Recruitment and Retention of Tumor Registrars the Missouri































- Slides: 37

Recruitment and Retention of Tumor Registrars: the Missouri experience J. Jackson-Thompson, MSPH, Ph. D Sue Vest, BA, CTR Missouri Cancer Registry, University of Missouri, Columbia

Missouri Cancer Registry • A collaborative partnership between the Missouri Department of Health & Senior Services (DHSS) and the University of Missouri - Columbia (MU); • Supported by CDC/NPCR Cooperative Agreement #U 55/CCU 721904 -04 and a contract between the University of Missouri and the Missouri Department of Health and Senior Services

Acknowledgments Sue Vest, CTR, MCR Project Manager Othr MCR operations staff – Nancy Cole, CTR & Audra Herkelman (MCR) Reporting facility staff Centers for Disease Prevention & Control National Program of Cancer Registries (CDC/NPCR)

Objectives Brief history of Missouri Cancer Registry (MCR); Introduce MCR operations staff; Identify some ways MCR has successfully dealt with recruitment & retention; and Outline some challenges still to be faced.

MCR Gold Celebration 2005

History of MCR Historical: 1972 - voluntary agreement State mandate – 1984, implemented ‘ 85 Received NPCR funding - 1995 – Reference year - 1996 Expanded reporting – 1999 – 192. 650 – 192. 657 RSMo NAACCR certification – 1998 dx. year NPCR quality indicators – ongoing ↑

MCR Database 36, 000 – 40, 000 records per year 28, 000 unduplicated MO cases per year >300, 000 unduplicated Missouri cases – 1996 and later Historical database - > 300, 000 records – 1995 and earlier

Challenges We All Face Timeliness: 90% at 12 months; 95% at 24 months Completeness: – ≥ 95% of expected cases – Treatment Accuracy: – Pass edits: 100% – Missing/unknown data elements: ranges from 2% 3% • Race, sex, age, county of residence – Death certificate only (DCE) cases ≤ 3%

Additional challenges many registries had (or still have) Early 1996: – Outdated hardware & software – Non-standard data elements & layout – Non-competitive salaries • Outdated job specifications (preelectronic) – Too few staff • Limited education & training opportunities • Difficulty retaining staff

1996 Assess the Situation

Need: Meet NPCR requirements Timeliness, completeness and quality Expanded cancer reporting statute Case-sharing agreements Advisory Board Data in standard NAACCR layout Collect required & recommended data elements

First Steps (Right off the Bat) Increase NPCR funding Upgrade hardware & buy commercial software Hire more CTRs – Rewrite job specs to reflect responsibilities – Obtain permission to hire at top of pay scale – Make working for MCR desirable Appoint Advisory Board Obtain case-sharing agreements

More Early Steps (Line Drives) Improve relationships with reporting facilities – From “There’s a law - our way or the highway” to “Partners for better patient outcomes” Provide service to reporting facilities – Offer training for hospital registry staff – Go to bat for hospital CTRs – talk to administrators

Meet the Challenges … And what do you get? More Challenges!

1999 (Extra base hit) Additional CTR positions funded by NPCR – No FTEs available Revise plan – Strengthen existing external relationship – Decrease state government – eliminate FTEs Outsource Operations! – Overnight, MCR operations staff became University staff

Next Steps (Home run) Impact of outsourcing – Greater flexibility in creating and filling positions – More understanding of need to maintain competitive salaries, retain staff – Obtained NAACCR certification – Met most NPCR requirements

How? (Home run #2) Flexible schedules for staff – Adjust hours to meet MCR staff needs – Added benefit – improved service to reporting facilities Allow telecommuting, working off-site – Find a good CTR, don’t let distance/circumstances be an issue Set standards – Must be CTR or CTR-eligible to be cancer data coordinator

Other Avenues (to the playoffs) Increase training opportunities – Send all MCR staff to training of their choice – Develop/improve training for hospital registrars Encourage staff to take advantage of tuition reimbursement Restructure operations to meet registry & staff needs

Further avenues (world series) Promote from within Annual salary increases Encourage staff to – Reach for the stars/achieve their dreams – Submit abstracts to NAACCR/make presentations – Get involved in research proposals Build a winning team

In Their Own Words

Database Management Unit Saba Yemane, BS Iris Zachary, BS, CTR Database Administrator Assistant Database Administrator

Quality Assurance Unit Electronic Deb Smith, CTR Senior Cancer Registrar Cate Ellis, CTR, BSN Cancer Data Coordinator

