PREIMPLANTATION GENETIC DIAGNOSIS PGD PGD involves the genetic













- Slides: 30


PREIMPLANTATION GENETIC DIAGNOSIS (PGD) PGD involves the genetic testing of biopsy material from embryos that are generated through in vitro fertilization (IVF) techniques and involves the transfer of chromosomally normal and disease-free embryos to the uterus PGD allows couples who have an increased risk of transmitting genetic disorders not only to have healthy children, but also to prevent complications such as health problems and the psychological and financial burdens that may result from the termination of a pregnancy

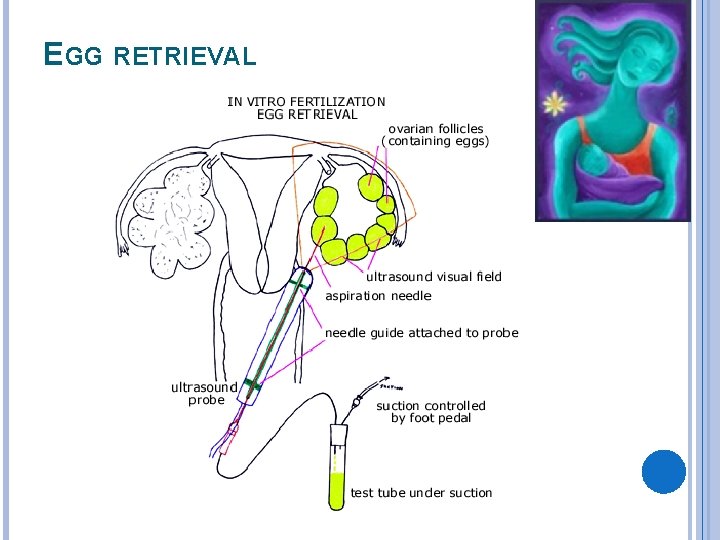
THE GENERAL METHOD OF IVF 1. Monitor egg maturation in the ovary - Ultrasound - Hormone levels 2. Collect eggs (mother’s own or from donor) - Injection of human chorionic gonadotropin (h. CG) and follicle stimulating hormone (FSH) to time egg ripening - Transvaginal aspiration using hollow needle

EGG RETRIEVAL

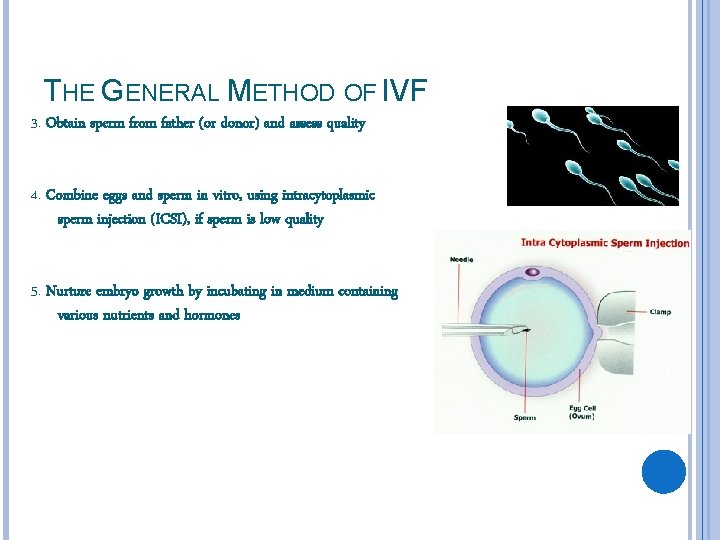
THE GENERAL METHOD OF IVF 3. Obtain sperm from father (or donor) and assess quality 4. Combine eggs and sperm in vitro, using intracytoplasmic sperm injection (ICSI), if sperm is low quality 5. Nurture embryo growth by incubating in medium containing various nutrients and hormones

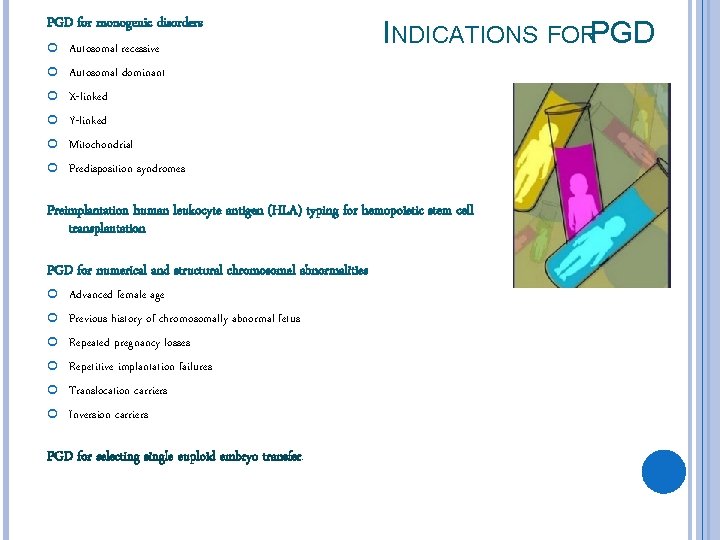
PGD for monogenic disorders Autosomal recessive Autosomal dominant X-linked Y-linked Mitochondrial Predisposition syndromes INDICATIONS FORPGD Preimplantation human leukocyte antigen (HLA) typing for hemopoietic stem cell transplantation PGD for numerical and structural chromosomal abnormalities Advanced female age Previous history of chromosomally abnormal fetus Repeated pregnancy losses Repetitive implantation failures Translocation carriers Inversion carriers PGD for selecting single euploid embryo transfer.

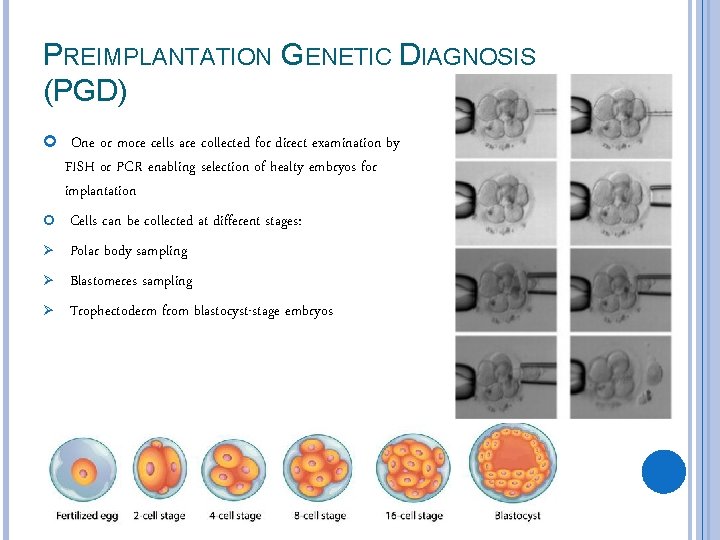
PREIMPLANTATION GENETIC DIAGNOSIS (PGD) One or more cells are collected for direct examination by Ø Ø Ø FISH or PCR enabling selection of healty embryos for implantation Cells can be collected at different stages: Polar body sampling Blastomeres sampling Trophectoderm from blastocyst-stage embryos

POLAR BODY BIOPSY Pre/Postfertilization Maternale inherited disorders Aneuploidies in advanced maternal age X-linked disease Maternal translocation Advantage: no negative effects on the subsequent steps of the embryo’s development longer time that is available for genetic analysis prior to embryo transfer Disadvantages: no information about the paternal genotype Cannot be used to diagnose: q paternal mutations q HLA typing q chromosomal abnormalities caused by paternal meiosis or mitotic errors qsex determination Only option available because of legal restrictions

