Predicting the emergence of antiretroviral therapy ART side












- Slides: 35

Predicting the emergence of antiretroviral therapy (ART) side effects: The role of psychological variables. Dr Rob Horne, Professor of Behavioural Medicine Dr Vanessa Cooper, Research Fellow Centre for Behavioural Medicine The School of Pharmacy, University of London rob. horne@pharmacy. ac. uk

Background • Despite advances in antiretroviral treatments, benefit is still accompanied by adverse effects (side-effects)1, 2 • Treatment side effects are frequently associated with nonadherence to ART 3, 4 • Improving coping skills for self-management for treatment side effects can reduce ART nonadherence – BALANCE intervention San Francisco (5 60 -minute individual counselling sessions)5. 1. Este JA et al (2010) Antivir Res: 85: 25 -33 2. Johnson MO et al (2005) J Pain Symptom Manage. 29: 193 -205 3. Ammassari A, et al (2001) JAIDS ; 28(5): 445 -9. 4. Johnson & Neilands TB (2007) AIDS Behav 11(4): 575 -585 5. Johnson MO (2011) Ann Behav Med. ; 41(1): 83 -91. Rob. horne@pharmacy. ac. uk

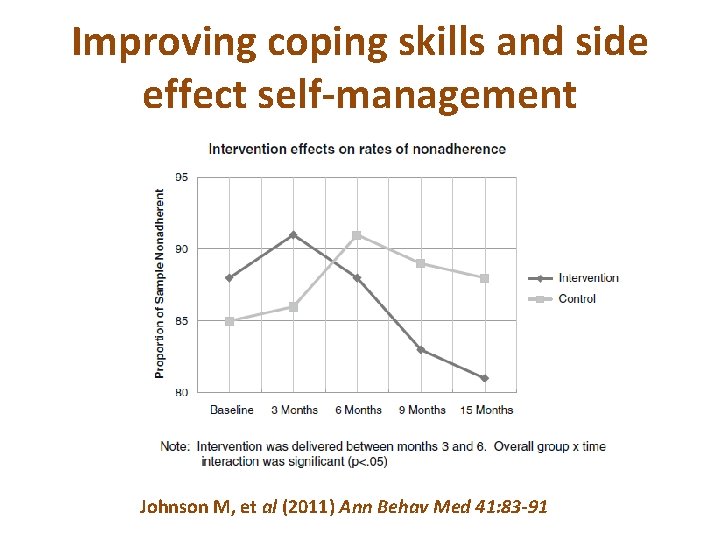
Improving coping skills and side effect self-management Johnson M, et al (2011) Ann Behav Med 41: 83 -91

Improving self-management of ART side effects: outstanding questions • Can we increase the effect if we intervene earlier (e. g. at start of treatment)? • Can we target interventions by identifying those most at risk of developing moderate to severe ART side effects? • Can we identify potentially modifiable factors associated with the subsequent emergence of moderate to severe ART side-effects? Rob. horne@pharmacy. ac. uk

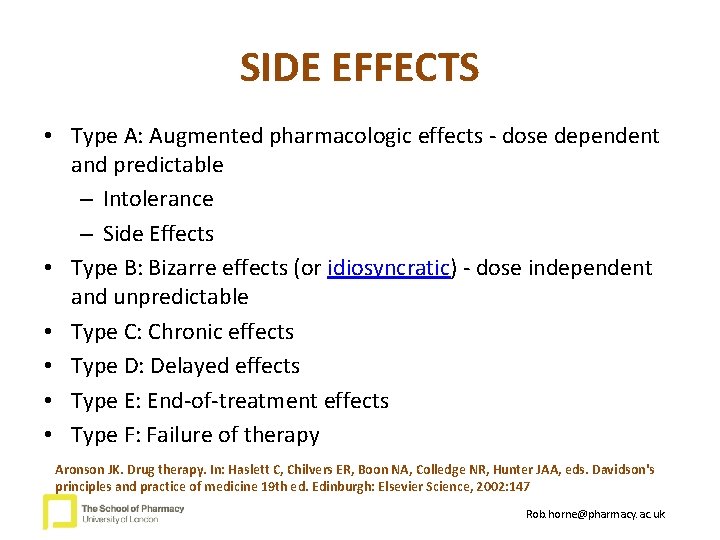
SIDE EFFECTS • Type A: Augmented pharmacologic effects - dose dependent and predictable – Intolerance – Side Effects • Type B: Bizarre effects (or idiosyncratic) - dose independent and unpredictable • Type C: Chronic effects • Type D: Delayed effects • Type E: End-of-treatment effects • Type F: Failure of therapy Aronson JK. Drug therapy. In: Haslett C, Chilvers ER, Boon NA, Colledge NR, Hunter JAA, eds. Davidson's principles and practice of medicine 19 th ed. Edinburgh: Elsevier Science, 2002: 147 Rob. horne@pharmacy. ac. uk

Side effects a question of attribution? 1, 2 1. Johnson MO, Stallworth T, Neilands TB (2003) AIDSBehav 7; 109 -117 2. Cooper, V. , Gellaitry, G. , Hankins, M. , Fisher, M. , Horne, R. (2009). AIDS Care, 21(4), 520 -528.

