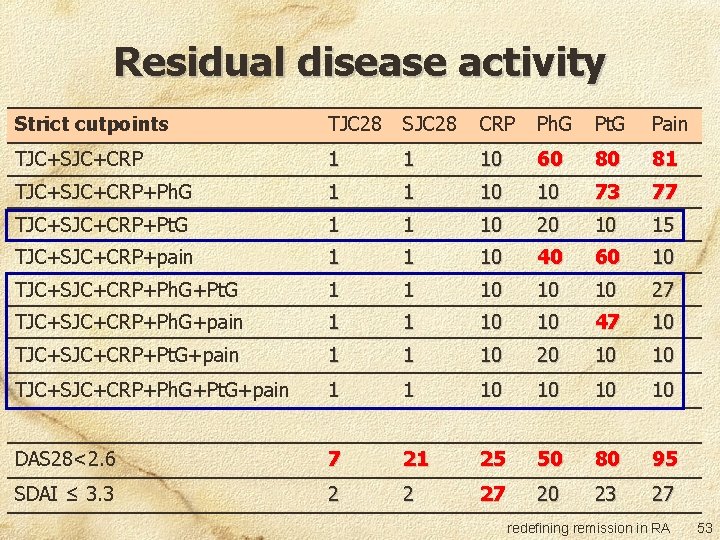
Residual disease activity Strict cutpoints TJC 28 SJC 28 CRP Ph. G Pt. G Pain TJC+SJC+CRP 1 1 10 60 80 81 TJC+SJC+CRP+Ph. G 1 1 10 10 73 77 TJC+SJC+CRP+Pt. G 1 1 10 20 10 15 TJC+SJC+CRP+pain 1 1 10 40 60 10 TJC+SJC+CRP+Ph. G+Pt. G 1 1 10 10 10 27 TJC+SJC+CRP+Ph. G+pain 1 1 10 10 47 10 TJC+SJC+CRP+Pt. G+pain 1 1 10 20 10 10 TJC+SJC+CRP+Ph. G+Pt. G+pain 1 1 10 10 DAS 28<2. 6 7 21 25 50 80 95 SDAI ≤ 3. 3 2 2 27 20 23 27 redefining remission in RA 50

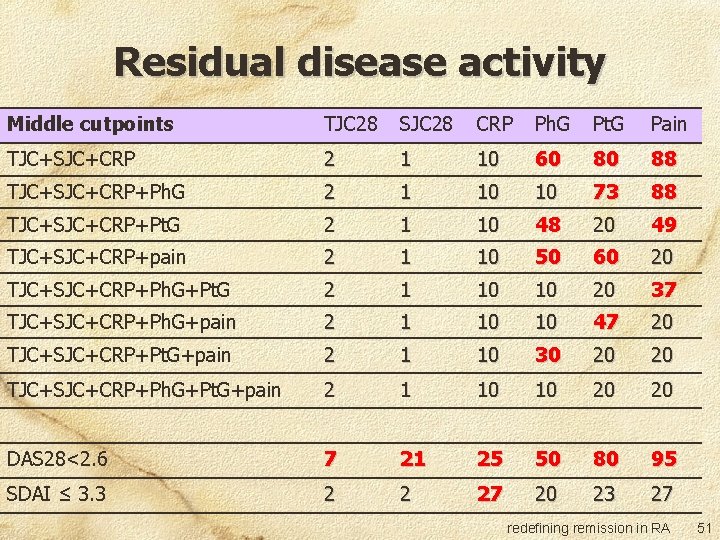
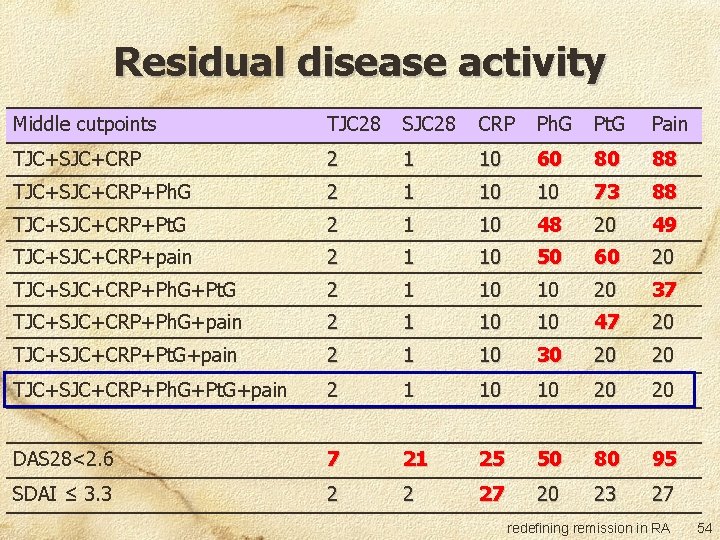
Residual disease activity Middle cutpoints TJC 28 SJC 28 CRP Ph. G Pt. G Pain TJC+SJC+CRP 2 1 10 60 80 88 TJC+SJC+CRP+Ph. G 2 1 10 10 73 88 TJC+SJC+CRP+Pt. G 2 1 10 48 20 49 TJC+SJC+CRP+pain 2 1 10 50 60 20 TJC+SJC+CRP+Ph. G+Pt. G 2 1 10 10 20 37 TJC+SJC+CRP+Ph. G+pain 2 1 10 10 47 20 TJC+SJC+CRP+Pt. G+pain 2 1 10 30 20 20 TJC+SJC+CRP+Ph. G+Pt. G+pain 2 1 10 10 20 20 DAS 28<2. 6 7 21 25 50 80 95 SDAI ≤ 3. 3 2 2 27 20 23 27 redefining remission in RA 51

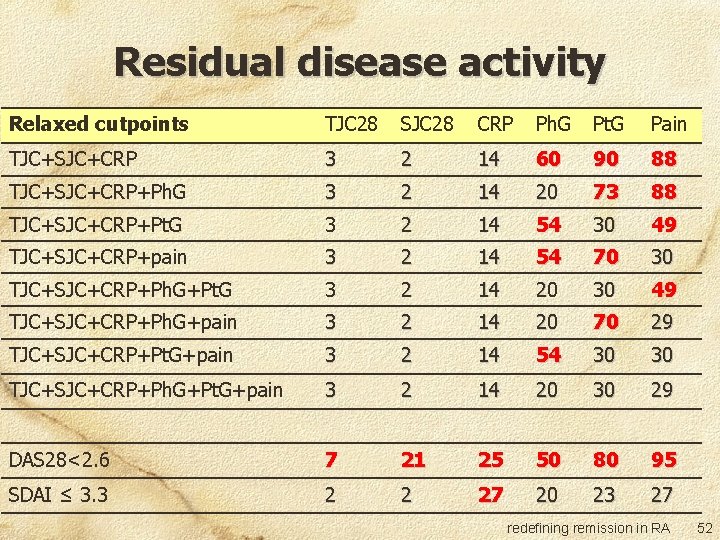
Residual disease activity Relaxed cutpoints TJC 28 SJC 28 CRP Ph. G Pt. G Pain TJC+SJC+CRP 3 2 14 60 90 88 TJC+SJC+CRP+Ph. G 3 2 14 20 73 88 TJC+SJC+CRP+Pt. G 3 2 14 54 30 49 TJC+SJC+CRP+pain 3 2 14 54 70 30 TJC+SJC+CRP+Ph. G+Pt. G 3 2 14 20 30 49 TJC+SJC+CRP+Ph. G+pain 3 2 14 20 70 29 TJC+SJC+CRP+Pt. G+pain 3 2 14 54 30 30 TJC+SJC+CRP+Ph. G+Pt. G+pain 3 2 14 20 30 29 DAS 28<2. 6 7 21 25 50 80 95 SDAI ≤ 3. 3 2 2 27 20 23 27 redefining remission in RA 52

Residual disease activity Strict cutpoints TJC 28 SJC 28 CRP Ph. G Pt. G Pain TJC+SJC+CRP 1 1 10 60 80 81 TJC+SJC+CRP+Ph. G 1 1 10 10 73 77 TJC+SJC+CRP+Pt. G 1 1 10 20 10 15 TJC+SJC+CRP+pain 1 1 10 40 60 10 TJC+SJC+CRP+Ph. G+Pt. G 1 1 10 10 10 27 TJC+SJC+CRP+Ph. G+pain 1 1 10 10 47 10 TJC+SJC+CRP+Pt. G+pain 1 1 10 20 10 10 TJC+SJC+CRP+Ph. G+Pt. G+pain 1 1 10 10 DAS 28<2. 6 7 21 25 50 80 95 SDAI ≤ 3. 3 2 2 27 20 23 27 redefining remission in RA 53

