EULAR recommendations for the management of psoriatic arthritis










- Slides: 17

EULAR recommendations for the management of psoriatic arthritis: 2015 update

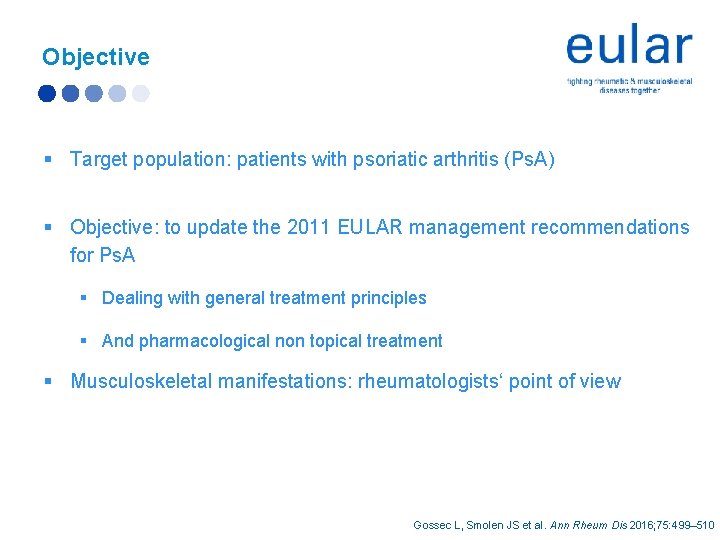
Objective § Target population: patients with psoriatic arthritis (Ps. A) § Objective: to update the 2011 EULAR management recommendations for Ps. A § Dealing with general treatment principles § And pharmacological non topical treatment § Musculoskeletal manifestations: rheumatologists‘ point of view Gossec L, Smolen JS et al. Ann Rheum Dis 2016; 75: 499– 510

Methods • Meeting of steering group (June 2014): decisions on scope of the literature review • Systematic literature review of drugs • General literature review of treatment strategies, prognosis and comorbidities • Taskforce full meeting (January 2015): § § • 3 Presentation of literature review Discussions based on the 2010 recommendations Elaboration of updated recommendations Determination of level of strength and grade of recommendations Votes by email on level of agreement of Taskforce members (0 -10 where 10 is full agreement) Oxford levels of evidence, 2009

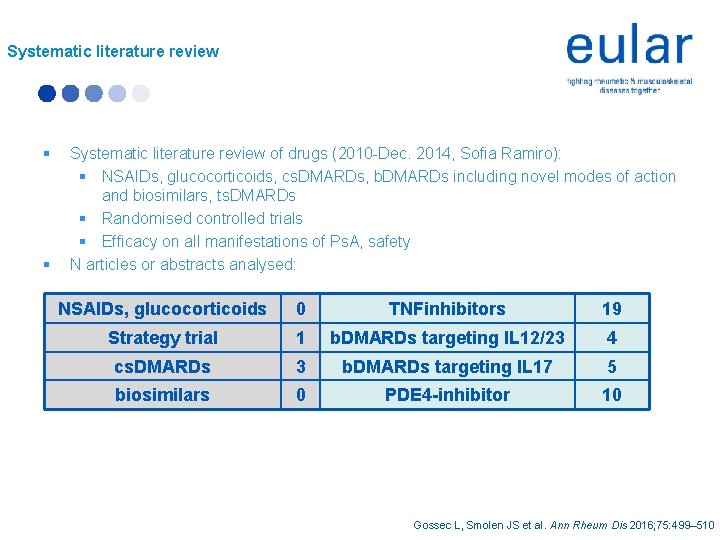
Systematic literature review § § Systematic literature review of drugs (2010 -Dec. 2014, Sofia Ramiro): § NSAIDs, glucocorticoids, cs. DMARDs, b. DMARDs including novel modes of action and biosimilars, ts. DMARDs § Randomised controlled trials § Efficacy on all manifestations of Ps. A, safety N articles or abstracts analysed: NSAIDs, glucocorticoids 0 TNFinhibitors 19 Strategy trial 1 b. DMARDs targeting IL 12/23 4 cs. DMARDs 3 b. DMARDs targeting IL 17 5 biosimilars 0 PDE 4 -inhibitor 10 Gossec L, Smolen JS et al. Ann Rheum Dis 2016; 75: 499– 510

EULAR recommendations for the management of psoriatic arthritis: 2015 update Five overarching principles a. Psoriatic arthritis is a heterogeneous and potentially severe disease, which may require multidisciplinary management. b. Treatment of psoriatic arthritis patients should aim at the best care and must be based on a shared decision between the patient and the rheumatologist, considering efficacy, safety and costs. c. Rheumatologists are the specialists who should primarily care for the musculoskeletal manifestations of patients with psoriatic arthritis; in the presence of clinically significant skin involvement a rheumatologist and a dermatologist should collaborate in diagnosis and management. d. The primary goal of treating patients with psoriatic arthritis is to maximise health-related quality of life, through control of symptoms, prevention of structural damage, normalisation of function and social participation; abrogation of inflammation is an important component to achieve these goals. e. When managing patients with psoriatic arthritis, extra-articular manifestations, metabolic syndrome, cardiovascular disease and other co-morbidities should be taken into account. Gossec L, Smolen JS et al. Ann Rheum Dis 2016; 75: 499– 510

EULAR recommendations for the management of psoriatic arthritis: 2015 update Recommendations Recommendation 1 Treatment should be aimed at reaching the target of remission or, alternatively, LDA or MDA, by regular monitoring and appropriate adjustment of therapy 2 In patients with Ps. A, NSAIDs may be used to relieve musculoskeletal signs and symptoms 3 In patients with peripheral arthritis, particularly those with many swollen joints, structural damage, high ESR/CRP and/or clinically relevant EAMs, cs. DMARDs should be considered, with MTX preferred in those with relevant skin involvement 4 Local injections of glucocorticoids should be considered as adjunctive therapy in Ps. A; systemic glucocorticoids may be used with caution at the lowest effective dose 5 In patients with peripheral arthritis and an inadequate response to at least one cs. DMARD, therapy with a b. DMARD, usually a TNF inhibitor, should be commenced LDA: Low disease activity; MDA: minimal disease activity; EAM: extra-articular manifestation Gossec L, Smolen JS et al. Ann Rheum Dis 2016; 75: 499– 510

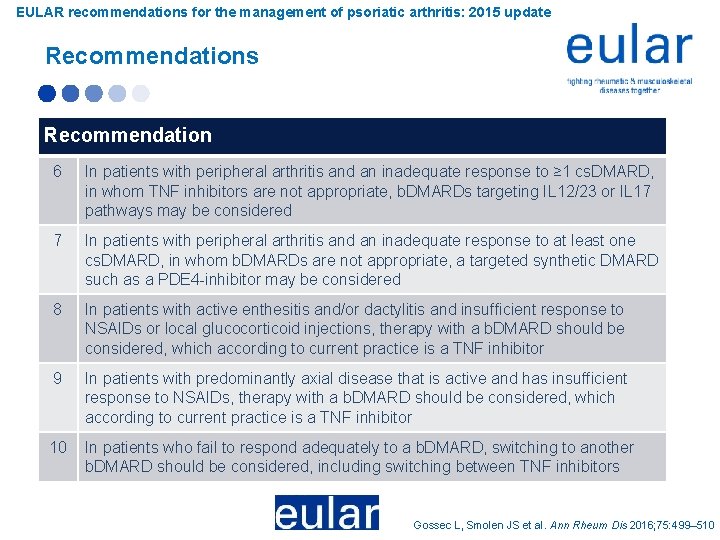
EULAR recommendations for the management of psoriatic arthritis: 2015 update Recommendations Recommendation 6 In patients with peripheral arthritis and an inadequate response to ≥ 1 cs. DMARD, in whom TNF inhibitors are not appropriate, b. DMARDs targeting IL 12/23 or IL 17 pathways may be considered 7 In patients with peripheral arthritis and an inadequate response to at least one cs. DMARD, in whom b. DMARDs are not appropriate, a targeted synthetic DMARD such as a PDE 4 -inhibitor may be considered 8 In patients with active enthesitis and/or dactylitis and insufficient response to NSAIDs or local glucocorticoid injections, therapy with a b. DMARD should be considered, which according to current practice is a TNF inhibitor 9 In patients with predominantly axial disease that is active and has insufficient response to NSAIDs, therapy with a b. DMARD should be considered, which according to current practice is a TNF inhibitor 10 In patients who fail to respond adequately to a b. DMARD, switching to another b. DMARD should be considered, including switching between TNF inhibitors Gossec L, Smolen JS et al. Ann Rheum Dis 2016; 75: 499– 510

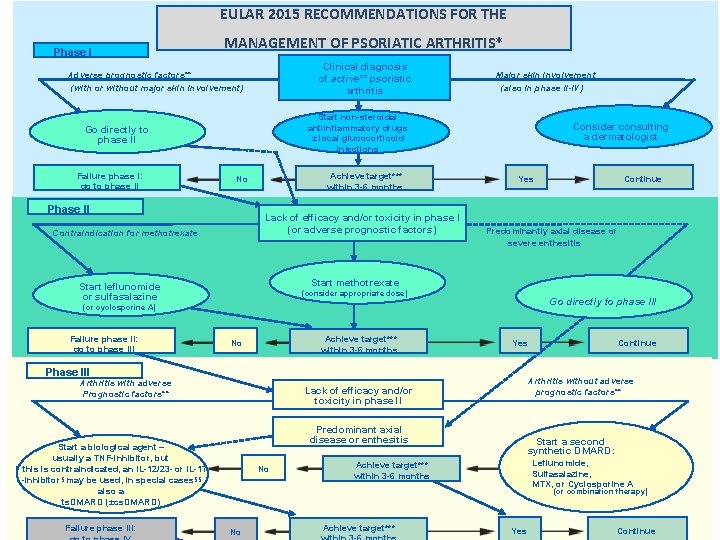
EULAR 2015 RECOMMENDATIONS FOR THE Phase I MANAGEMENT OF PSORIATIC ARTHRITIS* Clinical diagnosis of active** psoriatic arthritis Adverse prognostic factors** (with or without major skin involvement) Start non-steroidal antiinflammatory drugs ± local glucocorticoid injections Go directly to phase II Failure phase I: go to phase II Major skin involvement (also in phase II-IV) Achieve target*** within 3 -6 months No Phase II Lack of efficacy and/or toxicity in phase I (or adverse prognostic factors) Contraindication for methotrexate Consider consulting a dermatologist Yes Predominantly axial disease or severe enthesitis Start methotrexate Start leflunomide or sulfasalazine (consider appropriate dose) Go directly to phase III (or cyclosporine A) Failure phase II: go to phase III Achieve target*** within 3 -6 months No Yes Phase III Arthritis with adverse Prognostic factors** Predominant axial disease or enthesitis No Continue Arthritis without adverse prognostic factors** Lack of efficacy and/or toxicity in phase l. I Start a biological agent – usually a TNF-inhibitor, but If this is contraindicated, an IL-12/23 - or IL-17 -inhibitor§ may be used, in special cases§§ also a ts. DMARD (±cs. DMARD) Failure phase III: Continue Start a second synthetic DMARD: Leflunomide, Sulfasalazine, MTX, or Cyclosporine A Achieve target*** within 3 -6 months (or combination therapy) No Achieve target*** Yes Continue

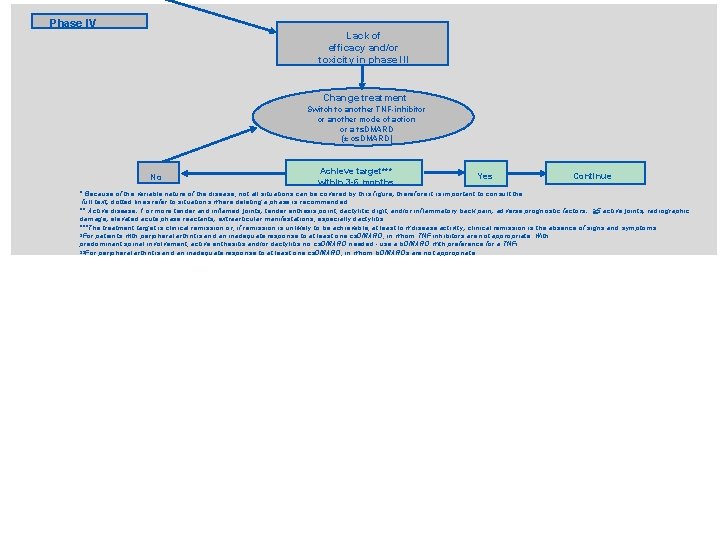
Phase IV Lack of efficacy and/or toxicity in phase l. II Change treatment Switch to another TNF-inhibitor or another mode of action or a ts. DMARD (± cs. DMARD) No Achieve target*** within 3 -6 months Yes Continue * Because of the variable nature of the disease, not all situations can be covered by this figure; therefore it is important to consult the full text; dotted lines refer to situations where deleting a phase is recommended. ** Active disease: 1 or more tender and inflamed joints; tender enthesis point, dactylitic digit, and/or inflammatory back pain; adverse prognostic factors: >5 active joints; radiographic damage; elevated acute phase reactants; extraarticular manifestations, especially dactylitis. ***The treatment target is clinical remission or, if remission is unlikely to be achievable, at least low disease activity; clinical remission is the absence of signs and symptoms. §For patients with peripheral arthritis and an inadequate response to at least one cs. DMARD, in whom TNF inhibitors are not appropriate. With predominant spinal involvement, active enthesitis and/or dactylitis no cs. DMARD needed - use a b. DMARD with preference for a TNFi. §§For peripheral arthritis and an inadequate response to at least one cs. DMARD, in whom b. DMARDs are not appropriate.

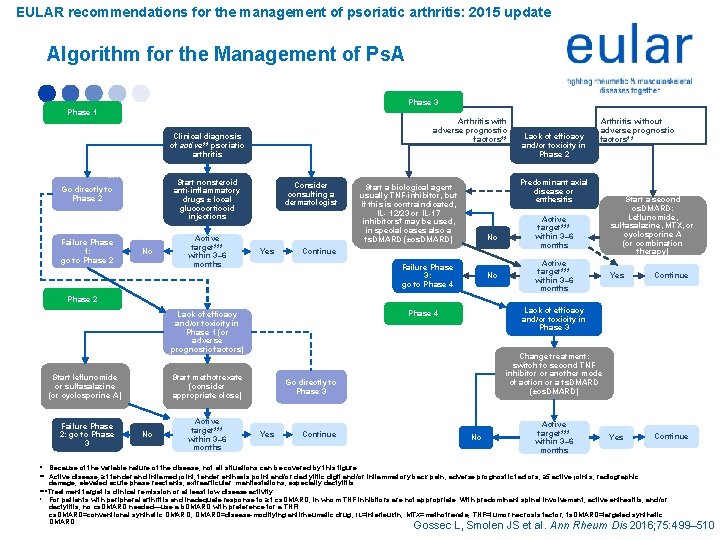
EULAR recommendations for the management of psoriatic arthritis: 2015 update Algorithm for the Management of Ps. A Phase 3 Phase 1 Adverse prognostic factors** (with or without major skin involvement) Go directly to Phase 2 Failure Phase 1: go to Phase 2 No Clinical diagnosis of active** psoriatic arthritis Active target*** within 3– 6 months Start leflunomide or sulfasalazine (or cyclosporine A) Failure Phase 2: go to Phase 3 Lack of efficacy and/or toxicity in Phase 1 (or adverse prognostic factors) Consider consulting a dermatologist Yes Active target*** within 3– 6 months No Continue Predominantly axial disease or severe enthesitis No Continue Arthritis without adverse prognostic factors** Active target*** within 3– 6 months Start a second cs. DMARD: Leflunomide, sulfasalazine, MTX, or cyclosporine A (or combination therapy) Yes Continue Lack of efficacy and/or toxicity in Phase 3 Phase 4 Change treatment: switch to second TNF inhibitor or another mode of action or a ts. DMARD (±cs. DMARD) Go directly to Phase 3 Yes Lack of efficacy and/or toxicity in Phase 2 Predominant axial disease or enthesitis Start a biological agent usually TNF-inhibitor, but if this is contraindicated, IL- 12/23 or IL-17 inhibitors† may be used, in special cases also a ts. DMARD (±cs. DMARD) Failure Phase 3: go to Phase 4 Start methotrexate (consider appropriate close) No Arthritis with adverse prognostic factors** (also in Phases 2– 4) Start nonsteroid anti-inflammatory drugs ± local glucocorticoid injections Phase 2 Contraindication for methotrexate Major skin involvement No Active target*** within 3– 6 months * Because of the variable nature of the disease, not all situations can be covered by this figure. ** Active disease, ≥ 1 tender and inflamed joint; tender enthesis point and/or dactylitic digit and/or inflammatory back pain; adverse prognostic factors, ≥ 5 active joints; radiographic damage; elevated acute phase reactants; extraarticular manifestations, especially dactylitis. ***Treatment target is clinical remission or at least low disease activity. † For patients with peripheral arthritis and inadequate response to ≥ 1 cs. DMARD, in whom TNF inhibitors are not appropriate. With predominant spinal involvement, active enthesitis, and/or dactylitis, no cs. DMARD needed—use a b. DMARD with preference for a TNFi. cs. DMARD=conventional synthetic DMARD; DMARD=disease-modifying antirheumatic drug; IL=interleukin; MTX=methotrexate; TNF=tumor necrosis factor; ts. DMARD=targeted synthetic DMARD. Gossec L, Smolen JS et al. Ann Rheum Dis 2016; 75: 499– 510

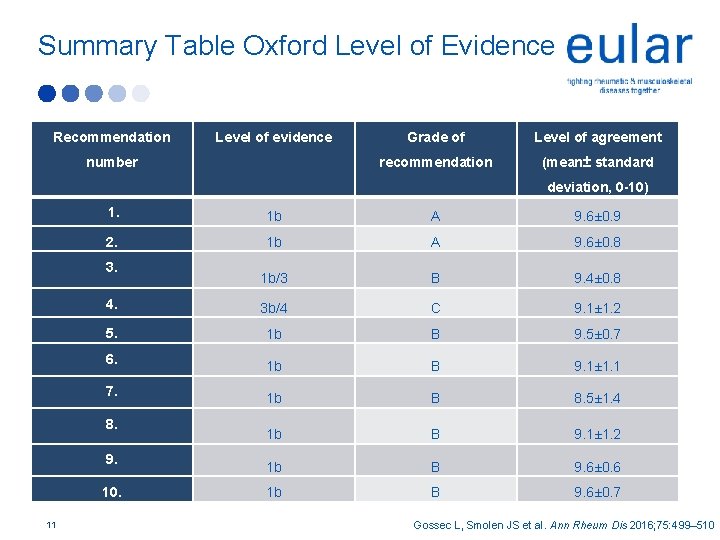
Summary Table Oxford Level of Evidence Recommendation Level of evidence number Grade of Level of agreement recommendation (mean± standard deviation, 0 -10) 1. 1 b A 9. 6± 0. 9 2. 1 b A 9. 6± 0. 8 1 b/3 B 9. 4± 0. 8 4. 3 b/4 C 9. 1± 1. 2 5. 1 b B 9. 5± 0. 7 1 b B 9. 1± 1. 1 1 b B 8. 5± 1. 4 1 b B 9. 1± 1. 2 1 b B 9. 6± 0. 6 1 b B 9. 6± 0. 7 3. 6. 7. 8. 9. 10. 11 Gossec L, Smolen JS et al. Ann Rheum Dis 2016; 75: 499– 510

EULAR recommendations for the management of psoriatic arthritis: 2015 update Lay version of recommendations Recommendation 1 Treatment should aim for remission or low disease activity; regular monitoring and adjustment of therapy will help achieve this goal. The goal of your treatment should be remission or low disease activity. Remission means that the disease is well-controlled, there is no longer any inflammation such as swollen joints and no signs of inflammation in blood tests. However, there may still be some symptoms even in remission. Low disease activity means that your levels of inflammation are minimal. Your doctor can help you to achieve these goals by keeping a close eye on your disease by examining you and asking for tests as needed. Your doctor may also adjust your treatment up or down as needed. 2 Non-steroidal anti-inflammatory drugs may be used. NSAIDs can be used to relieve signs and symptoms in your joints and muscles. They can help to reduce pain and improve mobility (movement) in your joints, but do not inhibit progression of joint damage. These drugs will not preserve long-term functioning and will not help the skin. 3 cs. DMARDs should be used at an early stage in people with arthritis in the joints of their arms, hands, legs or feet or other symptoms. Some people may have arthritis either in the large joints such as elbows, knees and wrists or in the small joints of their hands and feet (this is called peripheral arthritis). In these people, cs. DMARDs should be considered at an early stage, especially if they have many swollen joints, or symptoms in other parts of their body, such as their eyes. Methotrexate is the preferred drug, especially if you also have skin lesions as part of your disease. 4 Steroid injections should be considered, and systemic steroids may be used with caution at the lowest effective dose. Local injections of glucocorticoids (steroids) for example in joints or near tendons can be used in people with psoriatic arthritis to provide relief. Steroids taken by other means (e. g. as tablets) are not usually recommended but may be used with caution at the lowest possible dose. Gossec L, Smolen JS et al. Ann Rheum Dis 2016; 75: 499– 510