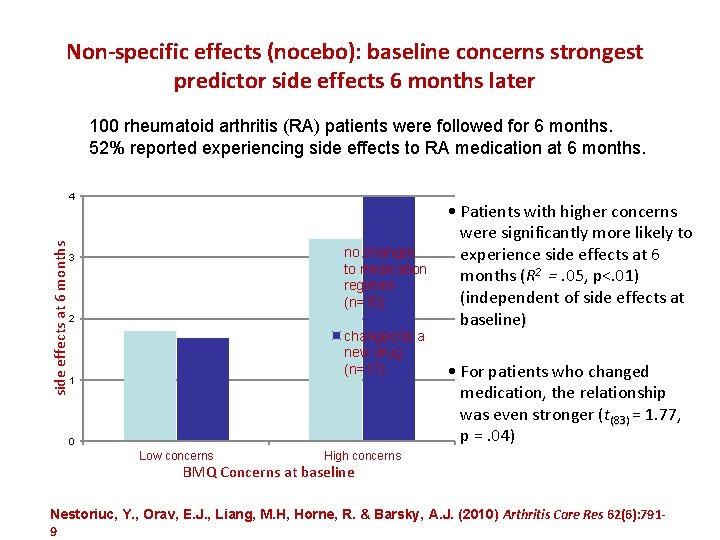
Non-specific effects (nocebo): baseline concerns strongest predictor side effects 6 months later 100 rheumatoid arthritis (RA) patients were followed for 6 months. 52% reported experiencing side effects to RA medication at 6 months. side effects at 6 months 4 no changes to medication regimen (n=70) 3 2 changed to a new drug (n=17) 1 0 Low concerns • Patients with higher concerns were significantly more likely to experience side effects at 6 months (R 2 =. 05, p<. 01) (independent of side effects at baseline) • For patients who changed medication, the relationship was even stronger (t(83) = 1. 77, p =. 04) High concerns BMQ Concerns at baseline Nestoriuc, Y. , Orav, E. J. , Liang, M. H, Horne, R. & Barsky, A. J. (2010) Arthritis Care Res 62(6): 7919

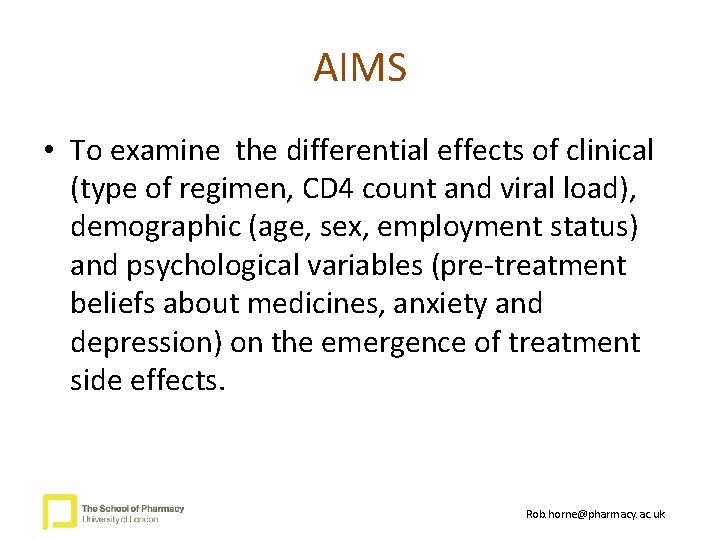
AIMS • To examine the differential effects of clinical (type of regimen, CD 4 count and viral load), demographic (age, sex, employment status) and psychological variables (pre-treatment beliefs about medicines, anxiety and depression) on the emergence of treatment side effects. Rob. horne@pharmacy. ac. uk


Methods • Prospective, follow-up study • Consecutive patients attending HIV clinics in Brighton, UK, completed validated questionnaires assessing beliefs about HAART (necessity and concerns), perceived sensitivity to adverse effects of medicines PSM), depression and anxiety before initiating treatment (baseline). • Self-reported symptoms were measured at 3 (3 M) and 6 months (6 M). • Adherence was assessed at 1, 3, 6 and 12 months follow up • Clinical, treatment and demographic data were recorded from medical files. Rob. horne@pharmacy. ac. uk

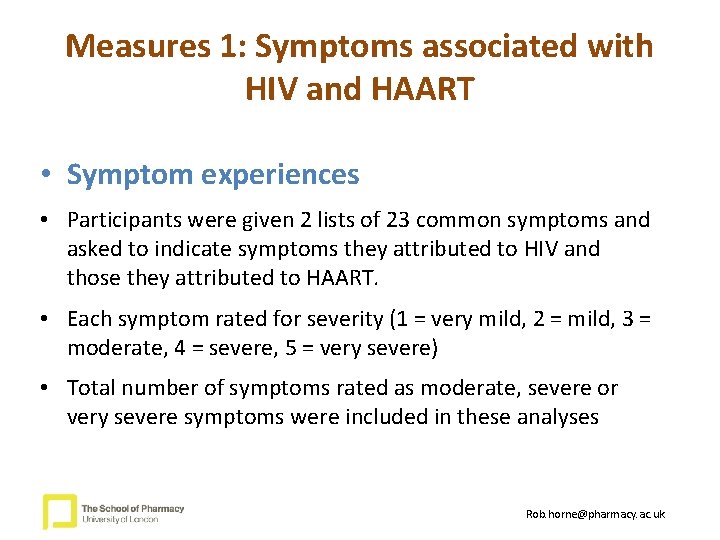
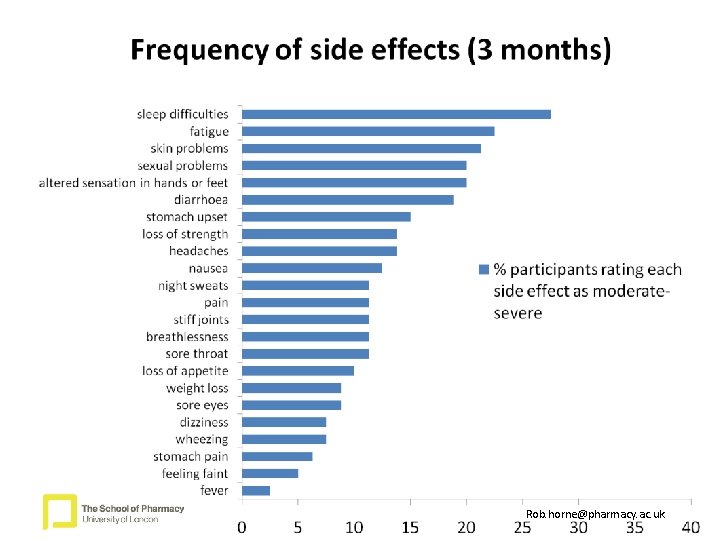
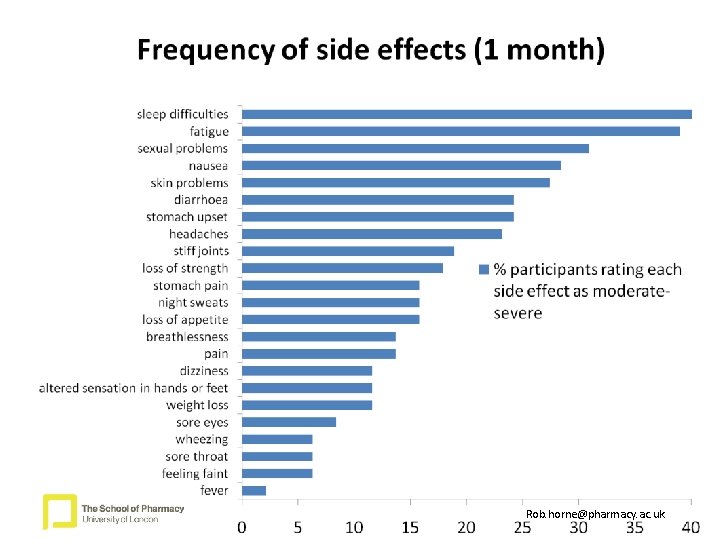
Measures 1: Symptoms associated with HIV and HAART • Symptom experiences • Participants were given 2 lists of 23 common symptoms and asked to indicate symptoms they attributed to HIV and those they attributed to HAART. • Each symptom rated for severity (1 = very mild, 2 = mild, 3 = moderate, 4 = severe, 5 = very severe) • Total number of symptoms rated as moderate, severe or very severe symptoms were included in these analyses Rob. horne@pharmacy. ac. uk


ART Adherence Low adherence identified by one or more: 1. Self-report of taking less that 95% of prescribed dose over previous month – Medication Adherence Self Report Inventory MASRI 1 and/or 2. Unilateral decision to stop treatment – and/or 3. Dropping out of study without notification Those in the low adherence group were significantly less likely to have an undetectable viral load at six months (Chi square= 10. 9, df=1, p<0. 001)2. 1. Walsh JC, Madalia S, Gazzard BG (2002) AIDS. 16(2): 269 -77 2. Horne R, Cooper V, Gellaitry G, Leake Date H, Fisher M. JAIDS. (2007) , 45(3): 334 -341 Rob. horne@pharmacy. ac. uk

ART Adherence Low adherence identified by one or more: 1. Self-report of taking less that 95% of prescribed dose over previous month – Medication Adherence Self Report Inventory MASRI 1 and/or 2. Unilateral decision to stop treatment – and/or 3. Dropping out of study without notification Those in the low adherence group were significantly less likely to have an undetectable viral load at six months (Chi square= 10. 9, df=1, p<0. 001)2. 1. Walsh JC, Madalia S, Gazzard BG (2002) AIDS. 16(2): 269 -77 2. Horne R, Cooper V, Gellaitry G, Leake Date H, Fisher M. JAIDS. (2007) , 45(3): 334 -341 Rob. horne@pharmacy. ac. uk

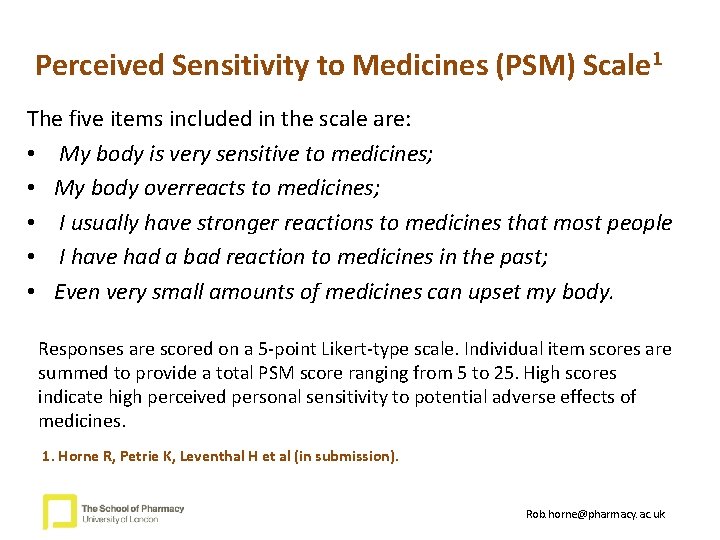
Perceived Sensitivity to Medicines (PSM) Scale 1 The five items included in the scale are: • My body is very sensitive to medicines; • My body overreacts to medicines; • I usually have stronger reactions to medicines that most people • I have had a bad reaction to medicines in the past; • Even very small amounts of medicines can upset my body. Responses are scored on a 5 -point Likert-type scale. Individual item scores are summed to provide a total PSM score ranging from 5 to 25. High scores indicate high perceived personal sensitivity to potential adverse effects of medicines. 1. Horne R, Petrie K, Leventhal H et al (in submission). Rob. horne@pharmacy. ac. uk

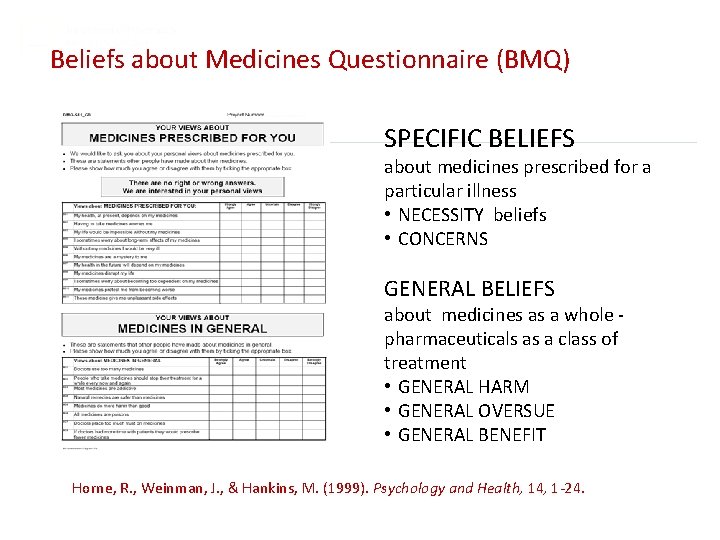
Beliefs about Medicines Questionnaire (BMQ) SPECIFIC BELIEFS about medicines prescribed for a particular illness • NECESSITY beliefs • CONCERNS GENERAL BELIEFS about medicines as a whole pharmaceuticals as a class of treatment • GENERAL HARM • GENERAL OVERSUE • GENERAL BENEFIT Horne, R. , Weinman, J. , & Hankins, M. (1999). Psychology and Health, 14, 1 -24.

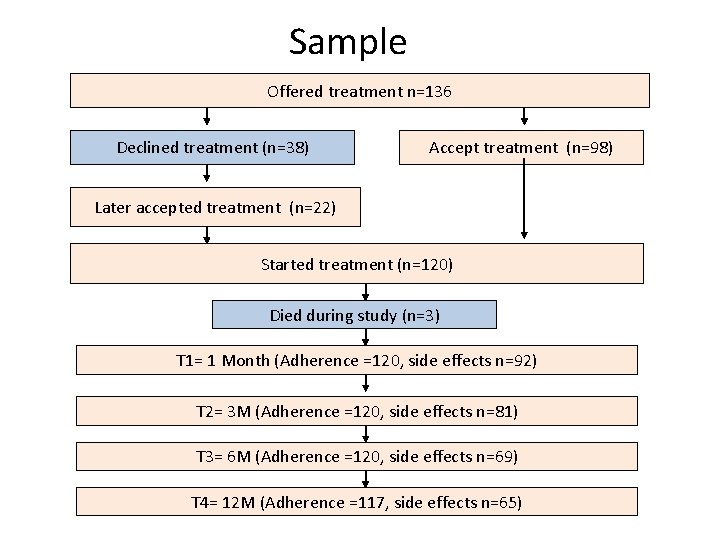
Sample Offered treatment n=136 Declined treatment (n=38) Accept treatment (n=98) Later accepted treatment (n=22) Started treatment (n=120) Died during study (n=3) T 1= 1 Month (Adherence =120, side effects n=92) T 2= 3 M (Adherence =120, side effects n=81) T 3= 6 M (Adherence =120, side effects n=69) T 4= 12 M (Adherence =117, side effects n=65)

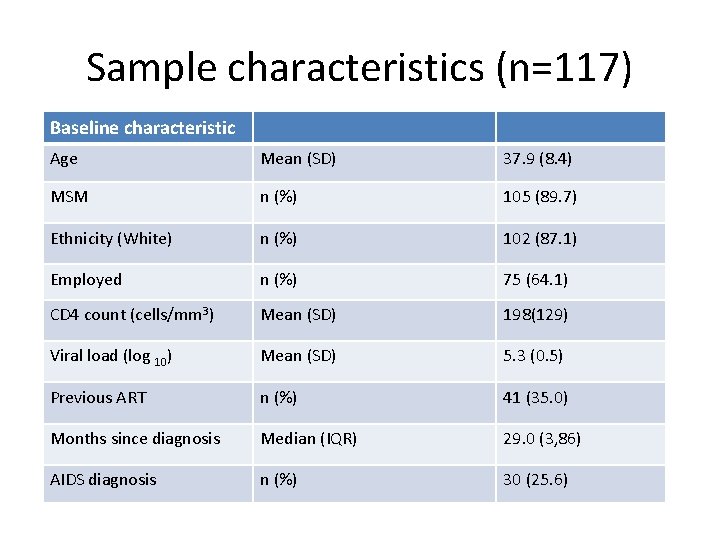
Sample characteristics (n=117) Baseline characteristic Age Mean (SD) 37. 9 (8. 4) MSM n (%) 105 (89. 7) Ethnicity (White) n (%) 102 (87. 1) Employed n (%) 75 (64. 1) CD 4 count (cells/mm 3) Mean (SD) 198(129) Viral load (log 10) Mean (SD) 5. 3 (0. 5) Previous ART n (%) 41 (35. 0) Months since diagnosis Median (IQR) 29. 0 (3, 86) AIDS diagnosis n (%) 30 (25. 6)

RESULTS

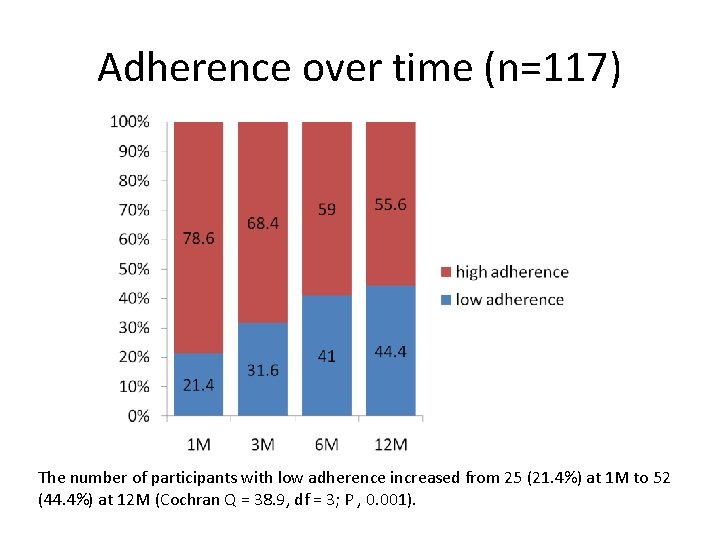
Adherence over time (n=117) The number of participants with low adherence increased from 25 (21. 4%) at 1 M to 52 (44. 4%) at 12 M (Cochran Q = 38. 9, df = 3; P , 0. 001).

Rob. horne@pharmacy. ac. uk

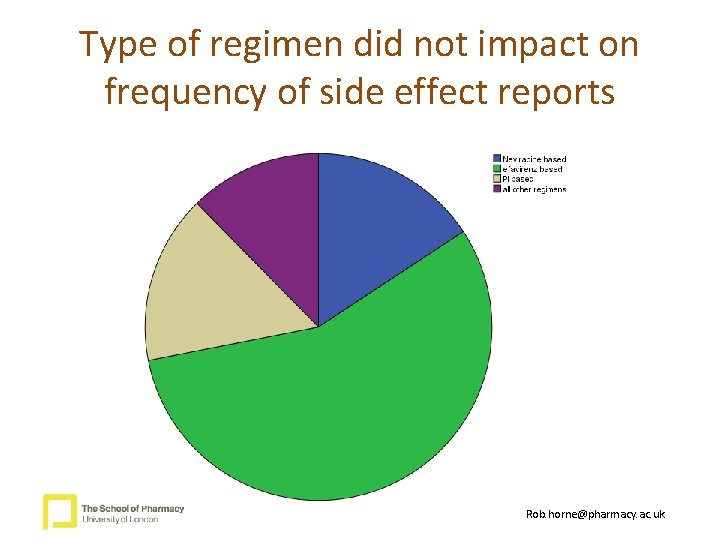
Type of regimen did not impact on frequency of side effect reports Rob. horne@pharmacy. ac. uk

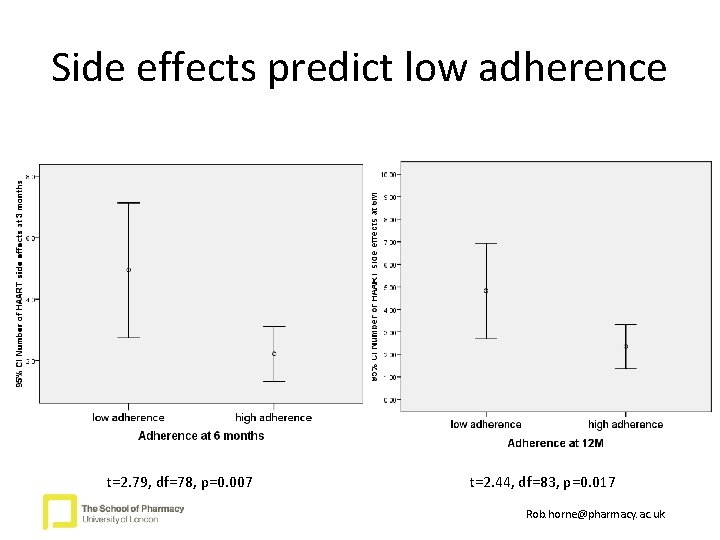
Side effects predict low adherence p=0. 011 t=2. 79, df=78, p=0. 007 t=2. 44, df=83, p=0. 017 Rob. horne@pharmacy. ac. uk

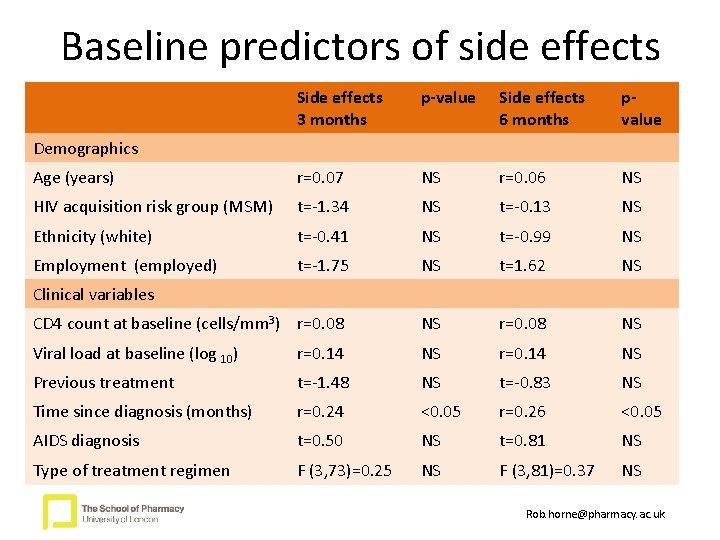
Baseline predictors of side effects Side effects 3 months p-value Side effects 6 months pvalue Age (years) r=0. 07 NS r=0. 06 NS HIV acquisition risk group (MSM) t=-1. 34 NS t=-0. 13 NS Ethnicity (white) t=-0. 41 NS t=-0. 99 NS Employment (employed) t=-1. 75 NS t=1. 62 NS CD 4 count at baseline (cells/mm 3) r=0. 08 NS Viral load at baseline (log 10) r=0. 14 NS Previous treatment t=-1. 48 NS t=-0. 83 NS Time since diagnosis (months) r=0. 24 <0. 05 r=0. 26 <0. 05 AIDS diagnosis t=0. 50 NS t=0. 81 NS Type of treatment regimen F (3, 73)=0. 25 NS F (3, 81)=0. 37 NS Demographics Clinical variables Rob. horne@pharmacy. ac. uk

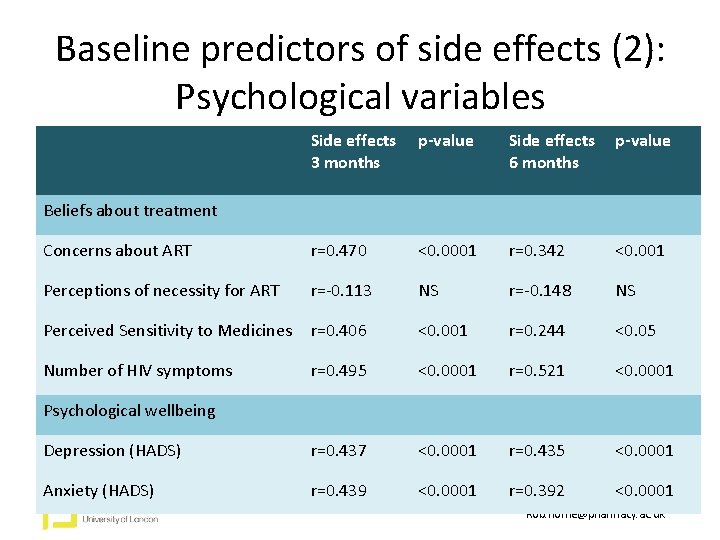
Baseline predictors of side effects (2): Psychological variables Side effects 3 months p-value Side effects 6 months p-value Concerns about ART r=0. 470 <0. 0001 r=0. 342 <0. 001 Perceptions of necessity for ART r=-0. 113 NS r=-0. 148 NS Perceived Sensitivity to Medicines r=0. 406 <0. 001 r=0. 244 <0. 05 Number of HIV symptoms r=0. 495 <0. 0001 r=0. 521 <0. 0001 Depression (HADS) r=0. 437 <0. 0001 r=0. 435 <0. 0001 Anxiety (HADS) r=0. 439 <0. 0001 r=0. 392 <0. 0001 Beliefs about treatment Psychological wellbeing Rob. horne@pharmacy. ac. uk

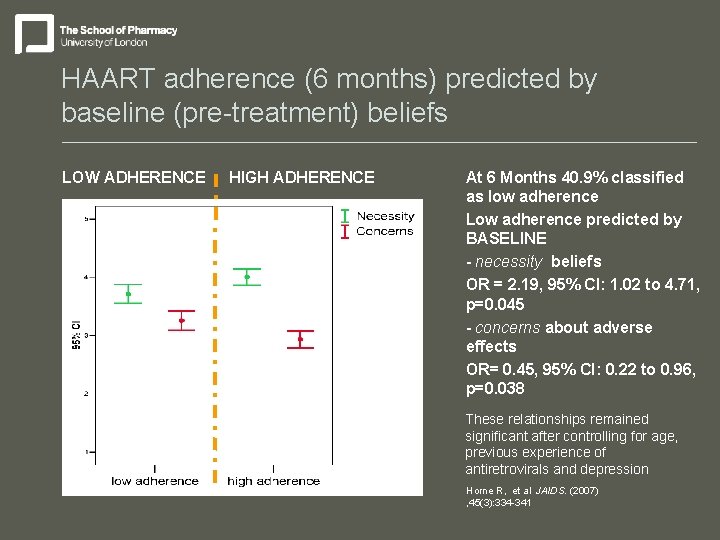
HAART adherence (6 months) predicted by baseline (pre-treatment) beliefs LOW ADHERENCE HIGH ADHERENCE At 6 Months 40. 9% classified as low adherence Low adherence predicted by BASELINE - necessity beliefs OR = 2. 19, 95% CI: 1. 02 to 4. 71, p=0. 045 - concerns about adverse effects OR= 0. 45, 95% CI: 0. 22 to 0. 96, p=0. 038 These relationships remained significant after controlling for age, previous experience of antiretrovirals and depression Horne R, et al JAIDS. (2007) , 45(3): 334 -341

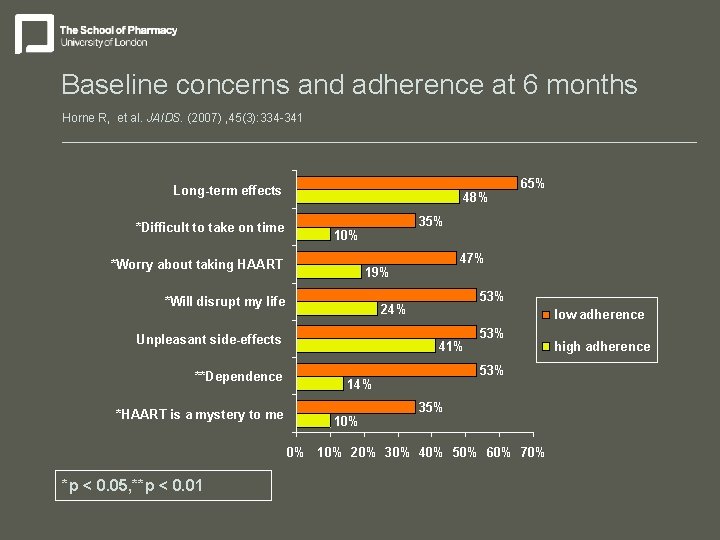
Baseline concerns and adherence at 6 months Horne R, et al. JAIDS. (2007) , 45(3): 334 -341 Long-term effects *Difficult to take on time 48% 35% 10% *Worry about taking HAART 53% 24% Unpleasant side-effects *HAART is a mystery to me 47% 19% *Will disrupt my life **Dependence low adherence 41% 53% 14% 10% 65% 35% 0% 10% 20% 30% 40% 50% 60% 70% *p < 0. 05, **p < 0. 01 high adherence

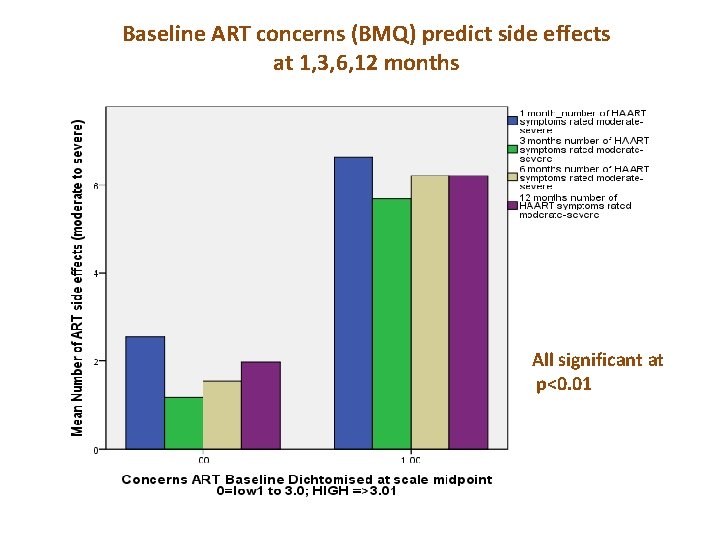
Baseline ART concerns (BMQ) predict side effects at 1, 3, 6, 12 months All significant at p<0. 01

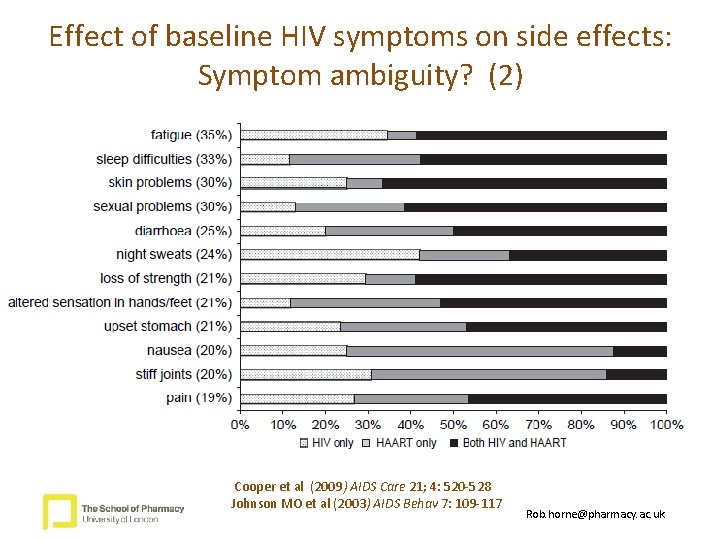
Effect of baseline HIV symptoms on side effects: Symptom ambiguity? (2) Cooper et al (2009) AIDS Care 21; 4: 520 -528 Johnson MO et al (2003) AIDS Behav 7: 109 -117 Rob. horne@pharmacy. ac. uk

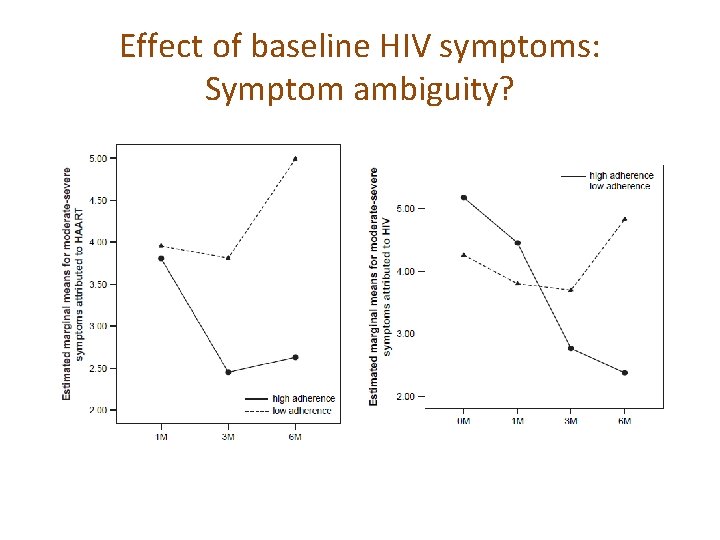
Effect of baseline HIV symptoms: Symptom ambiguity?

Limitations • Study under-powered for regression and structural equation modelling • Data collected from a single site • Sample predominately MSM Rob. horne@pharmacy. ac. uk

Conclusions • The emergence of ART side effects was associated with psychological variables including pre-treatment concerns about medicines, anxiety and depression, which may be amenable to change. • These results therefore have implications for the development of interventions to support patients and facilitate adherence. Rob. horne@pharmacy. ac. uk

Acknowledgements Study participants The staff at the Lawson Unit and Elton John Clinic Brighton. UK The study was supported by : National Institute for Health Research (RH) & an educational grant from Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Glaxo. Smith. Kline & Pharmacia. (VC)


Rob. horne@pharmacy. ac. uk
 Antiretroviral
Antiretroviral Antiretroviral
Antiretroviral Sss similarity theorem
Sss similarity theorem Side side side similarity
Side side side similarity Sss similarity definition
Sss similarity definition Similarity postulates
Similarity postulates Psychoanalytic therapy is to as humanistic therapy is to
Psychoanalytic therapy is to as humanistic therapy is to Bioness integrated therapy system occupational therapy
Bioness integrated therapy system occupational therapy Humanistic therapies aim to boost
Humanistic therapies aim to boost Radiation therapy side effects
Radiation therapy side effects Nicotine replacement therapy side effects
Nicotine replacement therapy side effects Emergence theory
Emergence theory The emergence of mass society
The emergence of mass society Ion storm
Ion storm Stages of second language development
Stages of second language development Emergence of scaling in random networks
Emergence of scaling in random networks Speech emergence stage
Speech emergence stage Plan emergence madagascar 2019-2023 pdf
Plan emergence madagascar 2019-2023 pdf Emergence netflix
Emergence netflix Entrepreneurship during post independence
Entrepreneurship during post independence The emergence of new values
The emergence of new values Plan emergence madagascar 2019-2023
Plan emergence madagascar 2019-2023 Itero göteborg
Itero göteborg So&e
So&e Landmarks in emergence of corporate governance
Landmarks in emergence of corporate governance Mass society
Mass society Perfect competition side by side graphs
Perfect competition side by side graphs Uil tea side by side
Uil tea side by side Sell side vs buy side
Sell side vs buy side Glass will break first on the weaker side, the side:
Glass will break first on the weaker side, the side: Side angle side theorem
Side angle side theorem Two wheels roll side by side
Two wheels roll side by side Root face
Root face Draw the projection of a regular hexagon of 25mm side
Draw the projection of a regular hexagon of 25mm side Lingual occlusion
Lingual occlusion Platzbedarf side by side melkstand
Platzbedarf side by side melkstand