Residual disease activity Middle cutpoints TJC 28 SJC 28 CRP Ph. G Pt. G Pain TJC+SJC+CRP 2 1 10 60 80 88 TJC+SJC+CRP+Ph. G 2 1 10 10 73 88 TJC+SJC+CRP+Pt. G 2 1 10 48 20 49 TJC+SJC+CRP+pain 2 1 10 50 60 20 TJC+SJC+CRP+Ph. G+Pt. G 2 1 10 10 20 37 TJC+SJC+CRP+Ph. G+pain 2 1 10 10 47 20 TJC+SJC+CRP+Pt. G+pain 2 1 10 30 20 20 TJC+SJC+CRP+Ph. G+Pt. G+pain 2 1 10 10 20 20 DAS 28<2. 6 7 21 25 50 80 95 SDAI ≤ 3. 3 2 2 27 20 23 27 redefining remission in RA 54

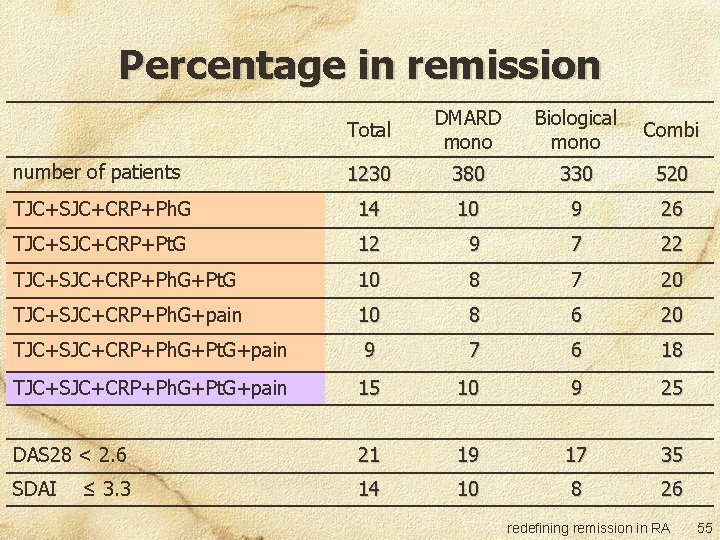
Percentage in remission Total DMARD mono Biological mono Combi number of patients 1230 380 330 520 TJC+SJC+CRP+Ph. G 14 10 9 26 TJC+SJC+CRP+Pt. G 12 9 7 22 TJC+SJC+CRP+Ph. G+Pt. G 10 8 7 20 TJC+SJC+CRP+Ph. G+pain 10 8 6 20 TJC+SJC+CRP+Ph. G+Pt. G+pain 9 7 6 18 TJC+SJC+CRP+Ph. G+Pt. G+pain 15 10 9 25 DAS 28 < 2. 6 21 19 17 35 SDAI 14 10 8 26 ≤ 3. 3 redefining remission in RA 55

Last Saturday TJC+SJC+CRP+Pt. G got highest marks 5 def’s to remain for further testing in observational datasets agenda at OMERACT 10 presentation of final validation exercises stability/reliability start development of patient ‘absence of disease’ definition and measurement collaborate with OMERACT RA Flare group redefining remission in RA 56