EULAR recommendations for the management of psoriatic arthritis: 2015 update Lay version of recommendations Recommendation 5 b. DMARDs should be used in people with peripheral arthritis who have not responded to at least one cs. DMARD. If you have peripheral arthritis and have not have good effects from treatment with at least one cs. DMARD for several months, you may benefit from therapy with a b. DMARD. This will usually be a TNF inhibitor since these are the b. DMARDs we have the most experience with. These work by blocking a molecule called TNF (which stands for tumour necrosis factor). TNF is involved in inflammation. 6 If TNF inhibitors are not appropriate, b. DMARDs targeting different cells or molecules may be considered for peripheral arthritis. If you have peripheral arthritis and are unable to take TNF inhibitors for some reason, a b. DMARD that targets a different cell or molecule may be considered. Some drugs have recently come on the market and seem to work as well as TNF inhibitors. 7 ts. DMARDs may be used for peripheral arthritis if b. DMARDs are not appropriate. *** If you have peripheral arthritis and are unable to take b. DMARDs for any reason, a ts. DMARD may be considered. These types of medicine work in a different way to b. DMARDs and are given as pills rather than injections or infusions. Gossec L, Smolen JS et al. Ann Rheum Dis 2016; 75: 499– 510

EULAR recommendations for the management of psoriatic arthritis: 2015 update Lay version of recommendations Recommendation 8 b. DMARDs should be considered for people with inflamed tendons, ligaments, and sausage-like fingers or toes. *** If you have inflamed tendons or ligaments or inflamed fingers or toes (sometimes called sausage-like), and have not had good effects from NSAIDs or steroids injections, a b. DMARD should be considered even if you have not been given a cs. DMARD. 9 b. DMARDs should be considered for people with axial disease. *** If your disease is mostly in your back (axial disease) and has not got better with NSAIDs, a TNF inhibitor should be considered without first prescribing a cs. DMARD. 10 People who do not respond to b. DMARDs should switch treatment. If your disease does not improve with a b. DMARD, you should be switched to another kind. This might mean switching to another brand of TNF inhibitor, or to a different type of b. DMARD that works on a different pathway, or in some cases to a ts. DMARD Gossec L, Smolen JS et al. Ann Rheum Dis 2016; 75: 499– 510

Conclusions § Update of the EULAR recommendations for the management of Ps. A § 5 overarching principles regarding treatment objectives and treatment strategy in Ps. A § 10 practical recommendations regarding pharmacological non-topical treatment § New drugs will allow rotations of drugs across different mechanisms of action, ultimately benefiting patients with Ps. A.

Acknowledgements: Ps. A update Taskforce Convenor: J. Smolen Epidemiologist: L. Gossec Fellow: S. Ramiro Steering group: M. Cutolo, M. de Wit, M Dougados, P. Emery, R. Landewé, S. Oliver, D. van der Heijde Other patient partners: M. Maccarone, N. Betteridge Other health professional: E. Haglund Other physicians: D. Aletaha, J. Braun, G. Burmester, J. D. Cañete, N Damjanov, O. Fitz. Gerald, P. Helliwell, T. K. Kvien, R Lories, T. Luger (dermatologist), H. Marzo-Ortega, D. Mc. Gonagle, I. B. Mc. Innes, I Olivieri, K Pavelka, G. Schett, J. Sieper, F. van den Bosch, D Veale, J Wollenhaupt, A. Zink Gossec L, Smolen JS et al. Ann Rheum Dis 2016; 75: 499– 510

DISCLOSURES • Many authors of these recommendations have received honoraria for talks and/or advisory board participation and/or research grants from, and/or were investigators of clinical trials performed by one or more pharmaceutical companies. These comprise: Abbvie, Amgen, Astra-Zeneca, BMS, Celgene, Celltrion, Glaxo, Janssen, Lilly, Medimmune, Merck-Serono, MSD, Novartis, Pfizer, Roche, Samsung, Sanofi, UCB • The development of these recommendations was funded by EULAR. No company representative was present at any time during the process.
 Gossec
Gossec What is esr
What is esr Graph discrete mathematics
Graph discrete mathematics Ra vs oa
Ra vs oa Eular
Eular Eular criteria
Eular criteria Eular
Eular Nursing management of arthritis
Nursing management of arthritis Poststreptococcal reactive arthritis
Poststreptococcal reactive arthritis Pictures of rheumatoid arthritis vs osteoarthritis
Pictures of rheumatoid arthritis vs osteoarthritis Steinbrocker stage
Steinbrocker stage Pauciarticular juvenile rheumatoid arthritis
Pauciarticular juvenile rheumatoid arthritis Gonorrhea symptoms female
Gonorrhea symptoms female Anatomi fisiologi gout arthritis
Anatomi fisiologi gout arthritis Septic arthritis complications
Septic arthritis complications Septic arthritis antibiotics
Septic arthritis antibiotics Soft tissue rheumatoid arthritis
Soft tissue rheumatoid arthritis Septic arthritis antibiotics
Septic arthritis antibiotics