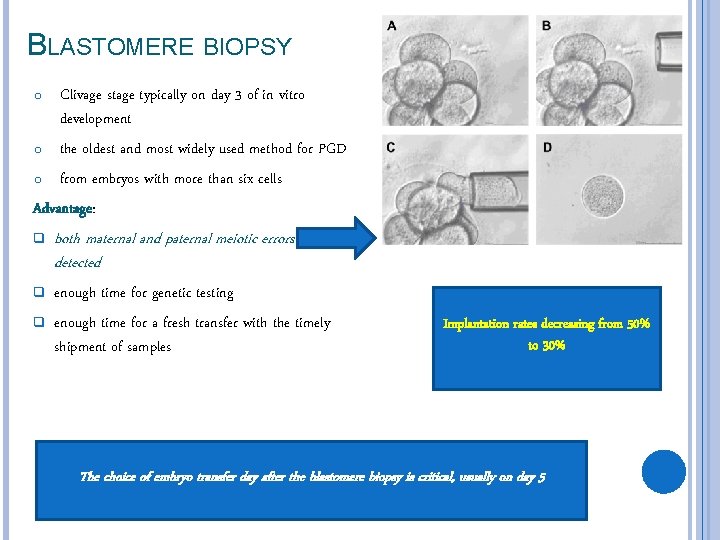
BLASTOMERE BIOPSY Clivage stage typically on day 3 of in vitro development o the oldest and most widely used method for PGD o from embryos with more than six cells Advantage: o q both maternal and paternal meiotic errors can be detected q enough time for genetic testing enough time for a fresh transfer with the timely shipment of samples q Implantation rates decreasing from 50% to 30% The choice of embryo transfer day after the blastomere biopsy is critical, usually on day 5

BLASTOCYST-STAGE BIOPSY Blastocyst-stage embryo biopsy can be defined as the retrieval of a small part of the trophectoderm from a hatching blastocyst Advantage: q. More than one cell to work with prevents most diagnostic errors that are caused by technical problems (hybridization failures, amplification failures, or the absence of a nucleus) q. Threefold reduction in the number of embryos that remained inadequately evaluated by the use of PGD for single-gene disorders q. No detrimental effect either on the blastocyst’s development or on the development of the fetus originating from the inner cell mass (ICM) The most common policy with blastocyst-stage biopsy is a day 5 biopsy with a day 6 transfer The trophectoderm karyotype is an excellent predictor of the Inner Cell Mass karyotype

ROLE OF NEW IVF TECHNIQUES: TIME-LAPSE IMAGING SYSTEMS Culturing embryos until day 5 to select the blastocyst with the best morphology ensures higher implantation rates and decreased miscarriage rates. Scoring embryos is a crucial step to select the best embryos for transfer, for cryopreservation, or for PGD to maintain high efficacy of the IVF cycle Time-lapse allows for the observation of the exact time points of cell divisions, compaction, blastocyst formation, generation and absorption of fragments, and multinucleation

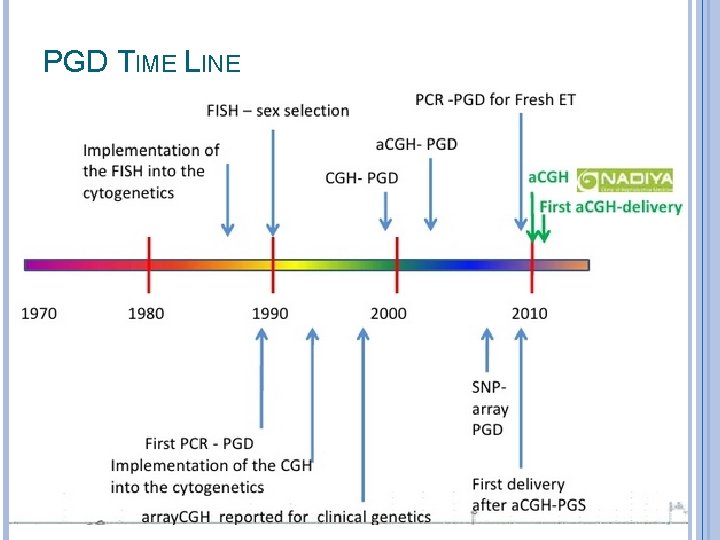
PGD TIME LINE

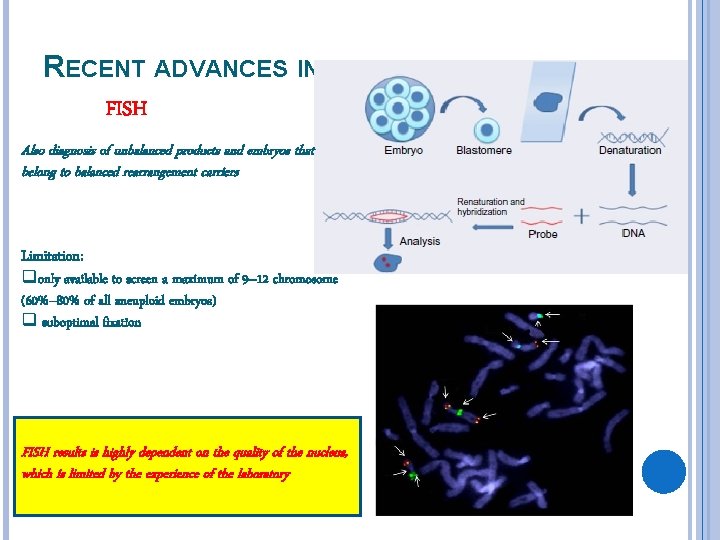
RECENT ADVANCES IN ANEUPLOIDY TESTING FISH Also diagnosis of unbalanced products and embryos that belong to balanced rearrangement carriers Limitation: qonly available to screen a maximum of 9– 12 chromosome (60%– 80% of all aneuploid embryos) q suboptimal fixation FISH results is highly dependent on the quality of the nucleus, which is limited by the experience of the laboratory

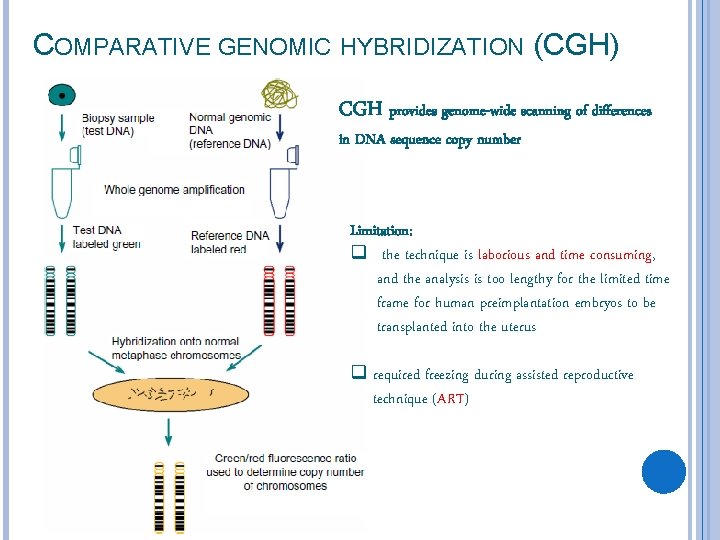
COMPARATIVE GENOMIC HYBRIDIZATION (CGH) CGH provides genome-wide scanning of differences in DNA sequence copy number Limitation: q the technique is laborious and time consuming, and the analysis is too lengthy for the limited time frame for human preimplantation embryos to be transplanted into the uterus q required freezing during assisted reproductive technique (ART)

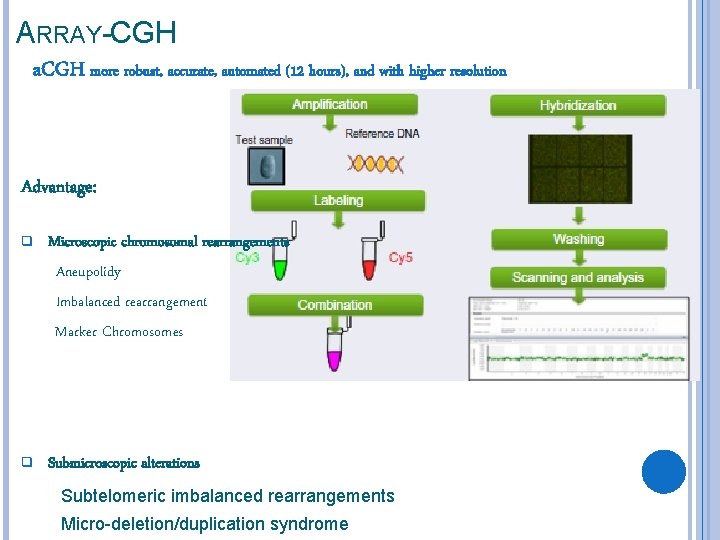
ARRAY-CGH a. CGH more robust, accurate, automated (12 hours), and with higher resolution Advantage: q Microscopic chromosomal rearrangements Aneupolidy Imbalanced rearrangement Marker Chromosomes q Submicroscopic alterations Subtelomeric imbalanced rearrangements Micro-deletion/duplication syndrome

ARRAY-CGH q Limitation: Cannot detect polyploidies, balanced chromosomal rearrangements (translocations, inversions), and uniparental disomies q cannot detect mosaicisms q cannot be detected because the total amount of DNA is the same as that of the control DNA Also, this method cannot be used to diagnose SGDs.

SNP-ARRAY Advantage: q Karyomapping is applicable to any inherited SGD within the informative SNP loci without the development of costly, time-consuming, and laborious patient- or disease-specific designs q In addition, SNP data enable the detection of chromosomal abnormalities (meiotic trisomies, monosomies, and deletions)

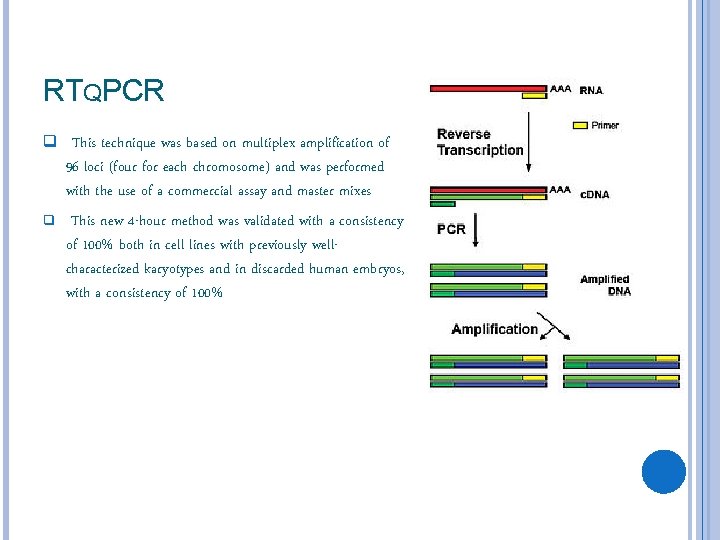
RTQPCR q This technique was based on multiplex amplification of q 96 loci (four for each chromosome) and was performed with the use of a commercial assay and master mixes This new 4 -hour method was validated with a consistency of 100% both in cell lines with previously wellcharacterized karyotypes and in discarded human embryos, with a consistency of 100%

OTHER TECHNIQUES NGS

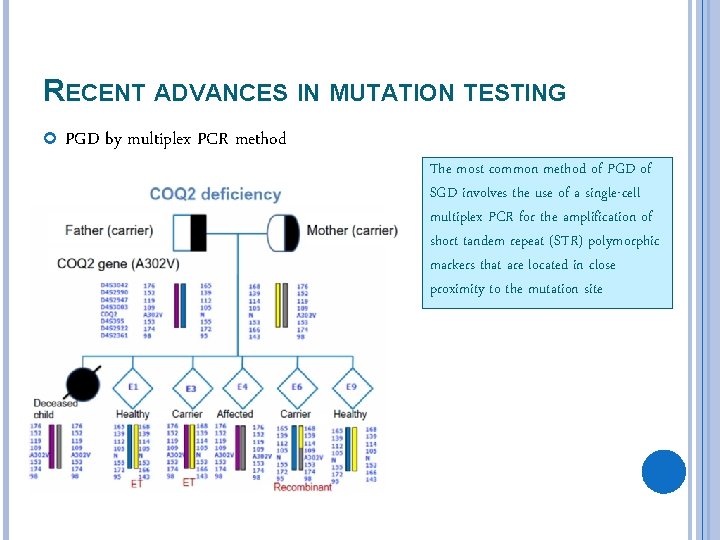
RECENT ADVANCES IN MUTATION TESTING PGD by multiplex PCR method The most common method of PGD of SGD involves the use of a single-cell multiplex PCR for the amplification of short tandem repeat (STR) polymorphic markers that are located in close proximity to the mutation site

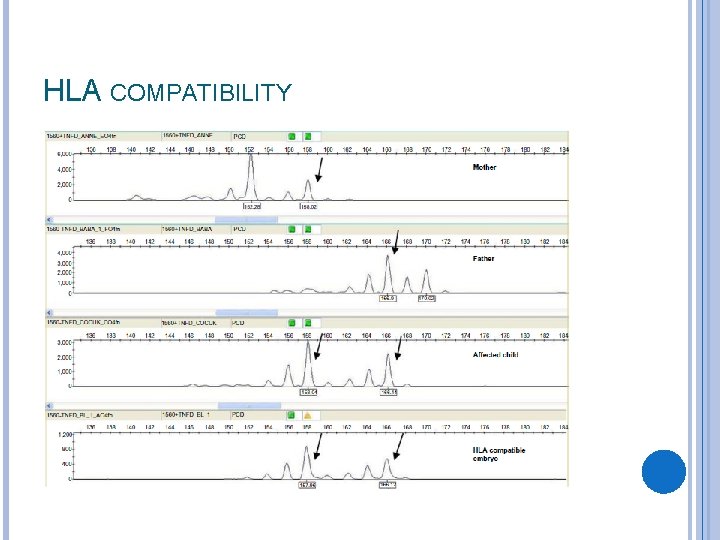
HLA COMPATIBILITY

RECENT ADVANCES IN MUTATION TESTING WGA (Whole genome amplification) o Some of the WGA protocols are PCR-based, such as primer extension PCR (ΡΕΡ) and degenerated oligonoucleotide primed PCR (DOP-PCR) o PEP was initially used on single sperms o MDA is a non-PCR, isothermal method for DNA amplification. o MDA, during its early use to amplify single blastomeres for PGD, was a long method (over 16 hours), and the ADO rates of the amplification products were as high as 34%. With this method, 12 different monogenic disorders with 38 different mutations were diagnosed successfully

RECENT ADVANCES IN MUTATION TESTING NGS Complex bioinformatic analyses are necessary to provide a sequence analysis Targeted, semiconductor technology-based NGS method, which was used with a bar-coding protocol that gave results in less than 24 hours

BENEFITS OF PGD Increased Implantation Rate Reduction in Pregnancy Losses Reduction in the Chance of Having a Child with Aneuploidy. Reduces the possibility of having to choose to terminate the pregnancy following a diagnosis of a probable genetic disorder.

RISKS Embryo damage Misdiagnosis The accuracy of the PGD for translocation is 90%. False negative result False positive result The chance for NO result The chance for mosaicism The use of special precautions to avoid exogenous DNA contamination has dramatically reduced the main causes of misdiagnosis IVF Risks Not Achieving Pregnancy There may not be any normal embryos available for transfer. The workup for PGD is expensive and labor intensive PGD can only detect a specific genetic disease in an embryo.

ALTERNATIVES TO PGD Conceive naturally and have prenatal diagnosis during pregnancy Consider assisted reproduction techniques in which one or both partners would not be the biological parent of the child. Adoption

FUTURE OF PGD Efforts continue to be focused on improving methods to obtain an accurate diagnosis. PGD holds great promise for the future as techniques and genetic tests are perfected. PGD may become routine in the next few years.

CONCLUSIONS After 10 years of PGD in Reproductive Medicine and the performance of 2500 cycles the main conclusions are : PGD is a reliable procedure in preventing the birth of affected children PGD of aneuploidy is effective and results in a high take home baby rate when implemented in certain categories of patients. Despite the efficiency of the PGD technique, conventional prenatal diagnosis is still required by most PGD laboratories

ANSA > Salute e Benessere > Speciali ed eventi > Legge 40: ad aprile sentenza Consulta su diagnosi preimpianto Per coppie fertili ma portatrici malattie legge 40 vieta esame Fibrosi Cistica Beta- Talassemia Distrofia muscolare di Duchenne

THANK YOU