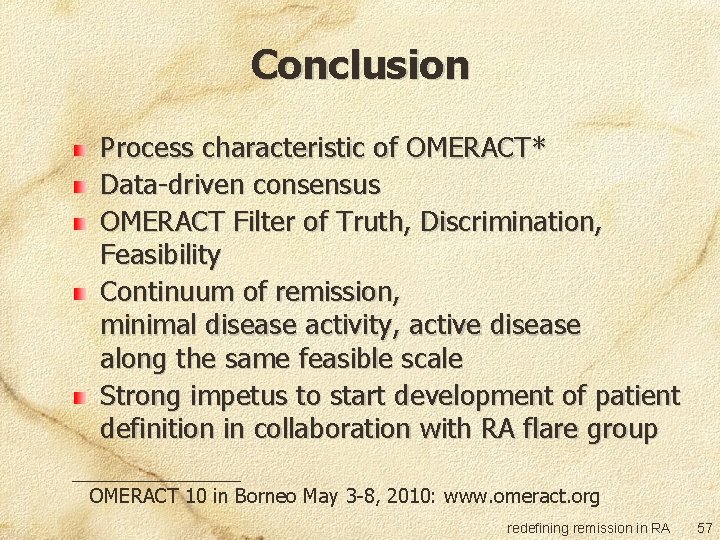
Conclusion Process characteristic of OMERACT* Data-driven consensus OMERACT Filter of Truth, Discrimination, Feasibility Continuum of remission, minimal disease activity, active disease along the same feasible scale Strong impetus to start development of patient definition in collaboration with RA flare group OMERACT 10 in Borneo May 3 -8, 2010: www. omeract. org redefining remission in RA 57
 Rheumatoid arthritis
Rheumatoid arthritis Diagnosing rheumatoid arthritis
Diagnosing rheumatoid arthritis Rheumatoid arthritis vs osteoarthritis
Rheumatoid arthritis vs osteoarthritis Deformities in rheumatoid arthritis
Deformities in rheumatoid arthritis Soft tissue rheumatoid arthritis
Soft tissue rheumatoid arthritis Barik meaning
Barik meaning Steinbrocker stage
Steinbrocker stage Boutonniere and swan neck deformity
Boutonniere and swan neck deformity Juvenile rheumatoid arthritis
Juvenile rheumatoid arthritis Haart side effects
Haart side effects Rheumatoid arthritis nursing interventions
Rheumatoid arthritis nursing interventions B t cells
B t cells Extra articular manifestations of rheumatoid arthritis
Extra articular manifestations of rheumatoid arthritis Nursing diagnosis for rheumatoid arthritis
Nursing diagnosis for rheumatoid arthritis Juvenile rheumatoid arthritis symptoms
Juvenile rheumatoid arthritis symptoms Factors that are reshaping and redefining the manager's job
Factors that are reshaping and redefining the manager's job Reclaiming and redefining the fundamentals of care
Reclaiming and redefining the fundamentals of care Redefining airmanship
Redefining airmanship Factors reshaping and redefining management
Factors reshaping and redefining management Rémission maladie de vaquez
Rémission maladie de vaquez Baptism for the remission of sins
Baptism for the remission of sins Ra factor range
Ra factor range Memorandum joint venture account
Memorandum joint venture account Break joint lamb
Break joint lamb Break joint vs spool joint
Break joint vs spool joint Nuchal ligament
Nuchal ligament Fibrous joints
Fibrous joints 5 types of permanent joints
5 types of permanent joints Gardening with arthritis
Gardening with arthritis Ankylosing spondylitis
Ankylosing spondylitis Kode icd 10 arthritis genu
Kode icd 10 arthritis genu Poststreptococcal reactive arthritis
Poststreptococcal reactive arthritis Anatomi fisiologi gout arthritis
Anatomi fisiologi gout arthritis Infusion therapy for arthritis
Infusion therapy for arthritis Jean luc meynard
Jean luc meynard Viral arthritis
Viral arthritis Satellitism in haemophilus influenzae
Satellitism in haemophilus influenzae Reactive arthritis
Reactive arthritis Arthritis foundation indiana
Arthritis foundation indiana Arthritis in shoulder nhs
Arthritis in shoulder nhs Septic arthritis workup
Septic arthritis workup Bakgil
Bakgil Eular psoriatic arthritis guidelines
Eular psoriatic arthritis guidelines Septic arthritis complications
Septic arthritis complications Arthritis and food allergies
Arthritis and food allergies Peripheral arthritis
Peripheral arthritis Haemophilus influenzae septic arthritis
Haemophilus influenzae septic arthritis Acute gout attack treatment
Acute gout attack treatment Septic arthritis complications
Septic arthritis complications Psoriasis arthritis nhs
Psoriasis arthritis nhs Rehaklinik psoriasis arthritis
Rehaklinik psoriasis arthritis Arthritis treatment
Arthritis treatment Haemophilus influenzae septic arthritis
Haemophilus influenzae septic arthritis Septic arthritis antibiotics
Septic arthritis antibiotics Kode icd 10 osteoarthritis genu
Kode icd 10 osteoarthritis genu Boutonniere nodes
Boutonniere nodes Seronegative arthritis
Seronegative arthritis Caprine arthritis encefalitis
Caprine arthritis encefalitis