Quality Assurance Unit – Manual and Circuit-riding Brenda Lee, CTR Keri Grier Senior Cancer Registrar Health Program Assistant

Audit Unit Debbie Douglas, CTR Senior Cancer Registrar

Non-hospital Unit Nancy Cole, CTR Senior Cancer Debra Eccleston Health Program Assistant

Administration Jeannette Jackson. Thompson, MSPH, Ph. D, Operations Director Sue Vest, CTR Project Manager

Surveillance, Research and Special Projects Unit Gonza Namulanda, MS Research Assistant Gentry White, BA, BS, MA, Ph. D Candidate Research Assistant

Audra Our Office Support Staff

Challenges Still to be Faced Hospital pay scales outstripping MCR pay scales – Department & University HR supportive Change from exempt to hourly for CTRs Need for additional positions Restrictions placed on contract

Staff Frustrations & Wishes More staff in QA, Non-hospital and Audit units Salary commensurate with responsibilities, training & expertise Space Two bureaucracies – MU & DHSS – Lack of knowledge about registry operations – Lack of support Not enough time

Where are we now?

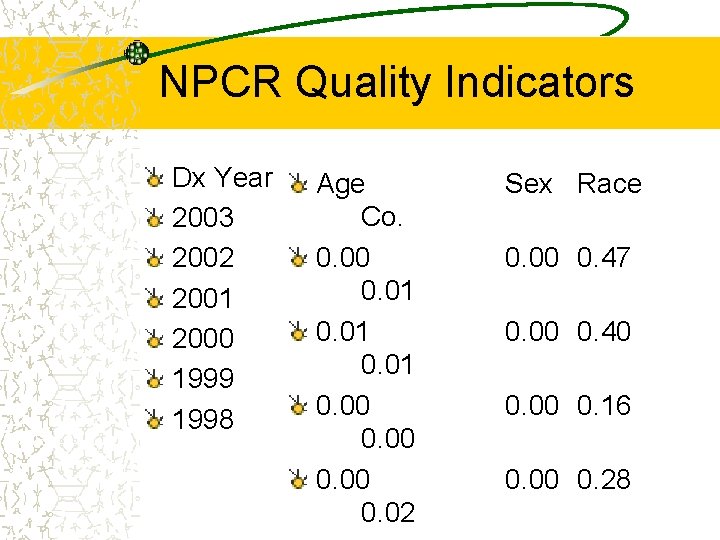
NPCR Quality Indicators Dx Year 2003 2002 2001 2000 1999 1998 Age Co. 0. 00 0. 01 0. 00 0. 02 Sex Race 0. 00 0. 47 0. 00 0. 40 0. 00 0. 16 0. 00 0. 28

Can we stop here? Overall completeness exceeds 95% at 24 months Completeness estimates vary – By race and sex – By site

The Future Electronic reporting capabilities Increased information on web site More non-hospital reporting More use of database for research

Research Trends – Incidence – Stage at Dx – Treatment – Age, sex, race/ethnicity Public health practice Outcomes

Contact Information Jeannette Jackson-Thompson, MSPH, Ph. D Operations Director, Missouri Cancer Registry and Research Assistant Professor, Health Management & Informatics, University of Missouri-Columbia Phone – 573 882 -7775 Toll-free for reporting facilities – 1 800 292 -2829 E-mail – jacksonthompsonj@health. missouri. edu MCR website: http: //mcr. umh. edu

MCR Gold Celebration 2005
 Northern and rural recruitment and retention initiative
Northern and rural recruitment and retention initiative Building a recruitment and retention plan
Building a recruitment and retention plan Onboarding portal upmc
Onboarding portal upmc Aja registrars
Aja registrars Meaning of recruitment
Meaning of recruitment Recruitment retention recovery
Recruitment retention recovery Oncogenes and tumor suppressor genes
Oncogenes and tumor suppressor genes Cachexia
Cachexia Missouri school counseling curriculum
Missouri school counseling curriculum Missouri economic research and information center
Missouri economic research and information center Compromise enables maine and missouri
Compromise enables maine and missouri Department of health and senior services missouri
Department of health and senior services missouri Missouri comprehensive guidance and counseling program
Missouri comprehensive guidance and counseling program Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Chụp tư thế worms-breton
Chụp tư thế worms-breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Các môn thể thao bắt đầu bằng tiếng nhảy
Các môn thể thao bắt đầu bằng tiếng nhảy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên?
Thế nào là giọng cùng tên? Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể